Abstract
Objective
To systematically review studies on the prevalence and complications of traditional male circumcision (i.e. circumcision by a traditional provider with no formal medical training), whose coverage and safety are unclear.
Methods
We systematically searched databases and reports for studies on the prevalence and complications of traditional male circumcision in youth 10–24 years of age in eastern and southern Africa, and also determined the ages at which traditional circumcision is most frequently performed.
Findings
Six studies reported the prevalence of traditional male circumcision, which had been practised in 25–90% of all circumcised male study participants. Most circumcisions were performed in boys 13–20 years of age. Only two of the six studies on complications reported overall complication rates (35% and 48%) following traditional male circumcision. The most common complications were infection, incomplete circumcision requiring re-circumcision and delayed wound healing. Infection was the most frequent cause of hospitalization. Mortality related to traditional male circumcision was 0.2%.
Conclusion
Published studies on traditional male circumcision in eastern and southern Africa are limited; thus, it is not possible to accurately assess the prevalence of complications following the procedure or the impact of different traditional practices on subsequent adverse events. Also, differences in research methods and the absence of a standard reporting format for complications make it difficult to compare studies. Research into traditional male circumcision procedures, practices and complication rates using standardized reporting formats is needed.
ملخص
الغرض
مراجعة منهجية للدراسات التي أجريت حول انتشار ومضاعفات ختان الذكور التقليدي (أي الذي يجريه معالجون شعبيون بدون تدريب طبي رسمي)، والذي لا يتضح مقدار سلامته ومدى التغطية به.
الطريقة
أجرى الباحثون بحثاً في قواعد المعطيات والتقارير عن الدراسات حول انتشار ومضاعفات ختان الذكور التقليدي بين الشباب في عمر 10-24 عاماً في شرق وجنوب أفريقيا، كما حددوا الأعمار التي يجرى غالباً عندها الختان التقليدي.
الموجودات
أبلغت ست دراسات عن انتشار ختان الذكور التقليدي، والذي أجرى لدى 25% إلى 90% من ختان جميع الذكور المشاركين في الدراسات، وأجري معظم الختان بين الفتيان في عمر 13 إلى 20 عاماً. وأبلغت دراستان فقط من الست دراسات عن المضاعفات وبلغ المعدلان الإجماليان للمضاعفات التي تلت ختان الذكور التقليدي (35% و 48%). وكانت أكثر المضاعفات شيوعاً هي العدوى، وعدم اكتمال الختان الذي استوجب إعادة الختان، وتأخر التئام الجرح. وكانت العدوى هي أكثر الأسباب في المعالجة داخل المستشفيات. وبلغ معدل الوفيات المتعلق بختان الذكور التقليدي 0.2%.
الاستنتاج
إن الدراسات المنشورة عن ختان الذكور التقليدي في شرق وجنوب أفريقيا مازالت محدودة؛ ولذلك لا يمكن قياس انتشار المضاعفات التالية لهذا الإجراء بدقة، ولا يمكن قياس تأثير الممارسات التقليدية المختلفة للأحداث الضائرة. كما أن اختلاف طرائق البحث وعدم وجود نموذج معياري للتبليغ عن المضاعفات يؤدي إلى صعوبة مقارنة الدراسات. وهناك حاجة لبحوث عن إجراءات وممارسات ختان الذكور التقليدية ومعدلات مضاعفاتها باستخدام نماذج تبليغ معيارية.
Resumen
Objetivo
Revisar sistemáticamente los estudios realizados sobre la prevalencia y las complicaciones de la circuncisión tradicional masculina (es decir, circuncisión realizada por curanderos sin ningún tipo de formación médica reglada), cuyo alcance y seguridad son inciertas.
Métodos
Se realizaron búsquedas sistemáticas en las bases de datos y en los informes de los estudios realizados sobre la prevalencia y las complicaciones de la circuncisión masculina tradicional en jóvenes de entre 10 y 24 años de edad en el África oriental y meridional. También se determinaron las edades en las que se suele realizar la circuncisión tradicional.
Resultados
Seis estudios informaron sobre la prevalencia de la circuncisión masculina tradicional, que se había practicado en el 25-90% de todos los participantes circuncidados del estudio. La mayoría de las circuncisiones se realizaron en jóvenes con edades comprendidas entre los 13 y los 20 años. De los seis estudios sobre las complicaciones, únicamente dos notificaron las tasas globales de las mismas (35% y 48%) tras la circuncisión masculina tradicional . Las complicaciones más frecuentes fueron: infección, circuncisión incompleta (requiriendo una segunda circuncisión) y retraso en la cicatrización. La causa más frecuente de hospitalización fue la infección. La mortalidad asociada a la circuncisión masculina tradicional fue del 0,2%.
Conclusión
Los estudios publicados sobre la circuncisión masculina tradicional en África oriental y meridional son escasos, por lo que no se puede evaluar con exactitud la prevalencia de complicaciones posteriores al procedimiento o las consecuencias de las distintas prácticas tradicionales en los acontecimientos adversos subsiguientes. Además, las diferencias existentes en los métodos de investigación y la ausencia de un formulario normalizado de notificación de complicaciones dificultan la comparación de los estudios. Es necesario realizar investigaciones sobre los procedimientos, las prácticas y los índices de complicaciones de la circuncisión tradicional masculina que utilicen formularios normalizados de notificación.
Resumé
Objectif
Évaluer de façon systématique les études sur la prévalence et les complications de la circoncision masculine traditionnelle (c’est-à-dire la circoncision par un prestataire traditionnel, sans aucune formation médicale officielle), pour laquelle la couverture et la sécurité sont incertaines.
Méthodes
Nous avons recherché de façon systématique dans les bases de données et les rapports des études sur la prévalence et les complications liées à la circoncision traditionnelle chez les jeunes hommes âgés de 10 à 24 ans dans l’Est et le Sud de l’Afrique. Nous avons également déterminé les âges auxquels la circoncision traditionnelle est la plus fréquemment réalisée.
Résultats
Six études ont rapporté la prévalence de la circoncision masculine traditionnelle, pratiquée chez 25 à 90% de tous les participants masculins circoncis étudiés. La plupart des circoncisions ont été effectuées sur des garçons âgés de 13 à 20 ans. Seules deux des six études sur les complications ont indiqué des taux de complications globaux (35% et 48%) suite à une circoncision masculine traditionnelle. Les complications les plus communes étaient une infection, une circoncision incomplète nécessitant une nouvelle circoncision ainsi que des retards de cicatrisation. L’infection était la cause d’hospitalisation la plus fréquente. La mortalité liée à la circoncision masculine traditionnelle s’élevait à 0,2%.
Conclusion
Les études publiées sur la circoncision masculine traditionnelle en Afrique orientale et méridionale sont limitées. Il est donc impossible d’aborder avec précision la prévalence des complications suite à l’opération ou l’impact des différentes pratiques traditionnelles sur les événements négatifs ultérieurs. De plus, les différences dans les méthodes de recherche et l’absence d’un format de rapport standard des complications rendent difficile la comparaison des études. Des recherches en matière d’opérations, de pratiques et de taux de complications de la circoncision masculine traditionnelle utilisant des formats de rapport normalisés sont donc nécessaires.
Резюме
Цель
Провести систематический обзор исследований о распространенности традиционной циркумцизии мужчин (т. е. циркумцизии, проводимой традиционным целителем, не имеющим формального медицинского образования) и связанных с ней осложнений. Охват и безопасность такой циркумцизии неясны.
Метод
Мы провели систематический обзор баз данных и отчетов об исследованиях, посвященных распространенности традиционной циркумцизии мужчин и связанным с ней осложнениям среди молодежи в возрасте 10–24 лет в восточных и южных районах Африки, а также определили возраст, в котором чаще всего проводится традиционная циркумцизия.
Результаты
В шести исследованиях сообщалось о распространенности традиционной циркумцизии мужчин, которую прошли от 25 до 90% всех охваченных исследованием мужчин, которым была сделана циркумцизия. В большинстве случаев циркумцизии подвергались мальчики и юноши в возрасте от 13 до 20 лет. Только в двух из шести исследований сообщалось об общем показателе осложнений (35 и 48%) после традиционной циркумцизии мужчин. Самыми распространенными видами осложнений были инфекция, неполная циркумцизия, после которой требовалось проведение повторной циркумцизии, и медленное заживление раны. Наиболее распространенной причиной госпитализации была инфекция. Смертность, связанная с традиционной циркумцизией мужчин, составляла 0,2%.
Вывод
Число опубликованных исследований, посвященных традиционной циркумцизии мужчин в восточной и южной частях Африки, ограничено; поэтому невозможно точно оценить распространенность осложнений после этой процедуры или воздействие различных традиционных практик на последующие неблагоприятные события. Кроме того, различия в методиках исследования и отсутствие стандартной формы учета осложнений затрудняют сравнение исследований. Необходимы исследования процедур и практик традиционной циркумцизии мужчин, а также показателей осложнений с использованием стандартных форм отчетности.
摘要
目的
旨在系统综述关于传统男性包皮环切(如传统的未经正规医疗培训的医生进行的包皮环切)的发病率和并发症的研究,该手术覆盖范围和安全性尚不清楚。
方法
我们系统调查了研究东南亚10-24岁年龄段传统男性包皮环切导致的发病率和并发症的数据库和报告,并且确定了传统包皮环切最常进行的年龄段。
发现
六项研究报告了传统男性包皮环切的发病率,在所有进行过包皮环切的男性研究对象中,25%到90%出现了发病率。大多数包皮环切术是在男孩13到20岁期间进行。六项关于并发症的研究中,仅有两项报告了传统男性包皮环切术后的总体并发症比率(35%和48%)。最常见的并发症有感染、要求再切割的不完全包皮环切和延迟伤口愈合。感染是最常见的住院原因。与传统男性包皮环切相关的死亡率是0.2%。
结论
已经发表的关于东南亚传统男性包皮环切的研究有限;因此,不可能准确地评估传统手术程序之后并发症的发病率或不同的传统操作对随后不良事件的影响。此外,因为研究方法上的差异和并发症标准报告格式的缺乏,很难进行比较研究。需要运用标准化的报告格式研究传统男性包皮环切的程序、操作和并发症发生率。
Introduction
Globally, 30% of men are circumcised, mostly for religious reasons.1 In many African societies, male circumcision is carried out for cultural reasons, particularly as an initiation ritual and a rite of passage into manhood. The procedure herein referred to as traditional male circumcision is usually performed in a non-clinical setting by a traditional provider with no formal medical training. When carried out as a rite of passage into manhood, traditional male circumcision is mainly performed on adolescents or young men. The self-reported prevalence of traditional male circumcision varies greatly between eastern and southern Africa, from 20% in Uganda and southern African countries to more than 80% in Kenya.2
Randomized controlled trials have shown a substantial protective effect of male circumcision with respect to female–to–male transmission of human immunodeficiency virus (HIV).3–5 In these studies, complications following male circumcision ranged from 1.7% to 7.6% and were mostly of minor clinical significance.6,7 However, serious complications and even deaths have been reported from traditional male circumcision carried out on adolescents.8,9 While medical male circumcision is increasingly being incorporated in comprehensive strategies for the prevention of HIV infection10, traditional providers will continue to be an important source of circumcision for many males in eastern and southern Africa and will not easily be replaced by male circumcision performed in a clinical setting for reasons that are both cultural and linked to health service capacity. Our aim in this systematic review was to evaluate traditional male circumcision in eastern and southern Africa in terms of its prevalence, the age at which the procedure is undertaken and the complications arising from it.
Methods
Search strategy
An initial search of African Healthline and African Index Medicus using the terms “traditional circumcision” and “traditional circumcisers” brought up no studies; we therefore excluded these databases from the subsequent search. We searched for primary studies in MEDLINE, Web of Science, Popline and African Journals OnLine using the terms “male circumcision AND traditional”, “traditional circumcisers”, “male circumcision AND anthropology”, “male circumcision AND complications”, “male circumcision AND history”, “male circumcision AND manhood/masculinity/rite of passage”. The search was limited to the period from January 1980 to February 2008 and covered articles published in any language. Additional reports were provided by key researchers and members of the Joint United Nations Programme on HIV/AIDS Working Group on Male Circumcision, and the East and Southern Africa Inter-Agency Task Team on Male Circumcision. We also searched all the references listed in the articles identified during the initial search.
Selection criteria
To be included in the review, articles had to describe original research studies from eastern and southern Africa that reported on the prevalence or complications of traditional male circumcision (as defined in the introduction) performed on youth 10–24 years of age, either specifically or in the context of a larger study. For assessing prevalence and age, we included cross-sectional, cohort and register studies; for assessing complications, we also included intervention studies. Studies reporting on male circumcision provided through medical facilities were excluded, as were studies focusing on newborn and infant circumcision.
Evaluation of studies
Two medically trained reviewers (AW, TK) independently evaluated identified studies in terms of methods, study design and representativeness of the study population. The reviewers then extracted the data relating to prevalence, age and complications of traditional male circumcision. Any discrepancies in the evaluation were resolved by consensus.
Results
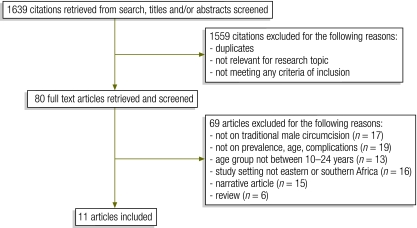
The review identified 11 articles reporting on 12 studies (Fig. 1). Of the included articles, six reported on the prevalence of traditional male circumcision,11–16 eight on age at the time of the procedure 11–18 and six on complications following the procedure.14,17–21
Fig. 1.
Study selection in systematic review of the literature on traditional male circumcision in eastern and southern Africa
Prevalence
Only one study reported national prevalence estimates of traditional male circumcision (Table 1). In that study, from Namibia, 21% of the males in the sample had been circumcised, and one-quarter of them indicated that they had been circumcised by a traditional provider.15In the remaining studies that provided information on the prevalence of traditional male circumcision, the information was collected at the district level.11–14,16 The percentage of men reportedly circumcised varied from 52% in an urban setting in Mbale district, Uganda,13 to 80% in rural areas of the Southern Rift Valley in Kenya,11 and 99% in rural and urban areas of Tarime district, in the United Republic of Tanzania.16 Rates of circumcision performed by traditional circumcisers in districts where male circumcision is widely practised were up to 90% in Uganda,13 74% in Kenya11 and 63% in the United Republic of Tanzania.16 In the townships of the Gauteng province of South Africa, 10% of males aged 14–24 years and 22% of those aged 19–29 years were reportedly circumcised, in 58–65% of cases by traditional circumcisers.12,14 The choice of providers depended on the affiliation to different ethnic groups; for example, 86% of Xhosa participants were circumcised by traditional providers compared with only 37% of Tswana men.12
Table 1. Studies on the prevalence and/or age of traditional male circumcision in eastern and southern Africa, as identified through a systematic review of the literature.
| First author | Publication year | Study setting | Study design | Data collection method | Study population |
Prevalence of MC |
Of circumcised males, % circumcised by traditional provider | Age at MC (in years) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tribe/residence | Male study participants (No.) | Age in years | % | 95% CIa | |||||||
| National Institute for Medical Research, United Republic of Tanzania16 | 2009 | United Republic of Tanzania, rural and urban areas, three regions. Only districts where most men are circumcised traditionally were considered (i.e. Mara region, Tarime district) | Cohort | Interview and clinical assessment of circumcision status | 77% Mkurya | 170 | 18–24 (29%) 25–34 (45%) 35–44 (26%) |
99 | 98–100 | 63 | By 18 (86.5%), by 21.5 (99.0%) |
| Peltzer17 | 2008 | South Africa, OR Tambo district, Eastern Cape, 17 initiation schools | Intervention | Interview 7 days post TMC; clinical examination 2, 4, 7 and 14 days post TMC | Xhosa | 192 | 18.7 (mean) | NA (MC was inclusion criterion for assessing complications after MC) | – | 100 | 18.7 ± 1.9 (mean ± SD) |
| Bailey18 | 2008 | Kenya, Bungoma district, Western province, 87% rural residence | Cohort | Direct observation at 3, 8 and 30 days post TMC (n = 24); interview and direct observation 62 days (median) post MC (n = 298); interview 46 days (median) post MC (n = 709) | Babukusu | 1007 | 12–16 (“majority”) | NA (MC was inclusion criterion for assessing complications after MC) | – | 44 | 15 (median), 14.7 (mean) |
| Shaffer11 | 2007 | Kenya, Southern Rift Valley, rural population | Cross-sectional | Questionnaire (self-completed) | Kalenjin, Kisii, Luhya, Luo | 1378 | 31.1 ± 8.8 (mean ± SD) | 80 | 78–82 | 74 | 12.7 ± 3.5 (mean ± SD) |
| DHS15 | 2006–07 | Namibia, nationally representative survey | Cross-sectional | Interview | Rural and urban | 5576 | 15–49 | 21 | 20–22 | 25 | < 13 (84%), 13–19 (9%) |
| Lagarde14 | 2003 | South Africa, North Central, Westonaria, Gauteng township | Cross-sectional | Interview | Sotho, Tswana, Xhosa and Zulu | 482 | 19–29 | 22 | 19–26 | 65 | 17 (median); 16–18 (IQR) |
| Rain-Taljaard12 | 2003 | South Africa, North Central, Gauteng township, mining area, urban | Cross-sectional | Interview | Sotho, Tswana, Xhosa and Zulu | 723 | 14–24 | 10 | 8–12 | 58 | 20 (median); 17–24 (IQR) |
| Bailey13 | 1999 | Uganda, Mbale district, industrial borough | Cross-sectional | Interview | Mainly Bagisu | 365 | 30.9 (mean) | 52 | 47–57 | 90 | 18 (median, non-Muslims), 13 (median, Muslims) |
CI, confidence interval; DHS, Demographic and Health Survey; IQR, interquartile range; MC, male circumcision; NA, not available; SD, standard deviation; TMC, traditional male circumcision.
a 95% CIs were calculated by the authors of the present review using the Wilson method.22
Age
Age at traditional male circumcision varied both within and among countries and ranged from 13 to 20 years (Table 1).11–14,17,18 In the United Republic of Tanzania, the period prevalence of circumcision was 86.5% at 18 and 99% at 21.5 years of age.16 In Namibia, 84% of boys were circumcised before the age of 13 years: in Omaheke and Kunene districts, most boys were below 2 years of age, and in Kavango district they were generally between 9 and 12 years of age.15
Complications
Overall complication rates
Only two out of six studies reported overall complication rates following traditional male circumcision; rates were 35% in Kenya (83% for 12 directly observed study participants)18 and 48% in South Africa (Table 2).14
Table 2. Studies on the complications of traditional male circumcision in eastern and southern Africa, as identified through a systematic review of the literature.
| First author | Publication year | Study setting | Study design | Data collection method | Study population |
Complications of TMC and types |
|||
|---|---|---|---|---|---|---|---|---|---|
| Male study participants circumcised by traditional provider (unless stated otherwise) (No.) | Age (in years) | Overall prevalence (%) | 95% CIa | Types | |||||
| Bailey 18 | 2008 | Kenya, Bungoma district, Western province, 87% rural residence | Cohort | Direct observation days 3, 8, 30 | 12 | 14.6 (mean) | 83 | 55–95 | 42% infection, 33% extensive circumcision, 33% permanent adverse sequelae, 25% loss of erectile function, 0% bleeding, 100% delayed wound healing (> 30 days) |
| a) Interview only (n = 272) 46 days (median) post MC; b) interview and direct observation (n = 173) 62 days (median) post MC | 445 | 14.7 (mean) 15.0 (median) | 35 | 31–40 |
Direct observation (n = 173 of 445): 21% delayed wound healing, 17% keloid scarring, 14% swelling, 12% crust still present, 12% foreskin remaining, 2% open wounds, 0% infection. Interview (n = 173 + 272): 24% delayed wound healing |
||||
| Peltzer17 | 2008 | South Africa, OR Tambo district, Eastern Cape, 17 initiation schools |
Intervention | Interview day 7, clinical examination days 2,4,7,14 post TMC | 192 | 18.7 (mean) | NR | – | 21% delayed wound healing, 16% infection, 11% pain, 10% incomplete circumcision, 4% dehydration |
| Meissner21 | 2006 | South Africa, Eastern Cape |
Hospital register | Review of data from the Department of Health, Eastern Cape | 10 609 | NR | NR | – | 0.2% mortality, 0.1% amputation/mutilation of penis |
| Lagarde14 | 2003 | South Africa, North Central, Westonaria, Gauteng |
Cross-sectional | Interview | 482 | 19–29 | 48 | 44–52 | 43% severe pain, 26% bleeding, 6% penile injury, 4% infection |
| Magoha20 | 1999 | Nigeria (3 hospitals in Lagos), Kenya (3 hospitals in Nairobi) | Cohort | Hospital record review (1981–1998) | 249 assessed for complications following medical MC | 13–24 (61%) | 11 | 8–15 | 3% wound infection, 1% severe haemorrhage, 1% urinary retention, 0.4% incomplete circumcision |
| Cross-sectional | Hospital record review | 50 admitted for complications after MC, 80% of them circumcised by traditional circumciser | NR | NR | – | 26% infection, 16% severe haemorrhage, 10% incomplete circumcision, 6% urinary retention, 4% septicaemia, 4% loss of glans, 2% loss of penis | |||
| Crowley19 | 1990 | South Africa, Cecilia Makiwane Hospital, Ciskei |
Cohort | Hospital record review | 45 admitted with “septic circumcision” | 21.5 (mean) | NR | – | 93% penile injury, 67% systemic infection, 19% severe dysuria, 9% mortality |
CI, confidence interval; MC, male circumcision; NR, not reported; TMC, traditional male circumcision.
a 95% CIs were calculated by the authors of the present review using the Wilson method.22
Types of complications
Two studies used direct observation to assess complications after traditional male circumcision.17,18 Infection and delayed wound healing were the most common complications. No severe bleeding occurred in the Kenyan (n = 12),18 and the South African study (n = 192).17 Excessive circumcision was reported as a primary complication after traditional male circumcision in the South African study17 and as a secondary result of incomplete initial circumcision in the Kenyan study.18 Re-circumcision resulted in excessive removal of skin and a deepened wound with prolonged wound healing, excessive scarring and loss of penile sensitivity. Delayed wound healing and keloid scarring were also associated with the use of a powder containing penicillin and talc that is used for wound care by traditional providers in Kenya.18 Fatalities did not occur in the South African study17 and one death was prevented by the research team in the Kenyan study.18
One study assessed complications based on recall by participants (n = 108).14 In contrast to results from direct observation, bleeding (26%) and severe pain (43%) were reported as being major adverse results, whereas delayed wound healing was not mentioned and local infections were reported in only 4% of cases.14
According to hospital admission records, infection was the most common reason for admission in Kenya, Nigeria and South Africa.19,20 In the South African study, two-thirds of the cases presented with systemic infection requiring treatment with antibiotics.19 Four of the 45 admitted patients had lost the glans of the penis and two patients lost the entire penis. In this study, 93% of the 45 subjects presented with some form of penile injury resulting not necessarily from the circumcision procedure itself but from poor post-operative wound care. Such care included tight bandages (traditionally believed to improve wound healing), which constricted the blood supply of the penile skin, in some cases causing occlusion of the deep dorsal arteries and leading to gangrene.19 The study from Kenya and Nigeria reported loss of the penis in 6% of all admitted cases.20 Dehydration was a frequent cause of death, due to fluid being restricted after the circumcision as a further test of the initiates’ endurance.19
Another study analysed circumcision-related complications from register data for 10 609 young men circumcised in the Eastern Cape province, South Africa, in June 2005.21 Of these, 3% were admitted for circumcision-related complications. Amputations or mutilations occurred in 0.1% of the cases and 0.2% of the 10 609 young men died. Septicaemia, pneumonia and dehydration were the most frequent causes of death.
Complications after circumcision by traditional versus medical providers
Three studies compared complications following circumcision by traditional and medical providers.14,18,20 Medical providers included surgeons,20 surgeons and general practitioners,14 and clinical officers,18 although “medical” circumcisions in the study by Bailey also included circumcisions by uncertified practitioners with little or no formal training in health care.18 In this study, directly observed complications occurred in 11 of 12 boys circumcised by a medical provider and in 10 of 12 boys circumcised traditionally (Table 2).18 However, more severe permanent adverse sequelae, such as loss of erectile function, persistent swelling and extensive scarring (n = 4), occurred in the traditionally circumcised group, whereas in the medical group, adverse sequelae were mostly cosmetic (pronounced torsion, jagged cut line with massive foreskin remaining, n = 3).18 Based on self-reporting by 445 medically circumcised boys, the overall rate of complications following male circumcision by medical providers was 18%, with infection and ruptured sutures being the most common acute complications. Among the 1007 study participants, infection was equally common among those circumcised traditionally and medically (data for 709 participants from self-report). Traditionally circumcised boys were less likely to access post-operative care (odds ratio: 0.67; 95% confidence interval: 0.45–0.99).18 Direct observation of 298 subjects on day 62 (median) after male circumcision revealed significant differences between the traditionally and medically circumcised groups.18 In the study of hospitals in Nigeria and Kenya, complete or partial amputation of the penis had occurred in 14% of the 50 hospital admissions after traditional male circumcision, but not once after medical male circumcision by surgeons (n = 249). The types of complications leading to admission after traditional male circumcision were not common after medical male circumcision, with rates of 3% for serious wound infection, 1% for severe bleeding and 0% for incomplete circumcision.20 In the study in the Gauteng township of South Africa, self-reported healing time (median: 3 weeks) did not differ among those who were circumcised traditionally or medically.14 However, the frequency of self-reported pain differed significantly between the two groups: 86% after traditional male circumcision and 61% after medical male circumcision.14
None of the studies reported on the assessment of confounders potentially related to the complications seen after traditional male circumcision, such as diabetes or coagulopathies.
Discussion
Main findings
National prevalence of traditional male circumcision is unknown for most countries in eastern and southern Africa. Data were available for Namibia, however, and indicated that one in four circumcisions is done by a traditional circumciser. Studies reporting on providers of male circumcision at district level were available from Kenya, South Africa, the United Republic of Tanzania and Uganda, with the prevalence of circumcisions performed by traditional circumcisers ranging from 37% to 90%.
The median age at circumcision ranged from 13 to 20 years, with considerable variation within and among countries, depending on the traditions of different ethnic groups.12,15 In some settings, circumcision may take place at an earlier age, especially when parents have their sons circumcised in a clinical setting in anticipation of fewer complications.18
The best available evidence on the complications following traditional male circumcision comes from a large cohort study in Kenya that reported a complication rate of 35%.18 Other studies were methodologically poor (e.g. retrospective assessments, lack of control group and self-reporting of complications) and most were cross-sectional.14,17,19–21 The included studies showed significantly higher rates of complications after traditional male circumcision than after male circumcision provided in a clinical setting. However, complications were also high for the clinical setting, perhaps because this type of circumcision was sometimes undertaken by untrained and underequipped health workers (18–25%).14,18
A comparison of the frequency of complications across studies was hampered by different research methods and lack of standardization in reporting. In general, poor postoperative wound care seemed to account for more complications than the circumcision itself,17–19 a finding that has important implications for the training of traditional circumcisers. Suturing the wound after traditional male circumcision is not a routine practice, but different traditional techniques (e.g. certain herb preparations) are being used to establish haemostasis.23 Analysis of hospital records in Kenya and Nigeria showed severe haemorrhage in 16% of the cases admitted after traditional male circumcision,20 but no severe bleeding was reported from the studies based on direct observation.17,18 More than 10% of males admitted to hospitals in Kenya, Nigeria and South Africa after traditional circumcision had partial or complete amputation of the penis, a condition that has serious life-long implications when it cannot be remedied through reconstructive surgery.19,20
Little information was available on the factors that may have contributed to the occurrence of complications, such as the technique, the setting (e.g. the initiate’s or the traditional circumciser’s home, an initiation school or mass circumcision at a public place), the instruments used or the methods of cleaning them. The exception was the study by Peltzer, which evaluated the impact of a training intervention for traditional circumcisers in the Eastern Cape province of South Africa.17 The authors reported continuous use of the traditional assegai (spear) by more than half of the traditional circumcisers in initiation schools, despite having been trained in safer techniques and provided with surgical blades. Similarly, one-third of the traditional nurses did not wear gloves for postoperative wound care, although the practice was recommended in their training.
Study limitations
Relevant studies may have been missed if they were not included in the databases searched for this review. Additional studies in the published literature were found, however, by contacting experts on male circumcision and by hand-searching for unpublished studies in the reference lists of all the publications identified in the initial search. We restricted our review to eastern and southern Africa because of the high prevalence of HIV infection in this region and the potential role traditional male circumcision providers could play in this context, especially in performing circumcision where access to formal health services is limited. The increasing demand for services by the population could generate a new “market”, with fly-by-night, self-declared “traditional circumcisers” with no training whatsoever seizing the opportunity to earn money.
Another possible limitation of our study is the exclusion of all Demographic and Health Survey (DHS) reports except for the one from Namibia, which was alone in providing prevalence estimates relating specifically to traditional male circumcision. The small sample sizes of some of the studies on the prevalence of traditional male circumcision limit the generalizability of the reported results. For all prevalence estimates of male circumcision, the calculated 95% confidence intervals were narrow. Subnational prevalence estimates vary considerably within most eastern and southern African countries. Therefore, district-level data cannot be interpreted as nationally representative, whatever the sample size.
Prevalence estimates based on data from self-report may lack validity; for example, up to 20% of the men in one study falsely reported having been circumcised.24 Comparability of the results across studies may also be hampered by the use of different terminology. Some languages lack a specific word for male circumcision and phrases such as “being a man”, or “having been initiated into manhood” are used instead (personal communication at regional consultation on young people and male circumcision in eastern and southern Africa, Johannesburg, South Africa, 2008). Men who report having been circumcised may be referring to the cultural initiation rites, with or without the surgical removal of the foreskin. No details have been provided about the techniques used to remove the foreskin partially or completely,25 although complication rates and the effectiveness of the procedure in preventing HIV infection vary with the type of surgical technique employed.
Implications for research and public health
Randomized controlled trials are the best sources of evidence on the safety of interventions. However, in the cultural context of traditional male circumcision, this type of study design is not feasible and carefully planned cohort studies are probably the best alternative.
More information on the providers of male circumcision is needed to improve the safety of the procedure. Male circumcision performed as a rite of passage is not necessarily carried out by a traditional circumciser; for example, half of the young men medically circumcised in Nigerian and Kenyan hospitals indicated “cultural initiation into manhood” as their reason for having been circumcised.20 Nevertheless, in most eastern and southern African countries, circumcisions are still carried out primarily by traditional circumcisers, although these vary in type from those whose role is handed down from generation to generation to health workers without specific training in male circumcision.26
One approach to minimizing complications following traditional male circumcision is to strengthen collaboration with the traditional sector by training traditional circumcisers.17 Further research is warranted to assess the feasibility and impact of such training interventions. Another alternative, practised in Kenya, is to carry out circumcision in hospitals, followed by a “modern” period of seclusion for receiving education on health, life skills and religious and cultural issues. In keeping with accepted traditions, these hospital programmes mostly adhere to male-only care throughout the operation and the period of instruction.27–29 In 2006, such programmes accounted for 4% of all boyhood circumcisions during the circumcision season in Kenya (every other year during a specific period of the year),27 where their high acceptability has reduced the stigmatization of boys not circumcised in traditional settings.29 In South Africa, the integration of medical male circumcision with traditional manhood initiation rituals still lacks acceptability; 70% of men fear being stigmatized if they are circumcised medically.30 Studies should be conducted on the acceptability of medical male circumcision in communities where male circumcision is carried out for traditional ritualistic purposes.
Prospective studies of better quality (e.g. with larger samples and a thorough assessment of outcomes and potential confounders) are needed to systematically assess the frequency of complications following traditional male circumcision in adolescents and young men. While hospital admission records can provide information on severe complications following the procedure, population estimates of complications cannot be calculated from studies based on hospital admission data. Furthermore, many initiates may not be able to seek post-circumcision care in medical facilities because dropping out of initiation school is regarded as shameful in some contexts.31 Initiates stay at these retreats or “initiation schools” not only for wound care after circumcision, but because this period of seclusion constitutes a very important part of the ritual for transmitting sociocultural norms and preparing for adult life.32 Also, contact with women is forbidden during the period of seclusion after traditional male circumcision, and going to a hospital would be likely to involve contact with female health workers.33
Conclusion
This is the first reported systematic review of studies on the prevalence and complications of traditional male circumcision. Most studies available from eastern and southern Africa were inadequate for assessing the prevalence or safety of traditional male circumcision in that region. Prevalence data should be collected in conjunction with information on providers of circumcision (e.g. through DHSs). High-quality prospective studies in different settings are urgently required to assess the complications of traditional male circumcision. Research on traditional male circumcision practices would also be useful to develop ways of strengthening collaboration with the medical sector, improving the safety of traditional male circumcision, and assessing the efficacy of training programmes for traditional circumcisers. Finally, studies should be conducted on the acceptability of medical male circumcision in communities where traditional circumcision is practised, and on how acceptability can affect the scaling up of medical male circumcision in such communities.
Competing interests:
None declared.
References
- 1.Weiss H, Polonsky J. Male circumcision: global trends and determinants of prevalence, safety and acceptability. Geneva: World Health Organization & United Nations Joint Programme on HIV/AIDS; 2007. [Google Scholar]
- 2.Drain PK, Halperin DT, Hughes JP, Klausner JD, Bailey RC. Male circumcision, religion, and infectious diseases: an ecologic analysis of 118 developing countries. BMC Infect Dis. 2006;6:172. doi: 10.1186/1471-2334-6-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey RC, Moses S, Parker CB, Agot K, Maclean I, Krieger JN, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–56. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 5.Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, Nalugoda F, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–66. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 6.Weiss HA, Halperin D, Bailey RC, Hayes RJ, Schmid G, Hankins CA. Male circumcision for HIV prevention: from evidence to action? AIDS. 2008;22:567–74. doi: 10.1097/QAD.0b013e3282f3f406. [DOI] [PubMed] [Google Scholar]
- 7.Muula AS, Prozesky HW, Mataya RH, Ikechebelu JI. Prevalence of complications of male circumcision in Anglophone Africa: a systematic review. BMC Urol. 2007;7:4. doi: 10.1186/1471-2490-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sidley P. Botched circumcisions lead to arrest for murder. BMJ. 1996;313:647. doi: 10.1136/bmj.313.7058.647a. [DOI] [PubMed] [Google Scholar]
- 9.Ncayiyana DJ. Astonishing indifference to deaths due to botched ritual circumcision. S Afr Med J. 2003;93:545. [PubMed] [Google Scholar]
- 10.New data on male circumcision and HIV prevention: policy and programme implications Geneva: World Health Organization & United Nations Joint Programme on HIV/AIDS; 2007. [Google Scholar]
- 11.Shaffer DN, Bautista CT, Sateren WB, Sawe FK, Kiplangat SC, Miruka AO, et al. The protective effect of circumcision on HIV incidence in rural low-risk men circumcised predominantly by traditional circumcisers in Kenya: two-year follow-up of the Kericho HIV Cohort Study. J Acquir Immune Defic Syndr. 2007;45:371–9. doi: 10.1097/QAI.0b013e318095a3da. [DOI] [PubMed] [Google Scholar]
- 12.Rain-Taljaard RC, Lagarde E, Taljaard DJ, Campbell C, MacPhail C, Williams B, et al. Potential for an intervention based on male circumcision in a South African town with high levels of HIV infection. AIDS Care. 2003;15:315–27. doi: 10.1080/0954012031000105379. [DOI] [PubMed] [Google Scholar]
- 13.Bailey RC, Neema S, Othieno R. Sexual behaviors and other HIV risk factors in circumcised and uncircumcised men in Uganda. J Acquir Immune Defic Syndr. 1999;22:294–301. doi: 10.1097/00126334-199911010-00012. [DOI] [PubMed] [Google Scholar]
- 14.Lagarde E, Dirk T, Puren A, Reathe RT, Bertran A. Acceptability of male circumcision as a tool for preventing HIV infection in a highly infected community in South Africa. AIDS. 2003;17:89–95. doi: 10.1097/00002030-200301030-00012. [DOI] [PubMed] [Google Scholar]
- 15.Namibia, Ministry of Health and Social Services. Demographic and Health Survey2006 Available from: http://www.measuredhs.com/hivdata/surveys/survey_detail.cfm?survey_id=482 [accessed 15 January 2008].
- 16.National Institute for Medical Research. Situation analysis for male circumcision in Tanzania Dar es Salaam: Ministry of Health and Social Welfare; 2009. [Google Scholar]
- 17.Peltzer K, Nqeketo A, Petros G, Kanta X. Traditional circumcision during manhood initiation rituals in the Eastern Cape, South Africa: a pre-post intervention evaluation. BMC Public Health. 2008;8:64. doi: 10.1186/1471-2458-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey RC, Egesah O, Rosenberg S. Male circumcision for HIV prevention: a prospective study of complications in clinical and traditional settings in Bungoma, Kenya. Bull World Health Organ. 2008;86:669–77. doi: 10.2471/BLT.08.051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowley IP, Kesner KM. Ritual circumcision (Umkhwetha) amongst the Xhosa of the Ciskei. Br J Urol. 1990;66:318–21. doi: 10.1111/j.1464-410X.1990.tb14936.x. [DOI] [PubMed] [Google Scholar]
- 20.Magoha GAO. Circumcision in various Nigerian and Kenyan hospitals. East Afr Med J. 1999;76:583–6. [PubMed] [Google Scholar]
- 21.Meissner O, Buso DL. Traditional male circumcision in the Eastern Cape–scourge or blessing? S Afr Med J. 2007;97:371–3. [PubMed] [Google Scholar]
- 22.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–72. doi: 10.1002/(SICI)1097-0258(19980430)17:8<857::AID-SIM777>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 23.Mogotlane SM, Ntlangulela JT, Ogunbanjo BG. Mortality and morbidity among traditionally circumcised Xhosa boys in the Eastern Cape Province, South Africa. Curationis. 2004;27:57–62. doi: 10.4102/curationis.v27i2.980. [DOI] [PubMed] [Google Scholar]
- 24.Weiss HA, Plummer ML, Changalucha J, Mshana G, Shigongo ZS, Todd J, et al. Circumcision among adolescent boys in rural northwestern Tanzania. Trop Med Int Health. 2008;13:1054–61. doi: 10.1111/j.1365-3156.2008.02107.x. [DOI] [PubMed] [Google Scholar]
- 25.Brown JE, Micheni KD, Grant EM, Mwenda JM, Muthiri FM, Grant AR. Varieties of male circumcision: a study from Kenya. Sex Transm Dis. 2001;28:608–12. doi: 10.1097/00007435-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Peltzer K, Nqeketo A, Petros G, Kanta X. Evaluation of a safer male circumcision training programme for traditional surgeons and nurses in the Eastern Cape, South Africa. Afr J Tradit Complement Altern Med. 2008;5:346–54. doi: 10.4314/ajtcam.v5i4.31289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown J, Micheni KD. Male adolescent circumcision in Kenya: teaching activities sponsored by FBOs in 2006. Report to the Catholic Medical Mission Board; 2007. Available from: http://www.cmmb.org/What/MaleAdolescentCircumcisioninKenya2006-Brown-Reportwith4annexes.pdf.pdf [accessed 26 October 2010].
- 28.Grant E, Brown J, Michen K, Grant A, Manuthu E, Njeru J. “Seizing the day”: right time, right place, and right messages for adolescent male reproductive sexual health: lessons from the Meru of Eastern Province Kenya. Int J Mens Health. 2004;3:189–96. doi: 10.3149/jmh.0303.189. [DOI] [Google Scholar]
- 29.Catholic Medical Mission Board. Meeting summary report of the Eastern and Southern Africa Faith-Based Organization Male Circumcision Consultation, Limuru, Kenya, 20 to 21September2007 Available from: http://www.malecircumcision.org/publications/documents/ESA_MC_Consultative_Conference_Report-20-21_September_2007_Final.pdf [accessed 17 June 2010]. [Google Scholar]
- 30.Peltzer K, Kanta X. Medical circumcision and manhood initiation rituals in the Eastern Cape, South Africa: a post intervention evaluation. Cult Health Sex. 2009;11:83–97. doi: 10.1080/13691050802389777. [DOI] [PubMed] [Google Scholar]
- 31.Kanta XGM. Traditional male circumcision and initiation into manhood: legal, health and environmental perspectives. Gonubie: Impilo Ya Bantu Health and Development Projects; 2004.
- 32.Vincent L. ‘Boys will be boys’: traditional Xhosa male circumcision, HIV and sexual socialisation in contemporary South Africa. Cult Health Sex. 2008;10:431–46. doi: 10.1080/13691050701861447. [DOI] [PubMed] [Google Scholar]
- 33.Mayatula V, Mavundla TR. A review on male circumcision procedures among South African blacks. Curationis. 1997;20:16–20. [PubMed] [Google Scholar]