Abstract
Yawning appears to be involved in arousal, state change, and activity across vertebrates. Recent research suggests that yawning may support effective changes in mental state or vigilance through cerebral cooling. To further investigate the relationship between yawning, state change, and thermoregulation, 12 Sprague-Dawley rats (Rattus norvegicus) were exposed to a total of two hours of ambient temperature manipulation over a period of 48 hours. Using a repeated measures design, each rat experienced a range of increasing (22→32°C), decreasing (32→22°C), and constant temperatures (22°C; 32°C). Yawning and locomotor activity occurred most frequently during initial changes in temperature, irrespective of direction, compared to more extended periods of temperature manipulation. The rate of yawning also diminished during constant high temperatures (32°C) compared to low temperatures (22°C). Unlike yawning, however, stretching was unaffected by ambient temperature variation. These findings are compared to recent work on budgerigars (Melopsittacus undulatus), and the ecological selective pressures for yawning in challenging thermal environments are discussed. The results support previous comparative research connecting yawning with arousal and state change, and contribute to refining the predictions of the thermoregulatory hypothesis across vertebrates.
Keywords: yawning, thermoregulation, ambient temperature, stretching, state change, arousal
Introduction
Phylogenetically old, yawning or yawn-like behaviors have been observed in all classes of vertebrates (Craemer, 1924; Luttenberger, 1975; Baenninger, 1987; Gallup et al., 2009), suggesting important and basic functions. It is physically similar across classes, and comparatively it has been characterized as an extended gaping of the mouth, followed by a more rapid closure (Provine, 1986; Baenninger, 1987). Provine (1986) first proposed the “state change hypothesis” based on the observations that yawning was associated with behavioral transitions. The general hypothesis was then extended to suggest that yawning facilitates a number of behavioral shifts, e.g., from boredom to alertness and changes from one activity to another (Provine, 1996; 2005). Through a thorough analysis of the behavioral correlates, temporal contexts, physiological mechanisms, and phylogenetic and ontogenetic aspects, Baenninger (1997) concluded that yawning might stimulate arousal during state change. For instance, comparative research shows that vertebrates yawn in anticipation of important daily events and behavioral transitions or during changes in activity levels. A recent report on captive chimpanzees (Pan troglodytes) provides strong support for this hypothesis, showing that locomotion increases after yawning (Vick & Paukner, 2009). Consistent with an arousing effect of yawning, it is also common when there are stressful events, threats, and increases in anxiety (Baenninger 1997; Gallup & Gallup, 2008). Perhaps the most regular periods of state change and locomotor activity occur during transitions between sleep and wakefulness, and the most frequent instances of yawning in humans occur prior to the onset of sleep and just after waking (Provine et al., 1987; Baenninger et al., 1996; Zilli et al., 2007). Yawning appears to have a circadian pattern in other animals as well; peaks in yawning frequency among laboratory rats occur during light to dark transitions, as activity increases (Anias et al., 1984).
The deep inhalation and constriction and relaxation of facial muscles during a yawn are hypothesized to modify levels of cortical arousal through enhanced cerebral blood flow (Askenasy 1989). Contrary to expectations from the arousal hypothesis, however, a recent study of electroencephalographic changes (EEG) describe no arousing effect (as measured by alpha power and activity) 10 seconds after yawning in patients suffering from excessive daytime sleepiness (Guggisberg et al., 2007). It is entirely possible, however, that yawning may increase alertness and vigilance over a longer time, and in ways that are not detected by EEG. For instance, yawning may increase arousal through the mechanical stimulation of the carotid body (Matikainen & Elo, 2008).
Likewise, recent research suggests that yawning may have a thermoregulatory function, and in particular may serve to cool the brain (Gallup & Gallup, 2007), thus maintaining mental efficiency and vigilance through thermal homeostasis. Shoup-Knox et al. (2010) recently explored the relationship between brain temperature and yawning by implanting thermocoupled probes in the prelimbic cortex of rats (Rattus norvegicus) to measure changes in brain temperature before, during and after yawning. Results showed yawning was preceded in all instances by rapid increases in brain temperature, but as soon as yawning occurred, brain temperatures began to decrease and there was a return to baseline following each yawn (Shoup-Knox et al., 2010). It is proposed that yawns may provide a metabolically inexpensive means of cooling by increasing venous blood flow, removing warmer blood from the brain and reducing temperature through convection. During hyperthermia in humans, blood flow is increased from the skin to the cranial cavity, and this change is essential for proper cooling of the brain (Cabanac & Brinnel, 1985). It is also hypothesized that the gaping of the mouth and deep inhalation during a yawn cools venous blood draining from the nasal and oral orifices into the cavernous sinus, which surrounds the internal carotid artery supplying blood to the rest of the brain (Gallup & Gallup 2007). Comparative research from birds, rats, and humans shows that yawning is followed by reductions in brain and body temperature, is suppressed by other methods of behavioral cooling, and is influenced by the direction and range of ambient temperature (reviewed by Gallup, 2010; in press). Consistent with the view that yawning is a thermoregulatory behavior, recent research has revealed a strong negative correlation between body temperature and the latency to yawn following a stressor in budgerigars (Melopsittacus undulatus) (Miller et al., 2010). This hypothesis complements those of arousal and state change, suggesting that the cooling component of yawning may facilitate these processes by reinstating brain thermal homeostasis.
The thermoregulatory hypothesis generates numerous testable predictions. One is that yawning will occur in a “thermal window”, or a narrow range of ambient temperatures (Gallup & Gallup, 2007). According to this model, the temperature of the air gives the yawn its utility. The model predicts that yawns should increase in frequency as ambient temperatures approach body temperature, diminish as temperatures continue to rise, and cease when ambient temperatures reach or exceed body temperature because they would no longer result in cooling. Among homeotherms, ambient air temperature provides an accurate index of the rate of heat transfer away from the body. During a rise in ambient temperature, the body is increasingly unable to lose heat, stimulating thermoregulatory mechanisms to control internal temperatures. Likewise, when temperatures fall below a certain point, and the brain is not overheated, further cooling is not beneficial.
Experimental work with budgerigars has recently tested the first prediction of the thermal window hypothesis (Gallup et al., 2009; 2010). Budgerigars yawned more frequently during increasing ambient temperatures (22–34°C), but as temperatures continued to rise and were held near body temperature (34–38°C), yawning rate diminished as more effective heat loss behaviors became prevalent (Gallup et al., 2009). A follow up study demonstrated that temperature change alone was not sufficient to influence yawning in budgerigars, as decreases across a similar range of ambient temperature (34–24°C) failed to produce associated increases in yawning frequency (Gallup et al., 2010). Thus, in this species, the physiological trigger for yawning appears to be related to increasing body temperatures rather than the detection of changing external temperatures. Deputte (1994) was the first to describe an association between yawning and ambient temperature in primates. In macaques (Macaca fasciularis), yawning was positively correlated with ambient temperature, and rising ambient temperature was correlated with heightened yawning while lying down (Deputte, 1994). Recent naturalistic research on capuchins (Cebus capucinus) showed patterns consistent with these findings; yawning occurred significantly more often when ambient temperatures were high and humidity low (Campos & Fedigan, 2009; for a reply see also Gallup, 2010). Given that fluctuations in ambient temperature can produce changes in state and activity, temperature manipulation provides an interesting avenue to further investigate the relationship between yawning, state change, and thermoregulation across vertebrate species.
The present study examined the incidence of yawning, associated behaviors (e.g., stretching), and activity in rats as a function of the range and direction of ambient temperature change. To our knowledge, this is the first study to experimentally investigate these associations in a mammalian species. As in our recent studies in budgerigars, we exposed rats to varying ambient temperatures, manipulating the direction and range of ambient temperature in a controlled thermal chamber. Animals experienced rapid changes in temperature of each direction (low-increasing: 22→27°; high-increasing: 27→32°; high-decreasing: 32→27°C; low-decreasing: 27→22°C), as well as periods of constant temperatures (cool: 22°C; warm: 32°C). Based on the results of Gallup et al. (2010) in budgerigars, we hypothesized that temperature manipulation would produce changes in yawning frequency, but that high-increasing ambient temperatures would generate the highest rates of yawning. We also predicted that lowest yawning rates would occur during low-decreasing temperatures. According to the predictions based on the thermal window, we expected yawning rates to be diminished in the constant high temperature condition in comparison to the low temperature condition. Although stretching is commonly associated with yawning and arousal and the two co-occur under many conditions (Baeninnger, 1997), we predicted that they would be disassociated in this experiment. Provine et al. (1987) first reported a disassociation between yawning and stretching in humans, showing that yawns are accompanied by stretches more so in the morning than in the evening. In addition, our past research using ambient temperature manipulation completely decoupled these behaviors in budgerigars, significantly impacting yawning rates while leaving stretching unaffected (Gallup et al., 2009; 2010). These results suggest differences in the functionality of these behaviors. Therefore, we also hypothesized that the frequency of stretching would not vary across different thermal environments, and that the rate of yawning and stretching would not be correlated during thermally challenging conditions. Lastly, we hypothesized that initial rapid changes in ambient temperature would increase arousal and alertness, and potentially produce highest locomotor activity.
Methods and Materials
Experimental Conditions
Twelve Sprague-Dawley rats (250–315g body weight) were individually tested in metal cages (0.25 × 0.20 × 0.18m) with a wire-mesh screen on one side. A similar screen served as a floor; floor and side allowed air circulation into the cage, which was covered by a wooden box. The box (0.96 × 0.43 × 0.61m) fully enclosed the cage, with holes allowing for ventilation. Three small infrared heat lamps within the box were used to adjust the ambient temperature. A viewing window (0.23 × 0.38 m) in the side of the box and covered with Plexiglas allowed viewing and filming of the rats inside the thermal chamber. A mirror was placed at the end of the cage opposite to the wire mesh, which allowed researchers/raters to see the rats yawn even when they were facing away. A Springfield PreciseTemp™ digital thermometer/hygrometer was positioned near the rats (0.5 m from heat lamps) in the upper ½ of the box, with the digital readout visible to the researcher. This was used to monitor and record the ambient temperature and humidity. Temperature recordings were collected every two minutes to the nearest 0.1°C. Humidity levels remained constant throughout the experimental procedure, never fluctuating by more than 1 percentage point (total range across experiments: 25–27%).
During each session, a researcher was present to monitor the rats and control the ambient temperature while the trial was recorded using a digital camcorder. Conditions included a rapidly increasing temperature range (22→32°C), and a rapidly decreasing temperature range (32→22°C). Following the methods of Gallup et al. (2010), these conditions were further divided into four 10-minute intervals, including a (i) low-increasing range (22→27°C) followed by a (ii) high-increasing (27→32°C), and a (iii) high-decreasing (32→27°C) followed by a (iv) low-decreasing range (27→22°C). Prior to each session and after the rats were placed within the thermal chamber, there was a 30 min acclimation period, during which the chamber temperature was held constant. This temperature served as the starting ambient temperature of each trial order (increasing: 22°C; decreasing: 32°C). Following the 30-minunte acclimation, the rat's behavior was recorded for an additional 20-minute period of constant temperature prior to temperature manipulation. A repeated measures design was used in which each testing session of 60 minutes included a 20-minute constant temperature period followed by both changing temperature conditions. Thus each session included a single constant temperature and two 20-minute changing temperature regimes, each of which was divided further into 10-minute blocks representing changes in the high temperature range and changes in the low temperature range. The trials were counterbalanced with respect to the order of temperature changes: (i) increasing then decreasing (ID) or (ii) decreasing then increasing (DI).
Each rat was tested twice, once in each trial order, with 24 hours between test sessions. Thus, each rat experienced two hours of temperature manipulation in total. Food and water were temporarily removed during each one-hour session, but all rats were given food and water ad libitum prior to and after testing. Rats were tested two at a time in individual adjacent cages; both visible through the Plexiglas window. Rats were randomly assigned to testing groups and the trial orders were alternated between groups. Rats were tested at one of three distinct times during the day (1130, 1400, 1630h), and time remained constant within pairs across the two days of testing. The heat lamps were turned on at the beginning of the increasing condition in both the ID and DI trial orders, and prior to the acclimation period in the DI trial. A small fluorescent light (0.33 m in length) remained on above the testing boxes to ensure constant lighting and visibility during the experimental trials. Decreasing temperature was achieved by turning off the heat lamps and opening a hinged door in the top of the thermal chamber at the end opposite to the cages, allowing heat to rapidly dissipate. To maintain a constant rate of temperature change and a maximum temperature of 32°C, an experienced researcher turned the lamps on and off at various stages of each trial (see Gallup et al., 2009; 2010). These methods have proven to be quite reliable, and overall temperature changes within ID and DI trial orders were highly consistent across trials (α > 0.99). This procedure was approved by the university Institutional Care and Animal Use Committee (Protocol # 653-09).
Analyses
A trained rater scored the video recordings for yawns, stretches, and total time spent active (in seconds). Individual cages were labeled and thus individual rats were recognizable on the tapes. All instances of yawning and stretching were recorded for each rat. Yawning was recognized as a wide opening of the mouth with tongue protrusion and eye closure, followed by a brief pause (the acme state), and more rapid mouth closure. Stretching consisted of either a strong, slow and sustained extension of one or more legs, or a slow apparent constriction of trunk muscles and arching of the back and neck. Rats were considered active when they were not lying still or resting. The behaviors and activity of each rat were summed across both trials of the same temperature change, and the distribution of behavioral observations was then paired with the appropriate time interval and temperature recordings. A repeated-measures ANOVA was used to investigate differences between trial orders, time of day, and temperature ranges (within subjects: trial condition; between subjects: trial order, time of day). Initial models include all variables of interest, but then are reduced to include only statistically significant relationships. A Pearson correlation test was used to measure the strength of the relationship between the rate of yawning and stretching among individual rats during the different temperature and time intervals.
Results
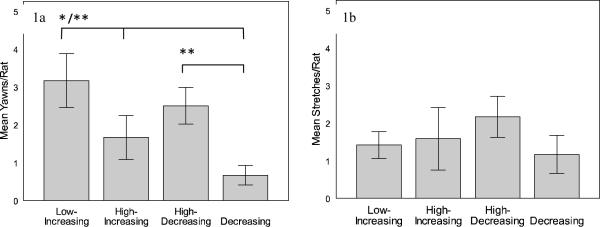
In total, 112 yawns were observed during 24 hours of observation (12 trials, 2 hours/rat). To evaluate differences in the frequency of yawning across the four 10-minute intervals, the model included trial order, time of day, and the interaction between these two factors. For this full model, there was no difference in yawning frequency between trials categorized by order (F3,18 = 1.712, p = 0.200, partial ) or time of day (F6,18 = 0.480, p = 0.814, partial ). There was also no interaction between these factors (F6,18 = 2.480, p = 0.063, partial ). After removing trial order and time of day from the model, yawning frequency differed significantly across time intervals (F3,33 = 6.507, p = 0.001, partial , Figure 1a). Pair-wise comparisons indicate that yawning occurred more often in the low-increasing condition compared to both the high-increasing (p = 0.032) and low-decreasing condition (p = 0.007). Yawning also occurred at higher rates during the high-decreasing condition compared to the low-decreasing condition (p = 0.006). No other comparisons were significant (p's > 0.1).
Figure 1.
Behavioral frequencies (mean ± SE) are from a total of 20 minutes in each condition (asterisks indicate significantly higher yawning for that condition, while bars represent pairwise comparisons). Yawning rates differed across the range and direction of ambient temperatures (1a). Yawning occurred more frequently during low-increasing temperatures in comparison to high-increasing and low-decreasing temperatures. In addition, high-decreasing temperatures produced more yawning than low-decreasing temperatures. Unlike yawning, stretching did not differ across temperature variation (1b).
* = significantly different at p < 0.05 level
** = significantly different at p < 0.01 level
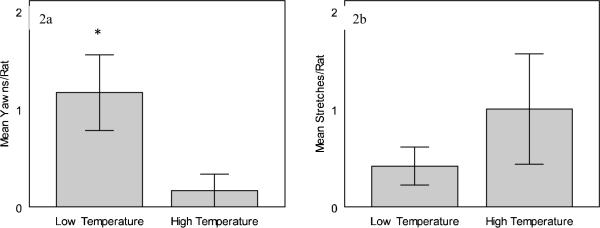
A similar model was run to investigate differences in yawning rates between high and low constant ambient temperatures. The full model found no difference in yawning frequency between trials categorized by order (F1,6 = 2.000, p = 0.207, partial ) or time of day (F2,6 = 1.167, p = 0.373, partial ), and there was also no interaction between these factors (F2,6 = 1.500, p = 0.296, partial ). After removing trial order and time of day from the model, the yawning frequency was significantly lower in the high temperature condition compared to the low condition (F1,11 = 6.600, p = 0.026, partial , Figure 2a).
Figure 2.
Behavioral frequencies (mean ± SE) are from a total of 20 minutes in each condition. Yawning behavior was diminished during constant high temperatures (32°C) in comparison to low temperatures (22°C) (2a). On the other hand, although occurring slightly more during high temperatures, stretching behavior did not differ as a function of constant temperature (2b).
* = significantly different at p < 0.05 level
A total of 86 stretches were observed during the 24 hours of testing. Unlike yawning, there was no difference in the rate of stretching across the range and direction of ambient temperature (F3,18 = 1.091, p = 0.378, partial , Figure 1b). In addition, there was no difference in stretch frequency between trial orders (F3,18 = 2.378, p = 0.104, partial ) or time of day (F6,18 = 1.332, p = 0.294, partial ), and there was no interaction between these factors (F6,18 = 2.577, p = 0.056, partial ). Likewise, there was no difference in the rate of stretching across the high and low constant temperatures (F1,6 = 1.324, p = 0.294, partial , Figure 2b). In addition, there was no difference in stretching frequency during constant temperatures between trials categorized by order (F1,6 = 0.243, p = 0.639, partial ) or time of day (F2,6 = 2.135, p = 0.199, partial ). There was also no interaction between these factors (F2,6 = 3.000, p = 0.125, partial ).
There were strong positive correlations between the frequency of yawning and stretching within the low-increasing (r = 0.655, p = 0.021) and high-increasing conditions (r = 0.884, p < 0.001). Correlations between yawning and stretching in the decreasing conditions were, however, not statistically significant (high-decreasing: r = 0.428, p = 0.165; low-decreasing: r = 0.566, p = 0.055). There was also a strong correlation between the frequency of yawning and stretching in the constant low temperature condition (r = 0.831, p = 0.001), but not in the high temperature condition (r = −0.161, p = 0.617), suggesting that these variables were independent of one another during a consistently warm ambient environment.
To evaluate differences in locomotor activity across the time/temperature conditions, the same model was used as above. Total activity across the four 10-minute time intervals did not vary as a function of trial order (F1,6 = 0.001, p = 0.979, partial ) or time of day (F2,6 = 0.229, p =0.802, partial ), and there were no significant interactions among factors (all p's > 0.1). After removing these from the model, total activity differed across the four time intervals (F3,33 = 16.087, p < 0.001, partial , Table 1). Pair-wise tests show that total time spent active was higher during the low-increasing condition compared to the high-increasing condition (p = 0.005). In addition, activity was higher during the high-decreasing condition in comparison to all other time intervals (all p's < 0.01). No other comparisons were significant (all p's > 0.05). Among high and low constant temperatures, total activity did not vary as a function of trial order (F1,6 = 0.429, p = 0.537, partial ), time of day (F2,6 = 1.848, p = 0.237, partial ), or temperature condition (F1,6 = 2.172, p = 0.191, partial ). Likewise, there were no significant interactions between these factors (all p's > 0.05).
Table 1.
Descriptive statistics (mean ± SE) for total activity in seconds per condition
| low-increasing | high-increasing | high-decreasing | low-decreasing | constant low | constant high | |
|---|---|---|---|---|---|---|
| Total Activity (Sec) | 230.4±43.3* | 70.8±27.3 | 481.3±46.0** | 196.3±63.4 | 185.0±76.5 | 282.5±50.8 |
Across the four 10-minute time intervals, highest activity occurred during initial changes in temperature. The low-increasing condition had more activity than the high-increasing condition, and the high-decreasing condition had more activity than all three other temperature change intervals. On the other hand, total time spent active did not differ between low and high constant temperatures.
significantly different at p < 0.05 level
significantly different at p < 0.01 level
Discussion
Among rats, yawning appears to be an initial response to loss of thermal homeostasis. Results indicate that initial changes in ambient temperature, irrespective of direction, trigger increased yawning and locomotor activity. As temperature change was extended, however, yawning frequency and activity began to drop or return to baseline. During the sustained directional changes in temperature, most yawning occurred during low-increasing temperatures, while the fewest yawns occurred during low-decreasing temperatures. When temperatures were held constant, yawning rates were nearly completely inhibited during high temperatures (32°C), while locomotor activity was unchanged. Unlike yawning and locomotor activity, stretching frequency was unrelated to ambient temperature variation. In addition, correlations between yawning and stretching diminished during thermally challenging, higher temperature conditions, particularly when temperatures were held constantly high (32°C). These results support previous research identifying yawning with arousal, activity, and state change, and also contribute to a growing literature connecting yawning and thermoregulation.
Similar to recent research on budgerigars (Gallup et al., 2009; 2010), this report reveals that ambient temperature manipulation influences yawning in rats. The triggers appear, however, to differ between the two species. Yawning appears to be related to increases in body temperature in budgerigars, whereas in rats it appears to be more closely associated with the detection of changing external temperatures. Water conservation is likely to influence how yawning is used to alleviate thermal stress, as has been suggested for budgerigars (Gallup et al., 2009). Thus different ecological conditions are likely to alter the way yawning occurs among homeothermic species. Budgerigars live in hot and dry climates and experience wide daily fluctuations in ambient temperature. They may be better adapted to dealing with initial changes in ambient temperature in the absence of yawning (e.g., through increased circulation and heat dissipation, or using other forms of behavioral thermoregulation). For instance, they are adapted to living at higher temperatures and conserving water, and they can escape heat and solar radiation by flocking to shade. The elevated frequency of yawning among budgerigars at high-increasing temperatures may be a product of the metabolically inexpensive nature of a yawn in comparison to more costly evaporative cooling behaviors (e.g., panting/gular fluttering) that take over at sustained high temperatures. Similarly, yawning has been witnessed at high ambient temperatures in capuchins (Campos & Fedigan, 2009), which are also adapted to water scarcity and live in seasonally hot tropical dry forest. In addition, the predictions of the thermal window rely on the relationship between ambient and body temperatures and birds have higher body temperatures than mammals. Nocturnal rodents may be more sensitive to acute changes in temperature, and thus yawning is triggered during initial deviations of thermal homeostasis. Likewise, yawning may not be involved in more extended behavioral cooling because water conservation is not as critical. As evidence for this, during higher ambient temperatures rats begin evaporative cooling behaviors (e.g., grooming) shortly after being exposed to thermally challenging conditions (Roberts et al., 1974). Extended periods of grooming were not witnessed within the narrow temperature range of this study.
Similarities between these two species exist, however, as yawning becomes less frequent in both rats and budgerigars when temperatures are held near body temperature, and when temperatures are low and decreasing. These results are consistent with the predictions of the thermal window framework, and with predictions that yawning is an initial and compensatory cooling mechanism (Gallup & Gallup, 2007). In the current study, the near complete inhibition of yawning at constant high temperatures (32°C), and the increase in yawning in the high-decreasing condition (32–27°C) may reflect a sensitive mechanism for maintaining thermal homeostasis. The predictions of the thermal window hypothesis rely on the specific relationship between ambient and body temperatures. At constant high temperatures, yawning may fail to produce effective cooling because the difference between ambient and body temperature is too small (4°C), and therefore it is inhibited. As ambient temperatures begin to fall, however, this provides a setting in which yawning regains utility. This may also explain why, unlike in budgerigars (which have higher body temperatures), yawning rate was not greater during high-increasing temperatures (27–32°C) among rats. Future research could explore this comparatively by exposing animals of different resting body temperatures to a wider range of ambient temperatures. In addition, although it has already been shown that brain temperature fluctuations are correlated with yawning events in rats during constant ambient temperatures (Shoup-Knox et al., 2010), future research should examine the incidence of yawning in response to core body temperature changes. Likewise, studies of yawning and thermoregulation in poikilotherms are badly needed to understand the evolutionary history of this behavior.
Our observations reveal that ambient temperature fluctuation also produced clear changes in locomotor activity levels during the experiments. Following acclimation and periods of constant ambient temperature, initial changes in temperature triggered increased yawning and locomotion among rats. These findings are consistent with research on both humans and chimpanzees, showing that yawning is related to modified activity or increased locomotion (Baenninger et al., 1996; Giganti et al., 2002; Vick & Paukner, 2009). Following continued temperature change in either direction, both yawning and locomotor activity fell precipitously, suggesting that yawning is not solely associated with drowsiness or rest. Instead, these findings support a wide range of comparative research showing a more general relationship between yawning, state change, and arousal (Provine, 1996; Baenninger, 1997; Provine, 2005). Homeothermic species are able to preserve a relatively constant body temperature as ambient temperature fluctuates, using a combination of autonomic and behavioral mechanisms controlled by the central nervous system (reviewed by Bicego et al., 2007). Among rats, yawning may be an adaptive response to early signs of thermal change, providing an increase in alertness and vigilance through cerebral thermoregulation.
Under thermally non-stressful conditions, stretching is associated with yawning in rats (Gessa et al., 1967) and a number of other species (Baenninger, 1997), and both are believed to be involved in arousal. On the other hand, past research on humans suggests that yawning and stretching may serve different functions (Provine et al., 1987), and it is likely that yawning is multifunctional. For instance, human stretching is accompanied by yawning, and after waking yawning and stretching co-occur, but yawns are less frequently accompanied by stretches at night before sleeping (Provine et al., 1987). Our results are consistent with this view, showing that stretching behavior was not affected by ambient temperature variation. Likewise, stretching was correlated with yawning only in a subset of the thermal conditions, specifically during the increasing and constant low temperatures. By separating these commonly associated behaviors, ambient temperature manipulations provide insight into their adaptive function. Although yawning and stretching have some similar physiological consequences (increased circulation), the regional differences in these effects are likely to have considerable implications for the purpose of each. Powerful stretching of jaw muscles during a yawn are believed to specifically increase local facial and cerebral blood flow (Askenasy, 1989), while body stretching is likely to have more global effects. In addition, unlike stretching, yawning is accompanied by a deep inhalation of air. Consistent with the thermoregulatory hypothesis, yawning is significantly altered by ambient temperature variation, while stretching remains unaffected. These findings parallel those of Gallup et al. (2009; 2010) for budgerigars, suggesting that stretching is not involved in thermoregulation.
In summary, the range of ambient temperature variation used in this study produced significant changes in yawning and locomotor activity among rats. Overall, this research provides novel support for the arousal and state change hypotheses while also refining the predictions of the thermoregulatory hypothesis. It is important that these hypotheses are not considered mutually exclusive, but closely integrated mechanisms for regulating behavior. That is, the thermoregulatory benefits resulting from yawning may provide the mechanism by which increased arousal is achieved. Future research should continue to comparatively investigate the association between yawning and ambient temperature variation. Although the effect of temperature change on yawning is hypothesized to vary across species, these differences should be predicted by underlying physiology and the unique evolutionary histories and ecological adaptations to thermal stress.
Acknowledgements
This research was supported by NIH 1047386/36047/MH33881 (to R.R.M.).
Literature Cited
- Anias J, Holmgren B, Urba-Holmgren R, Eguibar JR. Circadian variation of yawning behavior. Acta Neurobiologiae Experimentalis. 1984;44:179–186. [PubMed] [Google Scholar]
- Askenasy J. Is yawning an arousal defense reflex? Journal of Psychology. 1989;123:609–621. doi: 10.1080/00223980.1989.10543014. [DOI] [PubMed] [Google Scholar]
- Baenninger R. Some comparative aspects of yawning in Betta splendens, Homo sapiens, Panthera leo, and Papio sphinx. Journal of Comparative Psychology. 1987;101:349–354. [Google Scholar]
- Baenninger R. On Yawning and Its Functions. Psychonomic Bulletin & Review. 1997;4:198–207. doi: 10.3758/BF03209394. [DOI] [PubMed] [Google Scholar]
- Baenninger R, Binkley S, Baenninger M. Field observations of yawning and activity in humans. Physiology & Behavior. 1996;59:421–425. doi: 10.1016/0031-9384(95)02014-4. [DOI] [PubMed] [Google Scholar]
- Bicego KC, Barros RCH, Branco LGS. Physiology of temperature regulation: Comparative aspects. Comparative Biochemistry and Physiology A: Molecular & Integrative Physiology. 2007;147:616–639. doi: 10.1016/j.cbpa.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Cabanac M, Brinnel H. Blood flow in the emissary veins of the head during hyperthermia. European Journal of Applied Physiology. 1995;54:172–176. doi: 10.1007/BF02335925. [DOI] [PubMed] [Google Scholar]
- Campos FA, Fedigan LM. Behavioral adaptations to heat stress and water scarcity in white-faced capuchins (Cebus capucinus) in Santa Rosa National Park, Costa Rica. American Journal of Physical Anthropology. 2009;138:101–111. doi: 10.1002/ajpa.20908. [DOI] [PubMed] [Google Scholar]
- Craemer F. Über Sodbrennen und Gähnen. Gastroenterologia Archiv für Verdauungskrankheiten. 1924;33:149–162. [Google Scholar]
- Deputte BL. Ethological study of yawning in primates. I. Quantitative analysis and study of causation in two species of old world monkeys (Cercocebus albigena and Macaca fascicularis) Ethology. 1994;98:221–245. [Google Scholar]
- Gallup AC. The thermoregulatory hypothesis of yawning: time to reconsider terms such as “impossible” and “cannot” and evaluate theories based on evidence. Sleep Medicine. In press. [Google Scholar]
- Gallup AC. Yawning as a behavioral adaptation to heat stress and water scarcity in white-faced capuchins (Cebus capucinus) American Journal of Physical Anthropology. 2010;142:670–671. doi: 10.1002/ajpa.21323. [DOI] [PubMed] [Google Scholar]
- Gallup AC. A thermoregulatory behavior. In: Walusinski O, editor. The mystery of yawning in physiology and disease. Karger; Basel, Switzerland: 2010. pp. 84–89. [Google Scholar]
- Gallup AC, Gallup GG., Jr. Yawning as a brain cooling mechanism: nasal breathing and forehead cooling diminish the incidence of contagious yawning. Evolutionary Psychology. 2007;5:92–101. [Google Scholar]
- Gallup AC, Gallup GG., Jr. Yawning and thermoregulation. Physiology & Behavior. 2008;95:10–16. doi: 10.1016/j.physbeh.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Gallup AC, Miller ML, Clark AB. Yawning and thermoregulation in budgerigars (Melopsittacus undulatus) Animal Behaviour. 2009;77:109–113. [Google Scholar]
- Gallup AC, Miller ML, Clark AB. The direction and range of ambient temperature influences yawning in budgerigars (Melopsittacus undulatus) Journal of Comparative Psychology. 2010;124:133–138. doi: 10.1037/a0018006. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Pisano M, Vargiu L, Grabai F, Ferrari W. Stretching and yawning movements after intracerebral injectionof ACTH. Revue Canadian de Biologie. 1967;26:229–236. [PubMed] [Google Scholar]
- Giganti F, Hayes MJ, Akilesh MR, Salzarulo P. Yawning and behavioral states in premature infants. Developmental Psychobiology. 2002;41:289–296. doi: 10.1002/dev.10047. [DOI] [PubMed] [Google Scholar]
- Guggisberg AG, Mathis J, Herrmann US, Hess CW. The functional relationship between yawning and vigilance. Behavioural Brain Research. 2007;179:159–166. doi: 10.1016/j.bbr.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Luttenberger F. On yawning in reptiles. Zeitschrift für Tierpsychologie. 1975;37:113–137. [PubMed] [Google Scholar]
- Matikainen J, Elo H. Does yawning increase arousal through mechanical stimulation of the carotid body? Medical Hypotheses. 2008;70:488–492. doi: 10.1016/j.mehy.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Miller ML, Gallup AC, Vogel AR, Clark AB. Handling-stress initially inhibits, but then potentiates yawning in budgerigars (Melopsittacus undulatus) Animal Behaviour. 2010;80:615–619. [Google Scholar]
- Provine RR. Yawning as a stereotyped action pattern and releasing stimulus. Ethology. 1986;72:109–122. [Google Scholar]
- Provine RR. Contagious yawning and laughter: Significance for sensory feature detection, motor pattern generation, imitation, and the evolution of social behavior. In: Heyes CM, Galef BG, editors. Social Learning in Animals: The Roots of Culture. Academic Press; San Diego: 1996. pp. 179–208. [Google Scholar]
- Provine RR. Yawning. American Scientist. 2005;93:532–539. [Google Scholar]
- Provine RR, Hamernik HB, Curchack BB. Yawning: relation to sleeping and stretching in humans. Ethology. 1987;76:152–160. [Google Scholar]
- Roberts WW, Mooney RD, Martin JR. Thermoregulatory behaviors of laboratory rodents. Journal of Comparative and Physiological Psychology. 1974;86:693–699. doi: 10.1037/h0036138. [DOI] [PubMed] [Google Scholar]
- Shoup-Knox ML, Gallup AC, Gallup GG, Jr., McNay EC. Yawning and stretching predict brain temperature changes in rats: support for the thermoregulatory hypothesis. Frontiers in Evolutionary Neuroscience. 2010;2:1–5. doi: 10.3389/fnevo.2010.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick S, Paukner A. Variation and context of yawns in captive chimpanzees (Pan troglodytes) American Journal of Primatology. 2009;71:1–8. doi: 10.1002/ajp.20781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilli I, Giganti F, Salzarulo P. Yawning in morning and evening types. Physiology & Behavior. 2007;91:218–222. doi: 10.1016/j.physbeh.2007.02.015. [DOI] [PubMed] [Google Scholar]