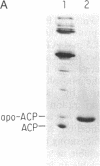
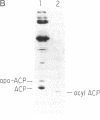
Abstract
An enzyme system catalyzing the synthesis of the beta 1,2-linked glucan backbone of the membrane-derived oligosaccharides of Escherichia coli from UDP-glucose has an essential requirement for the E. coli acyl carrier protein (ACP). This finding was surprising, because all other characterized functions of ACP involve acyl thioester residues linked to the phosphopantetheine moiety covalently bound to ACP. We now report that the activity of ACP in the synthesis of membrane-derived oligosaccharides is not altered by treatment with the sulfhydryl reagent N-ethylmaleimide nor by complete removal of the phosphopantetheine residue by treatment with a specific phosphodiesterase. The function of ACP in the synthesis of membrane-derived oligosaccharides is thus clearly different from that involved in lipid biosynthesis. We have nevertheless found that the same molecular species of ACP that undergo enzymic acylation with long-chain fatty acid residues also function in the synthesis of membrane-derived oligosaccharides.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dylan T., Ielpi L., Stanfield S., Kashyap L., Douglas C., Yanofsky M., Nester E., Helinski D. R., Ditta G. Rhizobium meliloti genes required for nodule development are related to chromosomal virulence genes in Agrobacterium tumefaciens. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4403–4407. doi: 10.1073/pnas.83.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Halverson L. J., Stacey G. Signal exchange in plant-microbe interactions. Microbiol Rev. 1986 Jun;50(2):193–225. doi: 10.1128/mr.50.2.193-225.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath B., Kondorosi E., John M., Schmidt J., Török I., Györgypal Z., Barabas I., Wieneke U., Schell J., Kondorosi A. Organization, structure and symbiotic function of Rhizobium meliloti nodulation genes determining host specificity for alfalfa. Cell. 1986 Aug 1;46(3):335–343. doi: 10.1016/0092-8674(86)90654-9. [DOI] [PubMed] [Google Scholar]
- Jackowski S., Edwards H. H., Davis D., Rock C. O. Localization of acyl carrier protein in Escherichia coli. J Bacteriol. 1985 Apr;162(1):5–8. doi: 10.1128/jb.162.1.5-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski S., Rock C. O. Ratio of active to inactive forms of acyl carrier protein in Escherichia coli. J Biol Chem. 1983 Dec 25;258(24):15186–15191. [PubMed] [Google Scholar]
- Kennedy E. P. Osmotic regulation and the biosynthesis of membrane-derived oligosaccharides in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1092–1095. doi: 10.1073/pnas.79.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. J., Kennedy E. P., Reinhold V. N. Osmotic adaptation by gram-negative bacteria: possible role for periplasmic oligosaccharides. Science. 1986 Jan 3;231(4733):48–51. doi: 10.1126/science.3941890. [DOI] [PubMed] [Google Scholar]
- Polacco M. L., Cronan J. E., Jr A mutant of Escherichia coli conditionally defective in the synthesis of holo-[acyl carrier protein]. J Biol Chem. 1981 Jun 10;256(11):5750–5754. [PubMed] [Google Scholar]
- Rock C. O., Cronan J. E., Jr Acyl carrier protein from Escherichia coli. Methods Enzymol. 1981;71(Pt 100):341–351. doi: 10.1016/0076-6879(81)71043-7. [DOI] [PubMed] [Google Scholar]
- Rock C. O., Cronan J. E., Jr Acyl-acyl carrier protein synthetase from Escherichia coli. Methods Enzymol. 1981;71(Pt 100):163–168. doi: 10.1016/0076-6879(81)71023-1. [DOI] [PubMed] [Google Scholar]
- Rock C. O., Jackowski S. Regulation of phospholipid synthesis in Escherichia coli. Composition of the acyl-acyl carrier protein pool in vivo. J Biol Chem. 1982 Sep 25;257(18):10759–10765. [PubMed] [Google Scholar]
- Shearman C. A., Rossen L., Johnston A. W., Downie J. A. The Rhizobium leguminosarum nodulation gene nodF encodes a polypeptide similar to acyl-carrier protein and is regulated by nodD plus a factor in pea root exudate. EMBO J. 1986 Apr;5(4):647–652. doi: 10.1002/j.1460-2075.1986.tb04262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therisod H., Weissborn A. C., Kennedy E. P. An essential function for acyl carrier protein in the biosynthesis of membrane-derived oligosaccharides of Escherichia coli. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7236–7240. doi: 10.1073/pnas.83.19.7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagelos P. R., Larrabes A. R. Acyl carrier protein. IX. Acyl carrier protein hydrolase. J Biol Chem. 1967 Apr 25;242(8):1776–1781. [PubMed] [Google Scholar]
- Weissborn A. C., Kennedy E. P. Biosynthesis of membrane-derived oligosaccharides. Novel glucosyltransferase system from Escherichia coli for the elongation of beta 1----2-linked polyglucose chains. J Biol Chem. 1984 Oct 25;259(20):12644–12651. [PubMed] [Google Scholar]