Abstract
Vitamin B12 (cobalamin, Cbl) is an essential nutrient in human metabolism. Genetic diseases of vitamin B12 utilisation constitute an important fraction of inherited newborn disease. Functionally, B12 is the cofactor for methionine synthase and methylmalonyl CoA mutase. To function as a cofactor, B12 must be metabolised through a complex pathway that modifies its structure and takes it through subcellular compartments of the cell. Through the study of inherited disorders of vitamin B12 utilisation, the genes for eight complementation groups have been identified, leading to the determination of the general structure of vitamin B12 processing and providing methods for carrier testing, prenatal diagnosis and approaches to treatment.
Vitamin B12, also known as cobalamin (Cbl), is a micronutrient that is synthesised only by microorganisms, yet is essential to human health. Cobalamin was first isolated by Smith (Ref. 1) and Rickes (Ref. 2), after Minot and Murphy (Ref. 3) showed that pernicious anaemia could be treated with oral liver extract. Later, vitamin B12 deficiency as a result of genetic disease was described despite adequate vitamin intake (Ref. 4). Some patients responded successfully to very high doses of vitamin B12, suggesting blocks in vitamin processing. These patients had homocystinuria and/or methylmalonic aciduria, implicating dysfunctional methionine synthase (MS) and/or methylmalonyl-CoA mutase (MUT or MCM). We now know that blocks in the intracellular processing of cobalamin into cofactor forms, methylcobalamin (MeCbl) for MS and adenosylcobalamin (AdoCbl) for MCM, or in the functional activity of MS or MCM result in inborn errors. These genetic blocks may be devastating in newborns or in early childhood. Understanding the genes, gene products and subcellular transport of vitamin B12 is important for minimising the disease burden from these disorders. This review outlines the present knowledge of cobalamin metabolism, with a focus on steps related to the intracellular human pathway and the initial cataloguing of cobalamin-utilisation disorders into complementation groups and biochemically distinct classes. Key to these discoveries have been the hundreds of patients who have been the source of cell cultures and DNA samples that have given us our current understanding of vitamin B12 utilisation in humans.
Vitamin B12 structure
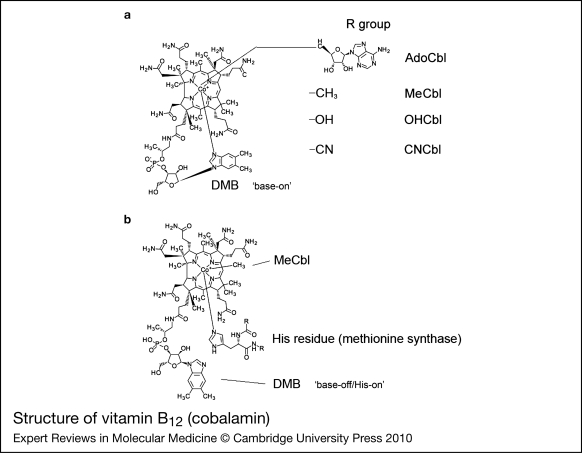
The structure of cobalamin was first solved by Hodgkin (Ref. 5) using x-ray crystallography. It is a large organometallic molecule, ~1300–1500 Da in size, and is the most chemically complex vitamin known. The focal point of vitamin B12 is the central cobalt atom, which has up to six ligands bound to it. Four of the ligands are the nitrogen atoms of the planar corrin ring that surround the cobalt atom (Fig. 1). The α-axial ligand, extending below the corrin ring, is a nitrogen of the 5,6-dimethylbenzimidazole (DMB) phosphoribosyl moiety that also attaches back to the corrin ring through one of its propionamide side chains. The upper or β-axial ligand varies, depending on the modification state of cobalamin (R-group in Fig. 1a). Functional β-axial ligands are methyl (MeCbl) or 5′-deoxyadenosyl (AdoCbl) groups. Additionally, a hydroxyl group (OHCbl) or a cyano group (CNCbl) can be bound as physiologically relevant β-axial ligands.
Figure 1.
Structure of vitamin B12 (cobalamin). (a) The ‘R group’ corresponds to substitutions at the upper or β-axial ligand (5′-deoxyadenosyl-, methyl-, hydroxo-, cyano-). The dimethylbenzimidazole constituent (DMB) is shown coordinated to the cobalt in the lower α-axial position (‘base-on’ structure). DMB is linked to the corrin ring through a phosphoribosyl attached to a propionamide side chain. (b) Structure of methylcobalamin (MeCbl) with DMB displaced from the cobalt by a histidine residue in methionine synthase (MS; the ‘base-off/His-on’ structure). A similar configuration is observed for adenosylcobalamin (AdoCbl) bound to methylmalonyl-CoA mutase. Structures are from http://www.genome.jp using the ‘SIMCOMP Search’ utility (query C00576, vitamin B12; C06410, MeCbl-MS).
There are three important, inter-related factors that contribute to cobalamin reactivity and function: the oxidation state of cobalt; whether the DMB is coordinated to cobalt in the lower axial position; and the identity of the R-group bound in the upper axial position. The cobalt atom of cobalamin may exist in the +3 [cob(III)alamin], +2 [cob(II)alamin] or +1 [cob(I)alamin] oxidation state. AdoCbl, MeCbl, CNCbl and OHCbl, all of which are cob(III)alamins, prefer to adopt a configuration where the DMB nitrogen base is coordinated to the cobalt in the lower axial position (referred to as ‘base-on’) (Fig. 1). Some enzymes, however, are able to shift these cob(III)alamins to the ‘base-off’ configuration. Interestingly, MS and MCM, which use MeCbl and AdoCbl as cofactors, respectively, bind the cobalamin so that the DMB nitrogen is displaced from the cobalt and replaced by a histidine of the enzyme (Fig. 1b). This type of binding is considered ‘base-off/His-on’ and is important for the catalytic activity of the enzymes. Cob(II)alamin generally has no R-group bound, binding only DMB as the lower axial ligand to make its preferred five-coordinate state. However, in the presence of ATP, MMAB, the human adenosyltransferase (ATR) enzyme, is able to bind cob(II)alamin in a novel four-coordinate state, where neither axial position is occupied (Ref. 51). Cob(I)alamin usually has no axial ligand. It is a highly reactive nucleophile that very easily oxidises to cob(II)alamin (Ref. 52). The challenge to the cell is how to productively produce cob(I)alamin, as in the reaction cycle of MS, without exposing the local environment to nucleophillic attack and risk of free-radical damage. The nature of the axial ligands also affects the ease with which the central cobalt is reduced. Strongly coordinating ligands stabilise cobalt against reduction, whereas weakly coordinating ligands allow cobalt to be reduced more easily (Ref. 53). Base-on cobalamin falls into the former category, protecting the cobalt from reduction because DMB has greater electron-donating character than the H2O molecule that binds in its absence (Ref. 54).
Vitamin B12 origins
Vitamin B12 is an extremely old molecule in evolution. It has even been suggested that B12 was synthesised prebiotically (Ref. 55). Accordingly, vitamin B12 utilisation is dispersed throughout evolution, occurring in both Eukaryota and Prokaryota, perhaps having been passed on from the ‘breakthrough organism’ – the last organism to use RNA as the sole genetically encoded catalyst (Ref. 56). Interestingly, although cobalamin utilisation is distributed widely among phyla, cobalamin synthesis seems limited to only a select few Archaea and Eubacteria. Perhaps this is because the synthesis of cobalamin is so complex – involving in excess of 25 steps, which can proceed either aerobically (cob genes) or anaerobically (cbi genes) (Ref. 57). Therefore, although mammals and other higher organisms require vitamin B12 for life, they ostensibly acquire it from their prokaryotic counterparts. Another interesting facet of the evolution of cobalamin is that vitamin B12 users seem more scattered than logically spread out through evolution, with whole phyla sometimes gaining or losing vitamin B12 dependency (Ref. 58). Additionally, although mammals and other higher eukaryotes are restricted to two cobalamin-dependent enzymes, MS and MCM, prokaryotes use a plethora of enzymes requiring this cofactor. These enzymes include three classes of AdoCbl-dependent mutases, the isomerases (e.g. MCM, ribonucleotide reductase, glutamate mutase), the eliminases (e.g. diol dehydratase) and the aminomutases (e.g. d-lysine-5,6-aminomutase), as well as the MeCbl-dependent methyltransferases (e.g. MS) and the vitamin B12-dependent reductive dehalogenases (e.g. 3–6 chloro-4-hydroxybenzoate dehalogenase) (Refs 59, 60). Therefore, because higher eukaryotes share common vitamin B12 ancestry with prokaryotes, but have apparently limited use of the vitamin and no biosynthesis, prokaryotes in general and a few specific bacteria and Archaea in particular have proved to be very useful models to understand vitamin B12 metabolism.
Human vitamin B12 ingestion and absorption
Because vitamin B12 is made by only a few microorganisms, it is acquired through dietary uptake in animals. Human dietary sources include milk, eggs, fish and meat in quantities in excess of a few micrograms a day (Ref. 61). In humans, the absorption, transport and cellular uptake of cobalamin is complex. Food-bound cobalamin is released in the stomach with the help of peptic activity, where it is subsequently bound by haptocorrin (HC) (Ref. 62). In the small intestine, cobalamin is released from HC by pancreatic protease digestion and bound by intrinsic factor (IF) to form an IF–Cbl complex. IF is very specific for cobalamin (i.e. for forms that have the lower DMB intact) and presumably acts as an early screening mechanism to prevent degraded cobalamins from intracellular access (Ref. 63). The IF–Cbl complex passes through the small intestine, where it is bound on the apical surface of ileal epithelial cells by a receptor composed of a heterodimer of amnionless and cubilin, called cubam, which aids in the endocytosis of IF–Cbl (Refs 64, 65). Once inside the cell, IF is degraded in the lysosomes and cobalamin is released into the cytosol (Ref. 66), where it is transported across the ileal receptor cell and released into the bloodstream, possibly by the recently identified (with respect to vitamin B12 transport) multidrug resistance protein MRP1 (Ref. 67). In the bloodstream, cobalamin binds to either HC or transcobalamin (TC) (Ref. 68). Although HC binds the bulk of plasma cobalamin (75–90%), it is not involved in cellular cobalamin uptake apart from uptake in hepatocytes (Ref. 68). Therefore, individuals who have deficient or absent HC have serum cobalamin values in the deficient range, but show no sign of cobalamin deficiency (Ref. 61). Although TC binds only a minor fraction of circulating cobalamins (10–25%), it is the protein responsible for facilitating the uptake of cobalamin by cells (Refs 69, 70). Mutations in the gene encoding TC (TCN1) result in severe tissue cobalamin insufficiency, megaloblastic anaemia, failure to thrive and often neurological complications, despite normal plasma cobalamin concentrations (Refs 71, 72). Additionally, TC acts as a final screening mechanism because, like IF, TC is very specific for cobalamin forms that have the lower DMB intact (Refs 73, 74). Treatment of TC deficiency requires very high serum cobalamin levels, ranging from 1000 to 10 000 pg/ml, achieved by oral or intramuscular delivery of 0.5–1.0 mg of CNCbl or HOCbl once or twice weekly (Refs 60, 75). There is some evidence that at sufficiently high concentrations, at least some tissues are capable of taking up unbound cobalamin (Ref. 61). From the bloodstream, cobalamin is taken up into cells through receptor-mediated endocytosis as a complex of Cbl–TC bound to the TC receptor (TCblR) (Refs 76, 77, 78). A mutation in the gene encoding TCblR (CD320) was recently identified in asymptomatic newborns whose fibroblasts showed decreased Cbl uptake, where restoration of the missing codon by site-directed mutagenesis (c.262–264) resulted in normal TCblR function (Ref. 79). In the lysosome, the Cbl–TC complex is digested to create free cobalamin, which is subsequently transported into the cytosol probably as a mixture of cob(III)alamin and cob(II)alamin. Once in the cytosol, cobalamin is processed by many proteins, some known, others perhaps still unknown, to produce the cofactors MeCbl and AdoCbl. Failure to produce the cobalamin cofactors results in a lack of functional enzymes and causes the constellation of biochemical, developmental and neurological manifestations associated with intracellular pathway defects.
Complementation analysis for cataloguing the intracellular pathway of vitamin B12 disorders
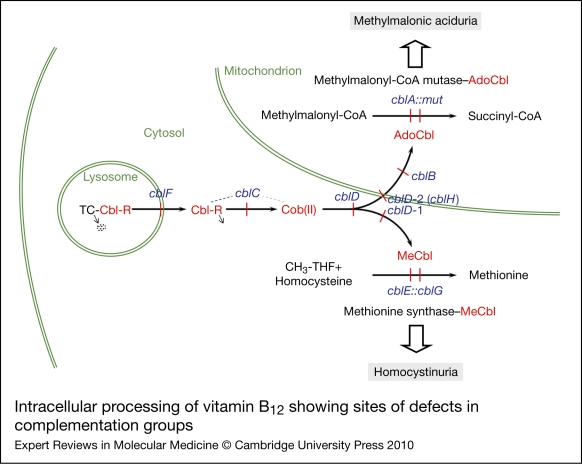
The considerable range of clinical and biochemical heterogeneity observed in patients with vitamin B12 pathway disorders led to a need to sort them into genetically defined groups. The question of whether severe and mild disease or B12-responsive and -unresponsive forms could be explained by mutations in different genes was addressed early on by complementation analysis. This is a powerful technique that permits the identification of specific genes through their expression in fibroblast heterokaryons, multinucleate cells produced by the fusion of fibroblast strains from different patients, which could then be tested for restoration of function. To examine complementation, the incorporation of [14C]propionate or [14C]methyltetrahydrofolate [or [14C]formate to methionine and serine (Ref. 22)] into trichloroacetic acid (TCA)-precipitable material was monitored by autoradiography of cells in situ or by direct scintillation counting of the TCA precipitate (Refs 80, 81). Initially, four distinct complementation groups were identified, cblA–cblC and mut. However, over the years, the method came to be used diagnostically with hundreds of cell lines being analysed, mainly in the McGill University laboratory of David Rosenblatt, which became a dominant diagnostic centre using these techniques. So far, eight complementation groups, cblA–cblG and mut, have been described that have blocks in the production or utilisation of MeCbl, AdoCbl or both cofactors. Although all the genes corresponding to these disorders have now been described, many of their functions remain unclear. Figure 2 illustrates the known or predicted functional location of the protein products of these genes. Three complementation groups – cblF, cblC and cblD – correspond to blocks in steps that are common to the synthesis of both cofactors with resulting deficiency of MS and MCM activities. Patients from these groups have combined homocystinuria and methylmalonic aciduria. Three groups, cblD variant 1, cblE and cblG, have blocks in the cytosolic pathway leading to MeCbl synthesis or apo-MS and result in deficient MS activity and homocystinuria. The final groups, cblD variant 2, cblA, cblB and mut, affect steps occurring in the mitochondrion leading to AdoCbl synthesis or apo-MCM and result in deficient MCM activity and methylmalonic aciduria.
Figure 2.
Intracellular processing of vitamin B12 showing sites of defects in complementation groups. Complementation groups are in blue and are positioned at sites of metabolic blocks (shown in red). Cobalamin intermediates are in red. Excreted metabolites due to genetic defects are in shaded boxes. Pathway details are described in the text. In the lysosome, cobalamin is released from transcobalamin (TC) through its degradation (arrow pointing to dots). In the cytosol, R groups are released by the cblC protein with the cob(II)alamin [Cob(II)] product remaining bound (dotted line emanating from the cblC protein denotes complex with cobalamin forms). The three versions of the cblD protein (cblD, cblD-1, cblD-2) illustrate the role of the protein in directing cobalamin to the mitochondrial or cytosolic pathway. In the mitochondrion, the cblB protein adds the 5′-deoxyadenosyl group, generating the active cofactor [adenosylcobalamin (AdoCbl)], which is transferred to the mut [methylmalonyl-CoA mutase (MCM)] protein. The cblA protein is proposed to act as a gatekeeper to ensure that the cofactor form that is accepted and retained by MCM is AdoCbl. In the cytosolic pathway, cob(II)alamin is bound to the cblG [methionine synthase (MS)] protein. The cblE [methionine synthase reductase (MSR)] protein catalyses generation of the active cofactor, methylcobalamin (MeCbl), or its regeneration if oxidised to cob(II)alamin during reaction cycles.
Complementation groups affecting both methionine synthase and methylmalonyl-CoA mutase
cblF
cblF was initially assigned to describe an infant with developmental delay and mild methylmalonic aciduria who was vitamin B12 responsive (Ref. 82). Her fibroblasts were found to accumulate free cobalamin, failing to attach to MS or MCM. By electron microscopy and subcellular fractionation, it was shown that most of the cobalamin was trapped in the lysosome, with only a small portion reaching the cytosol or mitochondria (Ref. 83). Complementation studies confirmed the genetically distinct disorder (Ref. 84). It was proposed that cblF represents a defect in the export of cobalamin out of the lysosome into the cytosol (Ref. 82). The gene responsible for cblF was very recently cloned by homozygosity mapping and microcell-mediated chromosome transfer and was found to correspond to LMBRD1, which encodes the lysosomal membrane protein LMBD1 (Ref. 27). LMBD1 shares homology with a family of membrane proteins that internalise lipocalins, which are carriers of small hydrophobic molecules such as steroids and lipids, leading to the suggestion that a lipocalin-like molecule may bind vitamin B12 in the lysosome on its release following TC degradation (Ref. 27). Transfection of cblF fibroblasts with intact cDNA corrected intracellular cobalamin processing and restored functional MS and MCM. The nature of the protein, with nine predicted transmembrane domains, and localisation of the protein to the lysosomal membrane, is consistent with a role in the export of cobalamin from the lysosome. Only 13 patients have been described with cblF (Refs 27, 28). Six mutations have been identified, and, interestingly, all of them have been chain-terminating frameshift mutations, with one, 1056delG, accounting for 18 of 26 analysed alleles (Fig. 3). Clinically, it is a highly variable disorder. Most patients show failure to thrive in infancy and mild to severe developmental delay, but respond to vitamin B12 therapy. Although most patients presented with homocystinuria and methylmalonic aciduria, the index case, mentioned above, showed no evidence of megaloblastic anaemia or homocystinuria. This individual was reportedly asymptomatic on B12 therapy in adulthood, despite being homoallelic for the 1056delG mutation (Ref. 27).
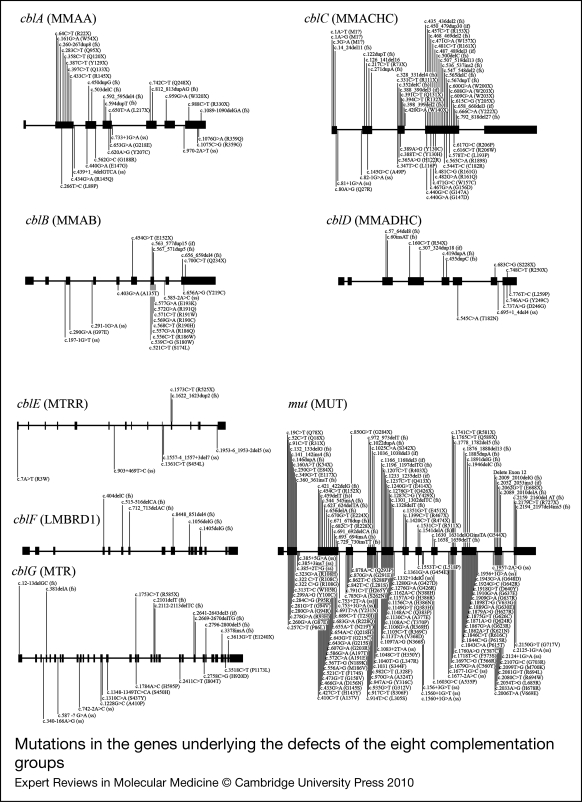
Figure 3.
Mutations in the genes underlying the defects of the eight complementation groups. For each complementation group, the gene name is given in brackets. Mutations are shown as cDNA position with corresponding amino-acid change in brackets. The numbering for each is based on the cDNA sequence: +1 corresponds to the A of the ATG translation initiation codon. Nonsense and frameshift (fs) mutations are displayed above the gene whereas missense and possible splice site or cryptic splice site (ss) mutations are displayed below. Mutations are based on cblA (Refs 6, 7, 8, 9, 10), cblB (Refs 11, 12, 13), cblC (Refs 14, 15, 16, 17, 18, 19), cblD (Refs 20, 21), cblE (Refs 22, 23, 24, 25, 26), cblF (Refs 27, 28), cblG (Refs 29, 30, 31, 32) and mut (Refs 9, 13, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50).
cblC
The cblC complementation group originally corresponded to the first set of patients who failed to produce either AdoCbl or MeCbl (Ref. 85). Since then, approximately 400 patients have been described with cblC, making it the most common disorder of intracellular vitamin B12 metabolism (Ref. 14). The gene responsible for the cblC group, called MMACHC, was identified by homozygosity mapping in 2006 (Ref. 15). More than 50 different disease-causing mutations have been identified and are summarised in Ref. 14 (Fig. 3). The most common is the c.271dupA mutation, which causes a frameshift truncation, accounting for 42% of pathogenic alleles (Ref. 14). Additionally, the c.394C > T (R132X) and c.331C > T (R111X) mutations are found commonly, at 20% and 5% of alleles, respectively. Although all cblC patients have combined homocystinuria and methylmalonic aciduria and often have haemotological, neurological and ophthalmic abnormalities to some degree, they tend to fall into either of two distinct phenotypes correlating with age of onset (Ref. 86). Early-onset patients present in the first year of life with severe disease and rarely respond clinically to treatment, whereas late-onset patients present in childhood to adulthood, are more likely to have less severe symptoms, and usually respond better to treatment (Ref. 86). A strong genotype–phenotype correlation can be found with some mutations: the c.271dupA and c.331C > T (R111X) mutations usually cause the much more prevalent early-onset disease, whereas some missense mutations [e.g. c.482G > A (R161Q)] and, bewilderingly, the c.394C > T (R132X) nonsense mutation usually result in late-onset disease (Refs 14, 15). The cblC protein (MMACHC) was predicted to have a vitamin B12 binding site and a TonB-like domain; the latter is a protein associated with bacterial cobalamin uptake (Ref. 15). Although initial studies suggested that MMACHC had base-on CNCbl binding (Ref. 87), it was later demonstrated to bind CNCbl in the base-off conformation (Ref. 88) and to reductively cleave the CN group to form MMACHC-bound cob(II)alamin (Ref. 87). It has also been shown to catalyse glutathione-dependent dealkylation of cobalamins containing C2–C6 alkanes, Ado-, or Me- as the upper axial ligand (Ref. 89). These results suggest that MMACHC might be involved in intracellular cobalamin transport and reductive dealkylation or decyanation, perhaps interacting with the cblF protein for export of cobalamin out of the lysosome and beginning the initial processing of cobalamin, yielding cob(II)alamin, before passing it along for distribution to the rest of the pathway (Refs 14, 62) (Fig. 2).
Late-onset cblC patients are cobalamin responsive but, as observed initially in fibroblasts and later in patients, they respond poorly to CNCbl compared with OHCbl, a phenomenon that is unique among the vitamin B12 disorders (Refs 86, 90, 91, 92). Investigations of cobalamin binding by wild-type and mutant protein, the latter containing the R161Q mutation commonly associated with OHCbl responsiveness in cblC patients, revealed reduced binding of CNCbl but not OHCbl by the mutant protein compared with the wild type (Ref. 93). Further, thermolability studies showed that MMACHC protein is strongly stabilised by cobalamin binding, whereas mutant protein was much less stabilised and only minimally or not at all with CNCbl (Ref. 87). These results suggest that OHCbl responsiveness in patients with the R161Q mutation is due to a combination of better affinity for OHCbl than CNCbl and a much better stabilisation of the mutant protein by OHCbl. It may well be that the high-dose OHCbl treatment generally used with cblC patients protects the protein from degradation in vivo. An interesting outcome of these studies was the finding that the cobalamin cofactors, AdoCbl and MeCbl, were far more protective of the mutant protein than OHCbl. Although treatment with AdoCbl or MeCbl has been tried before, it was found that they are not incorporated directly as cofactors of their cognate enzymes but are dealkylated and processed anew, results that have been confirmed in vitro with MMACHC (Refs 89, 94). However, the increased stabilisation of mutant protein afforded by the cofactors suggests that their use should be re-examined in some cases.
cblD
The cblD complementation group was first described in two siblings with combined homocystinuria and methylmalonic aciduria and deficiency of MCM and MS activities (Ref. 95), although the designation ‘cblD’ was not given until many years later (Ref. 81). For over 25 years, they remained the only cblD patients described, and biochemical analysis revealed that only this complementation group seemed to behave in a manner similar to cblC, but with less severe defects (Ref. 91). However, in 2004, Suormala et al. (Ref. 96) described three new cases of cblD: two had only MS deficiency (called cblD variant 1) and one had only MCM deficiency (cblD variant 2). These results suggested that the cblD protein might be responsible for branching of the cobalamin metabolism pathways to the cytosolic or mitochondrial compartments (Fig. 2). The same group cloned the cblD gene 4 years later (Ref. 20), naming it MMADHC. With an additional four patients they showed a clear genotype–phenotype relationship, whereby truncation mutations in the 5′ region resulted in only methylmalonic aciduria (MCM deficiency), truncation mutations in the middle and 3′ regions resulted in combined methylmalonic aciduria and homocystinuria (MCM and MS deficiency), and missense mutations in the 3′ region resulted in only homocystinuria (MS deficiency) (Fig. 3). Transfection experiments demonstrated correction of the defect in mutant fibroblasts. In particular, a cDNA constructed with a 5′-truncation mutation could correct the synthesis of MeCbl, suggesting that an internal translation initiation site is probably functional. Additionally, they demonstrated that the cblH complementation group, which had been previously described for one patient with unidentified methylmalonic aciduria (Ref. 97), was actually an example of cblD variant 2 (Fig. 2). MMADHC has a predicted mitochondrial leader sequence and a putative vitamin B12 binding sequence and shows limited homology to a bacterial ABC transporter (Ref. 20). Although no functional or biochemical data are yet available for MMADHC, it is currently speculated to interact with MMACHC as part of a chaperone role to present cobalamin to the cytosolic or mitochondrial pathways (Refs 20, 62).
Complementation groups affecting only methionine synthase
In 1984, Schuh and colleagues (Ref. 98) described an infant with homocystinuria, megaloblastic anaemia and developmental delay who, although treatable with cobalamin, showed no evidence of methylmalonic aciduria, a novel outcome at the time. This suggested that the infant had a unique block in MS or the synthesis of MeCbl. Subsequently, two other patients were described with the same symptoms (Refs 99, 100). Broken cell extracts from the original patient revealed that with added reducing agents, MS worked perfectly. However, this was not the case for MS from the next two patients. Their cell extracts were defective regardless of additions. Ultimately, Watkins and Rosenblatt (Ref. 101) used complementation analysis to show that the original patient, designated as cblE, had a block in a separate genetic locus to the other two patients, which they designated as cblG (Fig. 2).
cblG
The gene responsible for cblG was cloned by three separate groups based on the identification of human sequences homologous to the E. coli vitamin-B12-dependent MS, encoded by the metH gene, and other bacterial and Caenorhabditis elegans sequences (Refs 29, 102, 103). The human gene is designated MTR, for methyltransferase, as it was named when initially mapped to human chromosome 1 (Ref. 91), or more formally as 5-methyltetrahydrofolate:homocysteine methyltransferase. Twenty different mutations have been identified in MTR (Fig. 3). The most common is c.3518C > T (P1173L), which is present in 16 of 24 cblG cell lines surveyed (Ref. 30). The clinical disease is highly variable, with onset ranging from neonatal to adulthood, although most patients present with homocystinuria, hypomethioninaemia, megaloblastic anaemia and developmental delay (Refs 101, 104, 105, 106). In addition to its importance in protein synthesis, MS is a key enzyme of the methionine cycle, which maintains the cellular level of the methionine derivative, S-adenosylmethionine, the methyl donor in a wide array of cellular processes including DNA, RNA and protein methylation. It is also uniquely involved in the folate cycle, because it is the only mammalian enzyme to use 5-methyltetrahydrofolate as a methyl donor (Ref. 30). MS catalyses the methylation of homocysteine to form methionine in a reaction that requires the presence of enzyme-bound MeCbl for activity (Refs 107, 108). The reaction proceeds by methyl transfer from 5-methyltetrahydrofolate to MS-bound cob(I)alamin to form MeCbl, followed by transfer of the methyl group to homocysteine to form methionine and regeneration of cob(I)alamin (Refs 109, 110). Mammalian MS and Escherichia coli MetH are 55% identical (Ref. 103). The sequence homology extends to the domain structure of MetH in which linearly arrayed domains of the enzyme contain binding sites for the various substrates and cofactors (Refs 111, 112, 113). These domains seem to be faithfully maintained in mammalian MS.
cblE
Because the reaction catalysed by MS regenerates cob(I)alamin in every reaction cycle, the cofactor risks occasional oxidation to cob(II)alamin with consequent inactivation of the enzyme (Ref. 114). In E. coli, the restoration of a functional cofactor is dependent on two flavoproteins, flavodoxin and flavodoxin reductase, containing flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) prosthetic groups, respectively (Refs 115, 116). The corresponding human reducing system proved to be encoded by a single gene, MTRR, which is mutated in cblE patients (Fig. 3); it encodes a single protein containing FMN- and FAD/NADPH-binding sites (Ref. 117). This enzyme, named methionine synthase reductase (MSR), is a linear array of ‘flavodoxin’ at the N-terminus, an intervening linker sequence in the middle and ‘flavodoxin reductase’ at the C-terminus. It restores MS activity by catalysing the reductive methylation of cob(II)alamin on the inactivated MS in which S-adenosylmethionine is the source of the methyl group (Refs 118, 119). Additionally, MSR has been shown to catalyse the reduction of free B12 [as aquocob(III)alamin] to cob(II)alamin (Ref. 120), making it an aquocobalamin reductase and possibly functioning as such in the cytosolic pathway. MSR has also been suggested to function as a cob(II)alamin reductase in the mitochondrial pathway. This hypothesis was based on a potential mitochondrial leader sequence found by alternative splicing in the MTRR gene, as well as in vitro results demonstrating the production of cob(I)alamin through physical interaction with the mitochondrial MMAB protein (Refs 117, 121). However, evidence demonstrating MSR expression only in the cytosol (Ref. 122) and separate evidence suggesting that MMAB does not require a distinct cobalamin reductase (Ref. 123) have strongly countered this argument. The clinical presentation of cblE patients is similar to that of cblG patients. It is usually impossible to separate the two disorders, except for one patient who unexpectedly also had methylmalonic aciduria (Ref. 105), which remains unexplained.
Complementation groups affecting only methylmalonyl-CoA mutase
Patients with methylmalonic aciduria without elevated homocysteine or abnormalities of circulating vitamin B12 have defects in the mitochondrial pathway of AdoCbl synthesis and functional MCM. Clinically, patients have the following common features: failure to thrive, lethargy, vomiting of protein feeds, dehydration, respiratory distress and hypotonia (Ref. 124). Early on, three genes were implicated in the mitochondrial pathway, corresponding to complementation groups cblA, cblB and mut (Refs 80, 85) (Fig. 2). A detailed understanding of the mitochondrial processing of vitamin B12 is only now beginning to emerge, based largely on studies completed on bacterial model systems.
cblA
cblA was initially the designation for patient fibroblasts that failed to accumulate AdoCbl in intact cells, but showed restored AdoCbl synthesis in a broken-cell assay with OHCbl, ATP and a reducing system (Ref. 85). This block was hypothesised to correspond to a defect in mitochondrial cob(II)alamin reductase because of the ability of an external reducing system to alleviate the block (Ref. 85). The human gene, named MMAA, was identified in a search for genes clustered in proximity to MCM (mut) in microbial genomes and while searching for orthologous sequences in the human genome (Ref. 6). Examination of the sequence of MMAA, however, revealed that it did not encode a reductase, but rather a protein that belonged to the G3E family of P-loop GTPases, a group of proteins that participate in the assembly or function of the metal centres in metalloenzymes (Ref. 6). More than 30 cblA patient mutations have been described in the MMAA gene, with most of them corresponding to nonsense or frameshift mutations (Fig. 3). Although most cblA patients present in infancy or childhood with methylmalonic aciduria and potentially life-threatening acidotic crises (Ref. 104), they often respond to vitamin B12 therapy despite the severity of mutations (Refs 104, 124, 125). Reasons for this are becoming clearer because of our increased understanding of the role of MMAA. Studies have focused on the bacterial orthologues of MMAA, including key research by Banerjee and colleagues working with MeaB, the MMAA orthologue from Methylobacterium extorquens. Initial bacterial studies showed that MMAA orthologues form a complex with MCM and that GTP binding and hydrolysis contribute to cobalamin processing (Refs 126, 127, 128). Further, it was shown that MeaB protects MCM from inactivation and that the state of MeaB (apo, GDP or GTP bound) alters the affinity of MCM for AdoCbl (Ref. 129). Finally, recent studies suggest that MeaB acts as a regulator of MCM cofactor binding and ejection, where the binding and hydrolysis of GTP by MeaB are important in the discrimination of MCM binding to AdoCbl versus cob(II)alamin, and promotes ejection of the latter, inactive cofactor from MCM (Ref. 130). These studies are summarised below in the proposed model for cobalamin processing.
cblB
Patients in the cblB group are also deficient in AdoCbl synthesis and are metabolically similar to cblA (Ref. 86). However, cblB patients tend to present earlier, respond more poorly to vitamin B12 and, consequently, may produce a more severe clinical course with more profound neurological and metabolic complications (Refs 124, 125). The cblB disorder was separated biochemically from cblA by the failure to synthesise AdoCbl in broken cell extracts containing a reducing system (Ref. 85). On the basis of this early work, cblB had long been expected to correspond to a defect in the ATP:cob(I)alamin ATR. Like MMAA, the gene was identified in the survey of MCM-containing gene clusters in microbial genomes (see the previous section) and was named MMAB (Ref. 11). It was shown to correspond to an ATR based on sequence and functional similarity with a class of bacterial ATRs called PduO (Refs 11, 131). The MMAB protein catalyses the transfer of the 5′-deoxyadenosyl group of ATP to cob(I)alamin to form AdoCbl. To assess function, cob(II)alamin is the usual cobalamin added in vitro, with a reducing system, often MSR and NADPH, added to facilitate the reaction. Unexpectedly, in the presence of ATP, MMAB binds cob(II)alamin in an unusual base-off, four-coordinate state (Ref. 51). This unusual binding elevates the redox potential of cob(II)alamin to the physiological range of possible reduction by reduced flavin, perhaps in the form of an electron transfer protein, thus obviating the need for a specific cobalamin reductase to generate the reactive cob(I)alamin intermediate (Ref. 123). Most of the mutations identified in cblB disease were found to cluster in exon 7, which encodes the active site of the enzyme (Refs 12, 132) (Fig. 3). Several mutations have been modelled in human and microbial ATRs, identifying defects in substrate or cofactor binding, active site functions or protein dynamics (Refs 132, 133, 134, 135, 136). Interestingly, two of the mutations (R186W, E193K) resulted in absent protein in western blots of patient cell extracts, suggesting protein instability as a major contributor to disease phenotype (Ref. 137). Human MMAB has been crystallised in the presence of ATP (Ref. 132). It was found to exist as a trimer with three active sites, only two of which contained ATP. Crystallisation in the presence of both ATP and cob(II)alamin has been accomplished for the Lactobacillus reuteri ATR (LrPduO) (Ref. 135). The two enzymes are highly similar and, interestingly, AdoCbl was detected in LrPduO crystals, underscoring the capacity of reduced flavin, generated in the incubation mix, to drive the four-coordinate cob(II)alamin intermediate to cob(I)alamin and the formation of AdoCbl.
mut
The mut complementation group is representative of mutations in the MUT (MCM) gene. MCM is important for the metabolism of branched-chain amino acids, odd-chain fatty acids and cholesterol (Ref. 104). It catalyses the reversible isomerisation of l-methylmalonyl-CoA to l-succinyl-CoA (Fig. 2), which is important for the breakdown of propionate and for replenishing the tricarboxylic acid cycle. The MUT gene was the earliest of the human B12 pathway genes to be cloned, accomplished by antibody screening of a human liver λgt11 expression library (Ref. 138). Nearly 200 disease-causing mutations have been identified in MUT (Ref. 33) (Fig. 3). Two distinct classes of mutations have been described: mut− (‘mut-minus’), when there is residual enzyme activity or detectable [14C] propionate incorporation by mutant fibroblasts; and mut0 (‘mut-zero’), when protein or enzyme activity is not detected, as found, for example, in frameshift or chain-terminating mutations and some amino-acid substitutions (Refs 139, 140). Unsurprisingly, these two groups separate patients clinically. mut0 patients have a higher occurrence of morbidity, mortality and neurological complications than mut−, and mut− patients are more responsive to B12 therapy (Ref. 124). MCM from Proprionibacterium shermanii has been crystallised in the presence of AdoCbl and substrate (PDB accession number 4REQ) (Refs 141, 142, 143). The human enzyme (PDB accession number 3BIC) is structurally similar to the P. shermanii enzyme, with which it shares 60% identity in the α-subunit (Ref. 144). Human MCM exists as a homodimer in the mitochondrial matrix with 1 mol of AdoCbl bound per subunit (Refs 145, 146). Studies of the Methylobacterium extorquens enzyme, as described above, suggest that human MCM does not exist alone but functions as a complex with other proteins, notably MMAA and possibly MMAB.
Model for the intracellular processing of vitamin B12
The genes and proteins corresponding to all eight complementation groups defined in patients have provided most of the elements required to describe the intracellular processing of vitamin B12 (Fig. 2). Cobalamin, generally as OHCbl or CNCbl, is taken into the lysosome as a Cbl–TC complex, where digestion of the transcobalamin releases free cobalamin. The cobalamin is transported into the cytosol through the LMBD1 protein, possibly drawn through the lysosomal transporter by interaction of the cytosol face of LMBD1 with the MMACHC protein. It appears that cobalamins are bound to MMACHC in the base-off state, poised for cleavage of the upper axial ligand if one is present. MMACHC may act as an intracellular cobalamin carrier for delivery of the cofactor to the MMADHC protein for targeting to the cytosolic (MS) or mitochondrial (MCM) pathways. Evidence of interaction of MMACHC with either LMBD1 or MMADHC has yet to be demonstrated. In the cytosolic compartment, cob(II)alamin is expected to be bound to MS, where it is reductively methylated by MSR, using NADPH as an electron donor, to generate the MeCbl active cofactor of MS. MMADHC also participates in the targeting of cobalamin to the mitochondrial pathway, although the specific transporter has yet to be identified. Cob(II)alamin, on entry into the mitochondrial matrix, is bound by MMAB for the generation of AdoCbl, with the reducing equivalents probably coming from an electron transfer protein rather than a cobalamin reductase, as had been previously anticipated. The subsequent transfer of AdoCbl to MCM is predicted to be an exquisitely complicated process involving a complex of MMAA and MCM and possibly MMAB.
First, the complex of MCM:MMAA–GTP prevents the binding of cob(II)alamin, which would otherwise inactivate MCM. Second, AdoCbl is transferred directly from MMAB to the MCM–MeaB–GTP complex in a process requiring ATP binding to MMAB and GTP hydrolysis by MMAA. Third, in reaction cycles in which the radical 5′deoxyadenosyl intermediate is lost, leaving MCM with an inactive cob(II)alamin cofactor, the GTP-bound MMAA causes displacement of the cofactor, making the enzyme available for renewed AdoCbl binding. The proposed role for MMAA derives principally from studies of MeaB by Padovani and Banerjee (Refs 127, 129, 130). The crystal structure of MeaB has been determined and carries the expected nucleotide-binding domains and domains predicted to be involved in MCM binding at the N-terminus and a dimerisation domain at the C-terminus (PDB accession number 4REQ) (Ref. 147). The recently crystallised human MMAA (PDB accession number 2WWW), although it has a similar overall structure, seems to adopt a slightly different mode of assembly, which may have implications for the three-way interaction with MMAB and MCM.
Research in progress and outstanding research questions
Although all eight genes predicted through complementation analysis have been identified, additional genes are anticipated. Most notably, the mechanism of the mitochondrial transport of B12 remains unknown. The involvement of cblD defects in both the cytosolic and mitochondrial pathways suggests that the MMADHC protein is an accessory to the mitochondrial uptake of vitamin B12. The lysosomal delivery of vitamin B12 to the cytosol might also involve additional genes. The similarity of LMBD1 to the lipocalin family of membrane receptors predicts the involvement of a lipocalin-like molecule that might act as a vitamin B12 carrier after digestion of the Cbl–TC complex in the lysosome. Although a mitochondrial cobalamin reductase cannot be fully ruled out, studies on PduO-type ATRs argue against such a protein in human cells. Finally, if confirmed in human cells, the MMAA–MCM or MMAA–MCM–MMAB complex predicted by studies on MeaB proteins might account for the gene set required for mitochondrial cobalamin processing and utilisation. All these suggestions recognise the absence of additional disease states among human vitamin B12 processing disorders. The general view is that unidentified genes would probably not tolerate mutation (and therefore be lethal embryonically), might be associated with shared functions (and therefore might not reveal a vitamin B12 disease phenotype) or might be redundant with genes encoding proteins of similar function (allowing a bypass of a genetic defect). Therefore, completion of the human pathway requires either finding new genes or demonstrating that the pathway is fully functional without them. One approach might be to investigate the pathway in a model eukaryotic organism carrying orthologues of most (or all) the human genes, as suggested in studies of the methylmalonate pathway in C. elegans, an organism with knockout mutants of several vitamin B12 pathway genes (Refs 148, 149, 150).
Although we have gained much insight into the pathway of vitamin B12 metabolism, the goal of the medical geneticist has been to gain insight into managing the vitamin B12 disorders, providing access to carrier testing and prenatal diagnosis, and, in the best of outcomes, preventing or successfully treating symptomatic disease. The remarkable feature of vitamin B12 utilisation disorders has been their potential for treatment. The discovery that high-dose vitamin B12 can overcome pathway deficits in some patients has given new life to individuals with an otherwise potentially severe or fatal disease. The early discovery that OHCbl is effective in the treatment of cblC disorder while CNCbl is not is a powerful illustration of the complexity of vitamin B12 biochemistry. The more recent finding that AdoCbl or MeCbl may have a significant stabilising effect on MMACHC protein, despite ultimately being hydrolysed to cob(II)alamin, reminds us that there is still much to be learned on behalf of the patient. Strikingly, the most recent success with gene therapy to treat mice with knockout of the Mut gene (Ref. 151) has opened up a new avenue for treatment that might ultimately benefit patients with metabolically ‘unresponsive’ disorders. The application of widespread newborn screening for homocysteine and methylmalonate underscores the opportunity to identify and treat these patients before the onset of potentially irreversible disease.
Acknowledgements and funding
We are grateful to the peer reviewers for their helpful comments and corrections. Contributing research and the preparation of this review were supported by a Canadian Institutes for Health Research (CIHR) grant, MOP-44353, to R.A.G. Scholarship support to D.S.F. was provided by the CIHR Training Program in Genetics, Child Development and Health at the University of Calgary and by the Structural Genomics Consortium, Oxford University.
References
- 1.Smith E.L.. Purification of anti-pernicious anaemia factors from liver. Nature. 1948;161:638. doi: 10.1038/161638a0. [DOI] [PubMed] [Google Scholar]
- 2.Rickes E.L.. et al. Crystalline vitamin B12. Science. 1948;107:396–397. doi: 10.1126/science.107.2781.396. [DOI] [PubMed] [Google Scholar]
- 3.Minot G.R., Murphy W.P.. Treatment of pernicious anemia by a special diet. 1926. Yale Journal of Biology and Medicine. 1926;74:341–353. [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg L.E., Lilljeqvist A., Hsia Y.E.. Methylmalonic aciduria: metabolic block localization and vitamin B 12 dependency. Science. 1968;162:805–807. doi: 10.1126/science.162.3855.805. [DOI] [PubMed] [Google Scholar]
- 5.Hodgkin D.C.. et al. Structure of vitamin B12. Nature. 1956;178:64–66. doi: 10.1038/178064a0. [DOI] [PubMed] [Google Scholar]
- 6.Dobson C.M.. et al. Identification of the gene responsible for the cblA complementation group of vitamin B12-responsive methylmalonic acidemia based on analysis of prokaryotic gene arrangements. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:15554–15559. doi: 10.1073/pnas.242614799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lerner-Ellis J.P.. et al. Mutations in the MMAA gene in patients with the cblA disorder of vitamin B12 metabolism. Human Mutation. 2004;24:509–516. doi: 10.1002/humu.20104. [DOI] [PubMed] [Google Scholar]
- 8.Merinero B.. et al. Methylmalonic acidaemia: examination of genotype and biochemical data in 32 patients belonging to mut, cblA or cblB complementation group. Journal of Inherited Metabolic Disease. 2008;31:55–66. doi: 10.1007/s10545-007-0667-y. [DOI] [PubMed] [Google Scholar]
- 9.Martinez M.A.. et al. Genetic analysis of three genes causing isolated methylmalonic acidemia: identification of 21 novel allelic variants. Molecular Genetics and Metabolism. 2005;84:317–325. doi: 10.1016/j.ymgme.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Yang X.. et al. Mutation analysis of the MMAA and MMAB genes in Japanese patients with vitamin B(12)-responsive methylmalonic acidemia: identification of a prevalent MMAA mutation. Molecular Genetics and Metabolism. 2004;82:329–333. doi: 10.1016/j.ymgme.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Dobson C.M.. et al. Identification of the gene responsible for the cblB complementation group of vitamin B12-responsive methylmalonic acidemia. Human Molecular Genetics. 2002;11:3361–3369. doi: 10.1093/hmg/11.26.3361. [DOI] [PubMed] [Google Scholar]
- 12.Lerner-Ellis J.P.. et al. Mutation and biochemical analysis of patients belonging to the cblB complementation class of vitamin B12-dependent methylmalonic aciduria. Molecular Genetics and Metabolism. 2006;87:219–225. doi: 10.1016/j.ymgme.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Champattanachai V.. et al. Novel mutations in a Thai patient with methylmalonic acidemia. Molecular Genetics and Metabolism. 2003;79:300–302. doi: 10.1016/s1096-7192(03)00106-9. [DOI] [PubMed] [Google Scholar]
- 14.Lerner-Ellis J.P.. et al. Spectrum of mutations in MMACHC, allelic expression, and evidence for genotype-phenotype correlations. Human Mutation. 2009;30:1072–1081. doi: 10.1002/humu.21001. [DOI] [PubMed] [Google Scholar]
- 15.Lerner-Ellis J.P.. et al. Identification of the gene responsible for methylmalonic aciduria and homocystinuria, cblC type. Nature Genetics. 2006;38:93–100. doi: 10.1038/ng1683. [DOI] [PubMed] [Google Scholar]
- 16.Heil S.G.. et al. Marfanoid features in a child with combined methylmalonic aciduria and homocystinuria (CblC type) Journal of Inherited Metabolic Disease. 2007;30:811. doi: 10.1007/s10545-007-0546-6. [DOI] [PubMed] [Google Scholar]
- 17.Thauvin-Robinet C.. et al. The adolescent and adult form of cobalamin C disease: clinical and molecular spectrum. Journal of Neurology, Neurosurgery, and Psychiatry. 2008;79:725–728. doi: 10.1136/jnnp.2007.133025. [DOI] [PubMed] [Google Scholar]
- 18.Yuen Y.P.. et al. DNA-based diagnosis of methylmalonic aciduria and homocystinuria, cblC type in a Chinese patient presenting with mild developmental delay. Clinica Chimica Acta. 2007;375:171–172. doi: 10.1016/j.cca.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Nogueira C.. et al. Spectrum of MMACHC mutations in Italian and Portuguese patients with combined methylmalonic aciduria and homocystinuria, cblC type. Molecular Genetics and Metabolism. 2008;93:475–480. doi: 10.1016/j.ymgme.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Coelho D.. et al. Gene identification for the cblD defect of vitamin B12 metabolism. New England Journal of Medicine. 2008;358:1454–1464. doi: 10.1056/NEJMoa072200. [DOI] [PubMed] [Google Scholar]
- 21.Miousse I.R.. et al. Clinical and molecular heterogeneity in patients with the cblD inborn error of cobalamin metabolism. Journal of Pediatrics. 2009;154:551–556. doi: 10.1016/j.jpeds.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 22.Zavadakova P.. et al. CblE type of homocystinuria due to methionine synthase reductase deficiency: clinical and molecular studies and prenatal diagnosis in two families. Journal of Inherited Metabolic Disease. 2002;25:461–476. doi: 10.1023/a:1021299117308. [DOI] [PubMed] [Google Scholar]
- 23.Zavadakova P.. et al. cblE type of homocystinuria due to methionine synthase reductase deficiency: functional correction by minigene expression. Human Mutation. 2005;25:239–247. doi: 10.1002/humu.20131. [DOI] [PubMed] [Google Scholar]
- 24.Vilaseca M.A.. et al. CblE type of homocystinuria: mild clinical phenotype in two patients homozygous for a novel mutation in the MTRR gene. Journal of Inherited Metabolic Disease. 2003;26:361–369. doi: 10.1023/a:1025159103257. [DOI] [PubMed] [Google Scholar]
- 25.Wilson A.. et al. Molecular basis for methionine synthase reductase deficiency in patients belonging to the cblE complementation group of disorders in folate/cobalamin metabolism. Human Molecular Genetics. 1999;8:2009–2016. doi: 10.1093/hmg/8.11.2009. [DOI] [PubMed] [Google Scholar]
- 26.Kahleova R.. et al. Essential hypertension in adolescents: association with insulin resistance and with metabolism of homocysteine and vitamins. American Journal of Hypertension. 2002;15:857–864. doi: 10.1016/s0895-7061(02)02984-9. [DOI] [PubMed] [Google Scholar]
- 27.Rutsch F.. et al. Identification of a putative lysosomal cobalamin exporter altered in the cblF defect of vitamin B12 metabolism. Nature Genetics. 2009;41:234–239. doi: 10.1038/ng.294. [DOI] [PubMed] [Google Scholar]
- 28.Gailus S.. et al. A novel mutation in LMBRD1 causes the cblF defect of vitamin B(12) metabolism in a Turkish patient. Journal of Inherited Metabolic Disease. 2010;33:17–24. doi: 10.1007/s10545-009-9032-7. [DOI] [PubMed] [Google Scholar]
- 29.Leclerc D.. et al. Human methionine synthase: cDNA cloning and identification of mutations in patients of the cblG complementation group of folate/cobalamin disorders. Human Molecular Genetics. 1996;5:1867–1874. doi: 10.1093/hmg/5.12.1867. [DOI] [PubMed] [Google Scholar]
- 30.Watkins D.. et al. Hyperhomocysteinemia due to methionine synthase deficiency, cblG: structure of the MTR gene, genotype diversity, and recognition of a common mutation, P1173L. American Journal of Human Genetics. 2002;71:143–153. doi: 10.1086/341354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gulati S.. et al. Defects in human methionine synthase in cblG patients. Human Molecular Genetics. 1996;5:1859–1865. doi: 10.1093/hmg/5.12.1859. [DOI] [PubMed] [Google Scholar]
- 32.Wilson A.. et al. Functionally null mutations in patients with the cblG-variant form of methionine synthase deficiency. American Journal of Human Genetics. 1998;63:409–414. doi: 10.1086/301976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lempp T.J.. et al. Mutation and biochemical analysis of 19 probands with mut0 and 13 with mut- methylmalonic aciduria: identification of seven novel mutations. Molecular Genetics and Metabolism. 2007;90:284–290. doi: 10.1016/j.ymgme.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Worgan L.C.. et al. Spectrum of mutations in mut methylmalonic acidemia and identification of a common Hispanic mutation and haplotype. Human Mutation. 2006;27:31–43. doi: 10.1002/humu.20258. [DOI] [PubMed] [Google Scholar]
- 35.Cavicchi C.. et al. Mutational spectrum in ten Italian patients affected by methylmalonyl-CoA mutase deficiency. Journal of Inherited Metabolic Disease. 2005;28:1175–1178. doi: 10.1007/s10545-005-0191-x. [DOI] [PubMed] [Google Scholar]
- 36.Adjalla C.E.. et al. Seven novel mutations in mut methylmalonic aciduria. Human Mutation. 1998;11:270–274. doi: 10.1002/(SICI)1098-1004(1998)11:4<270::AID-HUMU3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 37.Jansen R., Ledley F.D.. Heterozygous mutations at the mut locus in fibroblasts with mut0 methylmalonic acidemia identified by polymerase-chain-reaction cDNA cloning. American Journal of Human Genetics. 1990;47:808–814. [PMC free article] [PubMed] [Google Scholar]
- 38.Qureshi A.A.. et al. Cloning and expression of mutations demonstrating intragenic complementation in mut0 methylmalonic aciduria. Journal of Clinical Investigation. 1994;93:1812–1819. doi: 10.1172/JCI117166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janata J., Kogekar N., Fenton W.A.. Expression and kinetic characterization of methylmalonyl-CoA mutase from patients with the mut- phenotype: evidence for naturally occurring interallelic complementation. Human Molecular Genetics. 1997;6:1457–1464. doi: 10.1093/hmg/6.9.1457. [DOI] [PubMed] [Google Scholar]
- 40.Crane A.M.. et al. Phenotype of disease in three patients with identical mutations in methylmalonyl CoA mutase. Human Genetics. 1992;89:259–264. doi: 10.1007/BF00220536. [DOI] [PubMed] [Google Scholar]
- 41.Crane A.M., Ledley F.D.. Clustering of mutations in methylmalonyl CoA mutase associated with mut- methylmalonic acidemia. American Journal of Human Genetics. 1994;55:42–50. [PMC free article] [PubMed] [Google Scholar]
- 42.Raff M.L.. et al. Genetic characterization of a MUT locus mutation discriminating heterogeneity in mut0 and mut- methylmalonic aciduria by interallelic complementation. Journal of Clinical Investigation. 1991;87:203–207. doi: 10.1172/JCI114972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Acquaviva C.. et al. Molecular basis of methylmalonyl-CoA mutase apoenzyme defect in 40 European patients affected by mut(o) and mut- forms of methylmalonic acidemia: identification of 29 novel mutations in the MUT gene. Human Mutation. 2005;25:167–176. doi: 10.1002/humu.20128. [DOI] [PubMed] [Google Scholar]
- 44.Peters H.L.. et al. Molecular studies in mutase-deficient (MUT) methylmalonic aciduria: identification of five novel mutations. Human Mutation. 2002;20:406. doi: 10.1002/humu.9074. [DOI] [PubMed] [Google Scholar]
- 45.Fuchshuber A.. et al. mut0 methylmalonic acidemia: eleven novel mutations of the methylmalonyl CoA mutase including a deletion–insertion mutation. Human Mutation. 2000;16:179. doi: 10.1002/1098-1004(200008)16:2<179::AID-HUMU17>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 46.Ogasawara M.. et al. Identification of two novel mutations in the methylmalonyl-CoA mutase gene with decreased levels of mutant mRNA in methylmalonic acidemia. Human Molecular Genetics. 1994;3:867–872. doi: 10.1093/hmg/3.6.867. [DOI] [PubMed] [Google Scholar]
- 47.Acquaviva C.. et al. N219Y, a new frequent mutation among mut(degree) forms of methylmalonic acidemia in Caucasian patients. European Journal of Human Genetics. 2001;9:577–582. doi: 10.1038/sj.ejhg.5200675. [DOI] [PubMed] [Google Scholar]
- 48.Benoist J.F.. et al. Molecular and structural analysis of two novel mutations in a patient with mut(-) methylmalonyl-CoA deficiency. Molecular Genetics and Metabolism. 2001;72:181–184. doi: 10.1006/mgme.2000.3122. [DOI] [PubMed] [Google Scholar]
- 49.Berger I.. et al. Mutation analysis of the MCM gene in Israeli patients with mut(0) disease. Molecular Genetics and Metabolism. 2001;73:107–110. doi: 10.1006/mgme.2001.3166. [DOI] [PubMed] [Google Scholar]
- 50.Touraine R.L.. et al. A 13-bp deletion (1952 del 13) in the methylmalonyl CoA mutase gene of an affected patient. Human Mutation. 1995;5:354–356. doi: 10.1002/humu.1380050417. [DOI] [PubMed] [Google Scholar]
- 51.Stich T.A.. et al. Spectroscopic evidence for the formation of a four-coordinate Co2+ cobalamin species upon binding to the human ATP:cobalamin adenosyltransferase. Journal of the American Chemical Society. 2005;127:7660–7661. doi: 10.1021/ja050546r. [DOI] [PubMed] [Google Scholar]
- 52.Schrauzer G.N., Deutsch E., Windgassen R.J.. The nucleophilicity of vitamin B12. Journal of the American Chemical Society. 1968;90:2441–2442. doi: 10.1021/ja01011a054. [DOI] [PubMed] [Google Scholar]
- 53.Krautler B.. Vitamin B12: chemistry and biochemistry. Biochemical Society Transactions. 2005;33:806–810. doi: 10.1042/BST0330806. [DOI] [PubMed] [Google Scholar]
- 54.Lexa D., Saveant J.M.. The electrochemistry of vitamin B12. Accounts of Chemical Research. 1983;16:235–243. [Google Scholar]
- 55.Eschenmoser A.. Vitamin B12: experiments concerning the origin of its molecular structure. Angewandte Chemie (International Edition in English) 1988;27:5–39. [Google Scholar]
- 56.Benner S.A., Ellington A.D., Tauer A.. Modern metabolism as a palimpsest of the RNA world. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:7054–7058. doi: 10.1073/pnas.86.18.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roth J.R., Lawrence J.G., Bobik T.A.. Cobalamin (coenzyme B12): synthesis and biological significance. Annual Review of Microbiology. 1996;50:137–181. doi: 10.1146/annurev.micro.50.1.137. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y.. et al. Comparative genomic analyses of nickel, cobalt and vitamin B12 utilization. BMC Genomics. 2009;10:78. doi: 10.1186/1471-2164-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown K.L.. Chemistry and enzymology of vitamin B12. Chemical Reviews. 2005;105:2075–2149. doi: 10.1021/cr030720z. [DOI] [PubMed] [Google Scholar]
- 60.Banerjee R., Ragsdale S.W.. The many faces of vitamin B12: catalysis by cobalamin-dependent enzymes. Annual Review of Biochemistry. 2003;72:209. doi: 10.1146/annurev.biochem.72.121801.161828. [DOI] [PubMed] [Google Scholar]
- 61.Rosenblatt D.S., Fenton W.A. Scriver C.R., Beaudet A.L., Valle D., Sly W.S. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York, USA: 2001. Inherited disorders of folate and cobalamin transport and metabolism; pp. 3897–3933. , eds), pp. [Google Scholar]
- 62.Quadros E.V.. Advances in the understanding of cobalamin assimilation and metabolism. British Journal of Haematology. 2010;148:195–204. doi: 10.1111/j.1365-2141.2009.07937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Banerjee R.. B12 trafficking in mammals: a case for coenzyme escort service. ACS Chemical Biology. 2006;1:149–159. doi: 10.1021/cb6001174. [DOI] [PubMed] [Google Scholar]
- 64.Moestrup S.K.. et al. The intrinsic factor-vitamin B12 receptor and target of teratogenic antibodies is a megalin-binding peripheral membrane protein with homology to developmental proteins. Journal of Biological Chemistry. 1998;273:5235–5242. doi: 10.1074/jbc.273.9.5235. [DOI] [PubMed] [Google Scholar]
- 65.Fyfe J.C.. et al. The functional cobalamin (vitamin B12)-intrinsic factor receptor is a novel complex of cubilin and amnionless. Blood. 2004;103:1573–1579. doi: 10.1182/blood-2003-08-2852. [DOI] [PubMed] [Google Scholar]
- 66.Kapadia C.R.. et al. Intrinsic factor-mediated absorption of cobalamin by guinea pig ileal cells. Journal of Clinical Investigation. 1983;71:440–448. doi: 10.1172/JCI110788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beedholm-Ebsen R.. et al. Identification of multidrug resistance protein 1 (MRP1/ABCC1) as a molecular gate for cellular export of cobalamin. Blood. 2010;115:1632–1639. doi: 10.1182/blood-2009-07-232587. [DOI] [PubMed] [Google Scholar]
- 68.Morkbak A.L.. et al. Effect of vitamin B12 treatment on haptocorrin. Clinical Chemistry. 2006;52:1104–1111. doi: 10.1373/clinchem.2005.061549. [DOI] [PubMed] [Google Scholar]
- 69.Hall C.A., Finkler A.E.. A second vitamin B12-binding substance in human plasma. Biochimica Biophysica Acta. 1963;78:234–236. doi: 10.1016/0006-3002(63)91633-0. [DOI] [PubMed] [Google Scholar]
- 70.Finkler A.E., Hall C.A.. Nature of the relationship between vitamin B12 binding and cell uptake. Archives of Biochemistry and Biophysics. 1967;120:79–85. doi: 10.1016/0003-9861(67)90600-5. [DOI] [PubMed] [Google Scholar]
- 71.Whitehead V.M.. Acquired and inherited disorders of cobalamin and folate in children. British Journal of Haematology. 2006;134:125–136. doi: 10.1111/j.1365-2141.2006.06133.x. [DOI] [PubMed] [Google Scholar]
- 72.Sennett C., Rosenberg L.E., Mellman I.S.. Transmembrane transport of cobalamin in prokaryotic and eukaryotic cells. Annual Review of Biochemistry. 1981;50:1053–1086. doi: 10.1146/annurev.bi.50.070181.005201. [DOI] [PubMed] [Google Scholar]
- 73.Allen R.H.. Human vitamin B12 transport proteins. Progress in Hematology. 1975;9:57–84. [PubMed] [Google Scholar]
- 74.Fedosov S.N.. et al. Comparative analysis of cobalamin binding kinetics and ligand protection for intrinsic factor, transcobalamin, and haptocorrin. Journal of Biological Chemistry. 2002;277:9989–9996. doi: 10.1074/jbc.M111399200. [DOI] [PubMed] [Google Scholar]
- 75.Cooper B.A., Rosenblatt D.S.. Inherited defects of vitamin B12 metabolism. Annual Review of Nutrition. 1987;7:291–320. doi: 10.1146/annurev.nu.07.070187.001451. [DOI] [PubMed] [Google Scholar]
- 76.Youngdahl-Turner P.. et al. Protein mediated vitamin uptake. Adsorptive endocytosis of the transcobalamin II-cobalamin complex by cultured human fibroblasts. Experimental Cell Research. 1979;118:127–134. doi: 10.1016/0014-4827(79)90590-1. [DOI] [PubMed] [Google Scholar]
- 77.Youngdahl-Turner P., Rosenberg L.E., Allen R.H.. Binding and uptake of transcobalamin II by human fibroblasts. Journal of Clinical Investigation. 1978;61:133–141. doi: 10.1172/JCI108911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Quadros E.V., Nakayama Y., Sequeira J.M.. The protein and the gene encoding the receptor for the cellular uptake of transcobalamin-bound cobalamin. Blood. 2009;113:186–192. doi: 10.1182/blood-2008-05-158949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Quadros E.V.. et al. Positive newborn screen for methylmalonic aciduria identifies the first mutation in TCblR/CD320, the gene for cellular uptake of transcobalamin-bound vitamin B(12) Human Mutation. 2010;31:924–929. doi: 10.1002/humu.21297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gravel R.A.. et al. Genetic complementation in heterokaryons of human fibroblasts defective in cobalamin metabolism. Proceedings of the National Academy of Sciences of the United States of America. 1975;72:3181–3185. doi: 10.1073/pnas.72.8.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Willard H.F., Mellman I.S., Rosenberg L.E.. Genetic complementation among inherited deficiencies of methylmalonyl-CoA mutase activity: evidence for a new class of human cobalamin mutant. American Journal of Human Genetics. 1978;30:1–13. [PMC free article] [PubMed] [Google Scholar]
- 82.Rosenblatt D.S.. et al. Defect in vitamin B12 release from lysosomes: newly described inborn error of vitamin B12 metabolism. Science. 1985;228:1319–1321. doi: 10.1126/science.4001945. [DOI] [PubMed] [Google Scholar]
- 83.Vassiliadis A.. et al. Lysosomal cobalamin accumulation in fibroblasts from a patient with an inborn error of cobalamin metabolism (cblF complementation group): visualization by electron microscope radioautography. Experimental Cell Research. 1991;195:295–302. doi: 10.1016/0014-4827(91)90376-6. [DOI] [PubMed] [Google Scholar]
- 84.Watkins D., Rosenblatt D.S.. Failure of lysosomal release of vitamin B12: a new complementation group causing methylmalonic aciduria (cblF) American Journal of Human Genetics. 1986;39:404–408. [PMC free article] [PubMed] [Google Scholar]
- 85.Mahoney M.J.. et al. Methylmalonicacidemia: biochemical heterogeneity in defects of 5′-deoxyadenosylcobalamin synthesis. Proceedings of the National Academy of Sciences of the United States of America. 1975;72:2799–2803. doi: 10.1073/pnas.72.7.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosenblatt D.S.. et al. Clinical heterogeneity and prognosis in combined methylmalonic aciduria and homocystinuria (cblC) Journal of Inherited Metabolic Disease. 1997;20:528–538. doi: 10.1023/a:1005353530303. [DOI] [PubMed] [Google Scholar]
- 87.Kim J., Gherasim C., Banerjee R.. Decyanation of vitamin B12 by a trafficking chaperone. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14551–14554. doi: 10.1073/pnas.0805989105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Froese D.S.. et al. Thermolability of mutant MMACHC protein in the vitamin B12-responsive cblC disorder. Molecular Genetics and Metabolism. 2010;100:29–36. doi: 10.1016/j.ymgme.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hannibal L.. et al. Processing of alkylcobalamins in mammalian cells: a role for the MMACHC (cblC) gene product. Molecular Genetics and Metabolism. 2009;97:260–266. doi: 10.1016/j.ymgme.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mudd S.H., Uhlendorf B.W., Hinds K.R.. Deranged B 12 metabolism: studies of fibroblasts grown in tissue culture. Biochemical Medicine. 1970;4:215–239. doi: 10.1016/0006-2944(70)90050-5. [DOI] [PubMed] [Google Scholar]
- 91.Mellman I.. et al. Cobalamin coenzyme synthesis in normal and mutant human fibroblasts. Evidence for a processing enzyme activity deficient in cblC cells. Journal of Biological Chemistry. 1979;254:11847–11853. [PubMed] [Google Scholar]
- 92.Andersson H.C., Shapira E.. Biochemical and clinical response to hydroxocobalamin versus cyanocobalamin treatment in patients with methylmalonic acidemia and homocystinuria (cblC) Journal of Pediatrics. 1998;132:121–124. doi: 10.1016/s0022-3476(98)70496-2. [DOI] [PubMed] [Google Scholar]
- 93.Froese D.S.. et al. Mechanism of vitamin B12-responsiveness in cblC methylmalonic aciduria with homocysteinuria. Molecular Genetics and Metabolism. 2009;98:338–343. doi: 10.1016/j.ymgme.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 94.Kim J.. et al. A human vitamin B12 trafficking protein uses glutathione transferase activity for processing alkylcobalamins. Journal of Biological Chemistry. 2009;284:33418–33424. doi: 10.1074/jbc.M109.057877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goodman S.I.. et al. Homocystinuria with methylmalonic aciduria: two cases in a sibship. Biochemical Medicine. 1970;4:500–515. doi: 10.1016/0006-2944(70)90080-3. [DOI] [PubMed] [Google Scholar]
- 96.Suormala T.. et al. The cblD defect causes either isolated or combined deficiency of methylcobalamin and adenosylcobalamin synthesis. Journal of Biological Chemistry. 2004;279:42742–42749. doi: 10.1074/jbc.M407733200. [DOI] [PubMed] [Google Scholar]
- 97.Watkins D., Matiaszuk N., Rosenblatt D.S.. Complementation studies in the cblA class of inborn error of cobalamin metabolism: evidence for interallelic complementation and for a new complementation class (cblH) Journal of Medical Genetics. 2000;37:510–513. doi: 10.1136/jmg.37.7.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schuh S.. et al. Homocystinuria and megaloblastic anemia responsive to vitamin B12 therapy. An inborn error of metabolism due to a defect in cobalamin metabolism. New England Journal of Medicine. 1984;310:686–690. doi: 10.1056/NEJM198403153101104. [DOI] [PubMed] [Google Scholar]
- 99.Hallam L.J.. et al. Vitamin B12-responsive neonatal megaloblastic anemia and homocystinuria with associated reduced methionine synthase activity. Blood. 1987;69:1128–1133. [PubMed] [Google Scholar]
- 100.Rosenblatt D.S.. et al. Vitamin B12 responsive homocystinuria and megaloblastic anemia: heterogeneity in methylcobalamin deficiency. American Journal of Medical Genetics. 1987;26:377–383. doi: 10.1002/ajmg.1320260216. [DOI] [PubMed] [Google Scholar]
- 101.Watkins D., Rosenblatt D.S.. Genetic heterogeneity among patients with methylcobalamin deficiency. Definition of two complementation groups, cblE and cblG. Journal of Clinical Investigation. 1988;81:1690–1694. doi: 10.1172/JCI113507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Y.N.. et al. Cloning, mapping and RNA analysis of the human methionine synthase gene. Human Molecular Genetics. 1996;5:1851–1858. doi: 10.1093/hmg/5.12.1851. [DOI] [PubMed] [Google Scholar]
- 103.Chen L.H.. et al. Human methionine synthase. cDNA cloning, gene localization, and expression. Journal of Biological Chemistry. 1997;272:3628–3634. [PubMed] [Google Scholar]
- 104.Fenton W.A., Scriver C.R., Beaudet A.L., Sly W.S., Valle D. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York, USA: 2001. Disorders of propionate and methylmalonate metabolism; pp. 2165–2193. , eds), pp. [Google Scholar]
- 105.Watkins D., Rosenblatt D.S.. Functional methionine synthase deficiency (cblE and cblG): clinical and biochemical heterogeneity. American Journal of Medical Genetics. 1989;34:427–434. doi: 10.1002/ajmg.1320340320. [DOI] [PubMed] [Google Scholar]
- 106.Harding C.O.. et al. Functional methionine synthase deficiency due to cblG disorder: a report of two patients and a review. American Journal of Medical Genetics. 1997;71:384–390. doi: 10.1002/(sici)1096-8628(19970905)71:4<384::aid-ajmg3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 107.Matthews R.G. Blakely R.L., Bencovic S.J. Folates and Pterins. J. Wiley & Sons, Inc.; New York, USA: 1984. Methionine biosynthesis; pp. 497–553. , eds), pp. [Google Scholar]
- 108.Taylor R.T. Dolphin D. B12. John Wiley & Sons, Inc.; New York: 1983. B12-dependent methionine biosynthesis; pp. 307–355. , ed.), pp. [Google Scholar]
- 109.Matthews R.G. Banerjee R. Chemistry and Biochemistry of B12. John Wiley & Sons; New York, USA: 1999. Cobalamin-dependent methionine synthase; pp. 681–706. , ed.), pp. [Google Scholar]
- 110.Matthews R.G., Ludwig M.L. Carmel R., Jacobsen D.W. Homocysteine in Health and Disease. Cambridge University Press; Cambridge, UK: 2001. Microbial modeling of human disease; pp. 100–112. , eds), pp. [Google Scholar]
- 111.Goulding C.W., Postigo D., Matthews R.G.. Cobalamin-dependent methionine synthase is a modular protein with distinct regions for binding homocysteine, methyltetrahydrofolate, cobalamin, and adenosylmethionine. Biochemistry. 1997;36:8082–8091. doi: 10.1021/bi9705164. [DOI] [PubMed] [Google Scholar]
- 112.Banerjee R.V.. et al. Cloning and sequence analysis of the Escherichia coli metH gene encoding cobalamin-dependent methionine synthase and isolation of a tryptic fragment containing the cobalamin-binding domain. Journal of Biological Chemistry. 1989;264:13888–13895. [PubMed] [Google Scholar]
- 113.Dixon M.M.. et al. The structure of the C-terminal domain of methionine synthase: presenting S-adenosylmethionine for reductive methylation of B12. Structure. 1996;4:1263–1275. doi: 10.1016/s0969-2126(96)00135-9. [DOI] [PubMed] [Google Scholar]
- 114.Drummond J.T.. et al. Assignment of enzymatic function to specific protein regions of cobalamin-dependent methionine synthase from Escherichia coli. Biochemistry. 1993;32:9290–9295. doi: 10.1021/bi00087a005. [DOI] [PubMed] [Google Scholar]
- 115.Fujii K., Huennekens F.M.. Activation of methionine synthetase by a reduced triphosphopyridine nucleotide-dependent flavoprotein system. Journal of Biological Chemistry. 1974;249:6745–6753. [PubMed] [Google Scholar]
- 116.Osborne C., Chen L.M., Matthews R.G.. Isolation, cloning, mapping, and nucleotide sequencing of the gene encoding flavodoxin in Escherichia coli. The Journal of Bacteriology. 1991;173:1729–1737. doi: 10.1128/jb.173.5.1729-1737.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leclerc D.. et al. Cloning and mapping of a cDNA for methionine synthase reductase, a flavoprotein defective in patients with homocystinuria. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3059–3064. doi: 10.1073/pnas.95.6.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Olteanu H., Banerjee R.. Human methionine synthase reductase, a soluble P-450 reductase-like dual flavoprotein, is sufficient for NADPH-dependent methionine synthase activation. Journal of Biological Chemistry. 2001;276:35558–35563. doi: 10.1074/jbc.M103707200. [DOI] [PubMed] [Google Scholar]
- 119.Wolthers K.R.. et al. Molecular dissection of human methionine synthase reductase: determination of the flavin redox potentials in full-length enzyme and isolated flavin-binding domains. Biochemistry. 2003;42:3911–3920. doi: 10.1021/bi027290b. [DOI] [PubMed] [Google Scholar]
- 120.Yamada K.. et al. Human methionine synthase reductase is a molecular chaperone for human methionine synthase. Proceedings of the National Academy of Sciences of the United States of America. 2006;20(103):9476–9481. doi: 10.1073/pnas.0603694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Leal N.A.. et al. Human ATP: Cob(I)alamin adenosyltransferase and its interaction with methionine synthase reductase. Journal of Biological Chemistry. 2004;279:47536–47542. doi: 10.1074/jbc.M405449200. [DOI] [PubMed] [Google Scholar]
- 122.Froese D.S.. et al. Restricted role for methionine synthase reductase defined by subcellular localization. Molecular Genetics and Metabolism. 2008;94:68–77. doi: 10.1016/j.ymgme.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mera P.E., Escalante-Semerena J.C.. Dihydroflavin-driven adenosylation of 4-coordinate Co(II) corrinoids: are cobalamin reductases enzymes or electron transfer proteins? Journal of Biological Chemistry. 2010;285:2911–2917. doi: 10.1074/jbc.M109.059485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Horster F.. et al. Long-term outcome in methylmalonic acidurias is influenced by the underlying defect (mut0, mut-, cblA, cblB) Pediatric Research. 2007;62:225–230. doi: 10.1203/PDR.0b013e3180a0325f. [DOI] [PubMed] [Google Scholar]
- 125.Matsui S.M., Mahoney M.J., Rosenberg L.E.. The natural history of the inherited methylmalonic acidemias. New England Journal of Medicine. 1983;308:857–861. doi: 10.1056/NEJM198304143081501. [DOI] [PubMed] [Google Scholar]
- 126.Korotkova N., Lidstrom M.E.. MeaB is a component of the methylmalonyl-CoA mutase complex required for protection of the enzyme from inactivation. Journal of Biological Chemistry. 2004;279:13652–13658. doi: 10.1074/jbc.M312852200. [DOI] [PubMed] [Google Scholar]
- 127.Padovani D., Labunska T., Banerjee R.. Energetics of interaction between the G-protein chaperone, MeaB, and B12-dependent methylmalonyl-CoA mutase. Journal of Biological Chemistry. 2006;281:17838–17844. doi: 10.1074/jbc.M600047200. [DOI] [PubMed] [Google Scholar]
- 128.Froese D.S.. et al. Sleeping beauty mutase (sbm) is expressed and interacts with ygfd in Escherichia coli. Microbiological Research. 2009;164:1–8. doi: 10.1016/j.micres.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Padovani D., Banerjee R.. Assembly and protection of the radical enzyme, methylmalonyl-CoA mutase, by its chaperone. Biochemistry. 2006;45:9300–9306. doi: 10.1021/bi0604532. [DOI] [PubMed] [Google Scholar]
- 130.Padovani D., Banerjee R.. A G-protein editor gates coenzyme B12 loading and is corrupted in methylmalonic aciduria. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21567–21572. doi: 10.1073/pnas.0908106106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Leal N.A.. et al. Identification of the human and bovine ATP:Cob(I)alamin adenosyltransferase cDNAs based on complementation of a bacterial mutant. Journal of Biological Chemistry. 2003;278:9227–9234. doi: 10.1074/jbc.M212739200. [DOI] [PubMed] [Google Scholar]
- 132.Schubert H.L., Hill C.P.. Structure of ATP-bound human ATP:cobalamin adenosyltransferase. Biochemistry. 2006;45:15188–15196. doi: 10.1021/bi061396f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fan C., Bobik T.A.. Functional characterization and mutation analysis of human ATP:Cob(I)alamin adenosyltransferase. Biochemistry. 2008;47:2806–2813. doi: 10.1021/bi800084a. [DOI] [PubMed] [Google Scholar]
- 134.Saridakis V.. et al. The structural basis for methylmalonic aciduria. The crystal structure of archaeal ATP:cobalamin adenosyltransferase. Journal of Biological Chemistry. 2004;279:23646–23653. doi: 10.1074/jbc.M401395200. [DOI] [PubMed] [Google Scholar]
- 135.St.Maurice M.. et al. Structural characterization of the active site of the PduO-type ATP:Co(I)rrinoid adenosyltransferase from Lactobacillus reuteri. Journal of Biological Chemistry. 2007;282:2596–2605. doi: 10.1074/jbc.M609557200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang J.. et al. Ligand-binding by catalytically inactive mutants of the cblB complementation group defective in human ATP:cob(I)alamin adenosyltransferase. Molecular Genetics and Metabolism. 2009;98:278–284. doi: 10.1016/j.ymgme.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 137.Zhang J.. et al. Impact of cblB mutations on the function of ATP:cob(I)alamin adenosyltransferase in disorders of vitamin B12 metabolism. Molecular Genetics and Metabolism. 2006;87:315–322. doi: 10.1016/j.ymgme.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 138.Ledley F.D.. et al. Molecular cloning of L-methylmalonyl-CoA mutase: gene transfer and analysis of mut cell lines. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:3518–3521. doi: 10.1073/pnas.85.10.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Willard H.F., Rosenberg L.E.. Inherited methylmalonyl CoA mutase apoenzyme deficiency in human fibroblasts: evidence for allelic heterogeneity, genetic compounds, and codominant expression. Journal of Clinical Investigation. 1980;65:690–698. doi: 10.1172/JCI109715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ledley F.D., Rosenblatt D.S.. Mutations in mut methylmalonic acidemia: clinical and enzymatic correlations. Human Mutation. 1997;9:1–6. doi: 10.1002/(SICI)1098-1004(1997)9:1<1::AID-HUMU1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 141.Mancia F.. et al. How coenzyme B12 radicals are generated: the crystal structure of methylmalonyl-coenzyme A mutase at 2 A resolution. Structure. 1996;4:339–350. doi: 10.1016/s0969-2126(96)00037-8. [DOI] [PubMed] [Google Scholar]
- 142.Mancia F., Evans P.R.. Conformational changes on substrate binding to methylmalonyl CoA mutase and new insights into the free radical mechanism. Structure. 1998;6:711–720. doi: 10.1016/s0969-2126(98)00073-2. [DOI] [PubMed] [Google Scholar]
- 143.Mancia F., Smith G.A., Evans P.R.. Crystal structure of substrate complexes of methylmalonyl-CoA mutase. Biochemistry. 1999;38:7999–8005. doi: 10.1021/bi9903852. [DOI] [PubMed] [Google Scholar]
- 144.Thoma N.H., Leadlay P.F.. Homology modeling of human methylmalonyl-CoA mutase: a structural basis for point mutations causing methylmalonic aciduria. Protein Science. 1996;5:1922–1927. doi: 10.1002/pro.5560050919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kolhouse J.F., Utley C., Allen R.H.. Isolation and characterization of methylmalonyl-CoA mutase from human placenta. Journal of Biological Chemistry. 1980;255:2708–2712. [PubMed] [Google Scholar]
- 146.Fenton W.A.. et al. Purification and properties of methylmalonyl coenzyme A mutase from human liver. Archives of Biochemistry and Biophysics. 1982;214:815–823. doi: 10.1016/0003-9861(82)90088-1. [DOI] [PubMed] [Google Scholar]
- 147.Hubbard P.A.. et al. Crystal structure and mutagenesis of the metallochaperone MeaB: insight into the causes of methylmalonic aciduria. Journal of Biological Chemistry. 2007;282:31308–31316. doi: 10.1074/jbc.M704850200. [DOI] [PubMed] [Google Scholar]
- 148.Chandler R.J.. et al. Propionyl-CoA and adenosylcobalamin metabolism in Caenorhabditis elegans: evidence for a role of methylmalonyl-CoA epimerase in intermediary metabolism. Molecular Genetics and Metabolism. 2006;89:64–73. doi: 10.1016/j.ymgme.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chandler R.J., Venditti C.P.. Genetic and genomic systems to study methylmalonic acidemia. Molecular Genetics and Metabolism. 2005;86:34–43. doi: 10.1016/j.ymgme.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kuhnl J.. et al. Functional analysis of the methylmalonyl-CoA epimerase from Caenorhabditis elegans. FEBS Journal. 2005;272:1465–1477. doi: 10.1111/j.1742-4658.2005.04579.x. [DOI] [PubMed] [Google Scholar]
- 151.Carrillo-Carrasco N.. et al. Liver-directed rAAV gene delivery rescues a lethal mouse model of methylmalonic acidemia and provides long-term phenotypic correction. Human Gene Therapy. 2010 doi: 10.1089/hum.2010.008. 2010 Aug 2 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading, resources and contacts
Reviews
There are many excellent reviews that address different aspects of vitamin B12 metabolism, the proteins involved and clinical correlates:
- Banerjee R., Gherasim C., Padovani D.. The tinker, tailor, soldier in intracellular B12 trafficking. Current Opinion in Chemical Biology. 2009;13:484–491. doi: 10.1016/j.cbpa.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Watkins D., Rosenblatt D.S.. Vitamin B(12) and birth defects. Molecular Genetics and Metabolism. 2009;98:166–172. doi: 10.1016/j.ymgme.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Fowler B., Leonard J.V., Baumgartner M.R.. Causes of and diagnostic approach to methylmalonic acidurias. Journal of Inherited Metabolic Disease. 2008;31:350–360. doi: 10.1007/s10545-008-0839-4. [DOI] [PubMed] [Google Scholar]
- Dali-Youcef N., Andres E.. An update on cobalamin deficiency in adults. Quarterly Journal of Medicine. 2009;102:17–28. doi: 10.1093/qjmed/hcn138. [DOI] [PubMed] [Google Scholar]
Websites
- http://www.oaanews.org/ http://www.oaanews.org/ The Organic Acidemia Association. A volunteer organisation for the provision of support and information on organic acidurias, including the methylmalonic acidurias:
- http://rarediseases.info.nih.gov/ http://rarediseases.info.nih.gov/ National Institutes of Health: Office of Rare Diseases Research. A highly informative site covering basic information, patient advocacy, research and clinical trials, and research resources for the public and scientific community:
- http://www.ncbi.nlm.nih.gov/omim http://www.ncbi.nlm.nih.gov/omim National Center for Biotechnology Information: Online Mendelian Inheritance in Man. Compilation of gene and phenotype information on all known Mendelian genetic disorders searchable by disease or gene name: