Abstract
Healthy aged individuals are more likely to suffer profound memory impairments following a challenging life event such as a severe bacterial infection, surgery, or an intense psychological stressor, than are younger adults. These peripheral challenges are capable of producing a neuroinflammatory response, (e.g., increased pro-inflammatory cytokines), and in the healthy aged brain this response is exaggerated and prolonged. Normal aging primes or sensitizes microglia and this appears to be the source of this amplified response. Among the outcomes of this exaggerated neuroinflammatory response is impairment in synaptic plasticity, and a reduction in key downstream mediators such as Arc and BDNF. Each of these mechanisms is important for long-term memory formation, and is compromised by elevated pro-inflammatory cytokines. Pharmacological, dietary and physical interventions are discussed as potential therapies to abrogate the challenge-induced neuroinflammatory response, thereby preventing or reducing memory deficits in aged subjects.
Keywords: Interleukin-1, memory consolidation, aging, glial sensitization, neuroinflammatory response
By the year 2030, roughly 20% of the population will be over 65 years of age [1]. As life expectancy continues to increase, it is important to understand the factors underlying the decline in memory and cognition that occurs with normal healthy aging, not just those that result from neurodegenerative disorders.
Although cognitive function declines with age, there is considerable variability among individuals in the extent of this decline [2]. Many older individuals show very little decline, while others show extensive decline. An important observation is that the cognitive function of otherwise normal older individuals can be severely impaired if they experience what can be called a challenging life event such as an infection [3], surgery [4], or psychological stress [5]. One effect of normal aging is an increase in the vulnerability to the cognitive effects of these challenges [2, 6–8]. Recently, there have been several advances toward understanding a) the mechanisms that mediate this vulnerability, b) the resulting memory impairments associated with vulnerable individuals presented with such challenges, and c) potential treatments and interventions to buffer against the effects of these challenges. The purpose of this article is to review those advances.
How do peripheral challenges, such as an infection or a surgical manipulation, impact cognitive functioning? The narrative will have a number of steps. First, we will suggest that the first step in a cascade of processes is that these challenges activate innate immune cells, thereby inducing a peripheral inflammatory response. Pro-inflammatory cytokines that are released as part of this response signal the brain, and so we next discuss communication between the periphery and the brain, as it is the key to understanding how an immune challenge to the periphery can potentially impair memory function, which is mediated in the brain. Next we will introduce the brain’s resident immune cells, microglia. This is because microglia become activated upon peripheral inflammatory signaling to the brain, and are key to the memory impairments that follow. Microglia have the main job of immunosurveillance. Upon detection of a pathogen, microglia become activated and release pro-inflammatory cytokines that organize host defense and restore homeostasis. Under basal conditions, microglia in the healthy aged brain exhibit phenotypic signs of activation, but do not always release elevated levels of pro-inflammatory cytokines (although this is somewhat dependent on the age and strain of the animal being studied). As we shall describe, in the face of a challenge, the microglia from an aged animal exhibit a sensitized pro-inflammatory response. This exaggerated pro-inflammatory response in the brain can then either directly or indirectly impact neuronal functioning to ultimately impair hippocampal-dependent memory. We will then discuss a handful of putative mechanisms of pro-inflammatory cytokine-induced neuronal dysfunction. Finally, pharmacological, dietary, and behavioral interventions will be described that have aimed to ameliorate or block the initial pro-inflammatory surge in the aged brain, to protect against memory impairments. See Figure 1 for schematic representation of the perspective described.
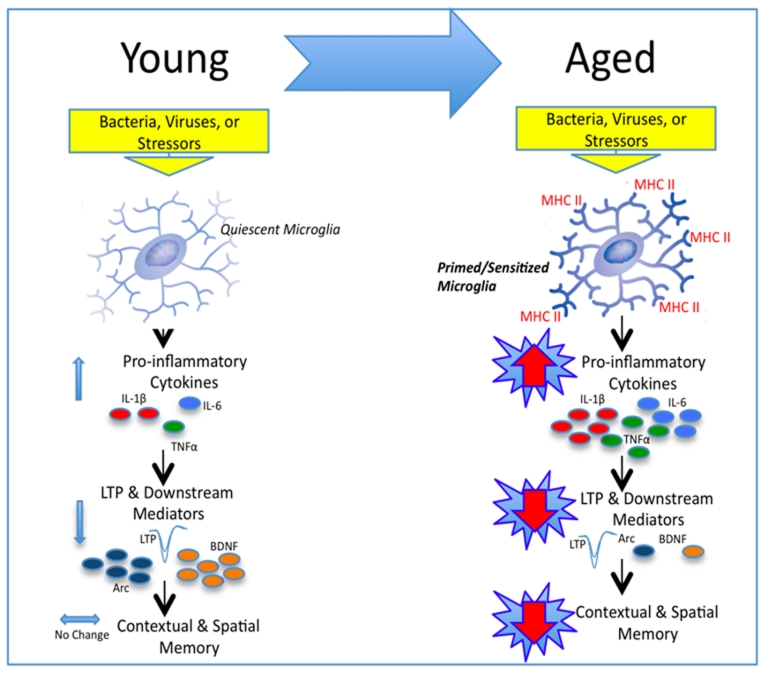
Figure 1.
Schematic depicting our working hypothesis. Upon a peripheral inflammatory, or stressor challenge in the young adult animal, once quiescent microglia are rapidly and transiently activated. Pro-inflammatory cytokines are released resulting in only moderate elevations above basal levels, and lasting no longer than 24 hours. Synaptic facilitation (LTP) and downstream mediators such as BDNF and Arc are moderately decreased resulting in mild to negligible memory impairments. In contrast, unchallenged aged microglia exhibit phenotypic markers of activation (i.e., MHCII), rendering them primed for a subsequent challenge. Upon a peripheral challenge, these microglia produce a sensitized neuroinflammatory response. Pro-inflammatory cytokine release is exaggerated and prolonged, lasting at least 8 days. Synaptic facilitation (LTP) and downstream mediators are profoundly reduced, and long-term contextual, and spatial memory is significantly impaired.
Communication between peripheral pro-inflammatory cytokines and the brain:
An important recent advance in understanding the biological basis of behavior is the recognition that there is extensive communication between the immune system and the central nervous system. As a result of this communication, neural activity is altered quite dramatically during peripheral infection [9], implying that there must be pathways of communication from the immune system to the brain. Over the last 15 years it has become clear that this communication indeed occurs, and by more than one pathway. Both blood-borne and neural routes have been identified [10–15]. Pro-inflammatory cytokines play a key role in this communication. Pro-inflammatory cytokines are regulators of host responses to infection, immune responses, inflammation, and reactions to trauma. Interleukin-1 beta (IL-1β) plays a principal role in immune-to-brain communication. It is released by a variety of cell types distributed throughout the periphery and brain (e.g. macrophages, epithelial cells, fibroblasts, endothelial cells, glia) in response to pathogens and injury. IL-1β can also induce the production of other cytokines such as interleukin-6 (IL-6), and tumor necrosis factor alpha (TNFα), which in turn have secondary effects on other cells. These pro-inflammatory cytokines accumulate in the blood during infection, and have been shown to passively cross into the brain parenchyma at “leaky” areas, the circumventricular organs of the blood brain barrier, so to enter cerebrospinal fluid and interstitial fluid spaces of the brain and spinal cord [11, 16]. There is also active transport of cytokines across the barrier [16, 17], as well as binding of cytokines to receptors on epithelial cells of the brain blood vessels, with consequent production of mediators such as prostaglandins, that then diffuse into the parenchyma [18]. Neuronal communication is thought to involve the vagus nerve. IL-1β and perhaps other pro-inflammatory cytokines bind to receptors on vagal afferents or sensory paraganglia associated with vagal afferents [14], thereby activating afferent vagal fibers [13] that terminate in the nucleus tractus solitarius (NTS). A neural cascade of activity then ascends from the NTS to regions such as the hippocampus [12].
Importantly, this immune-to-brain signaling results in the de novo production of pro-inflammatory cytokines within the brain, primarily by microglia [19–22]. That is, part of the neural cascade that follows peripheral inflammation includes the activation of microglia and a shift of the microglia to an inflammatory phenotype. A determination of the signals to the microglia during peripheral challenge is a topic of current investigation.
Microglial phenotype in normal aging: a shift towards an immunologically primed state
Microglia, as part of the myelomonocytic lineage, constitute the predominant innate immune cell in the brain parenchyma and serve many functions including immunosurveillance of the brain microenvironment for pathogen invasion, cellular debris, apoptotic cells, and alterations in neuronal phenotype [23]. It is important to note that perivascular macrophages, which reside outside the brain parenchyma and are also of myelomonocytic origin, also serve a critical role in the brain’s innate immune response [24]. Our focus here is on evidence showing that microglia undergo profound immunophenotypic and functional changes with normal brain aging. Based on this evidence, we will explore the notion that the aging-related change in microglia immunophenotype represents a progressive shift in the activation state of microglia from quiescent to primed, that is to say from a state of maintaining homeostasis in CNS microenvironments to a state skewed towards exaggerated pro-inflammatory immune responses to endogenous (e.g. sterile injury) and exogenous (e.g. pathogens) danger signals. Finally, we will examine evidence suggesting that sensitization of microglial pro-inflammatory responses may be a consequence of a loss of neuronal inhibitory control over microglia immune function.
In normal brain aging, the immunophenotype of microglia is characterized by up-regulation of glial activation markers including major histocompatibility complex II (MHC II) and complement receptor 3 (CD11b), a finding that has been reported in several species including human post-mortem tissue [25–29], rodent [30–35], canine, [28], and non-human primates [36–38]. It should be noted that astroglia also undergo considerable immunophenotypic changes with age [39], but have received little attention as to their role in aging-induced sensitization of pro-inflammatory responses. An important issue that merits attention here is the distinction between “normal” brain aging and “pathological” brain aging. Our work, as well as the preponderance of studies reviewed here, has focused on studying normal aging wherein older animals are capable of exhibiting a sensitized response—be it a pro-inflammatory response, or a behavioral response—to challenge, compared to younger control animals. Outside the scope of the present review, a considerable literature has studied senescent animals, which basally typically exhibit behavioral and brain cytokine profiles dramatically different from younger animals and whose brains are generally classified under the heading of “neurodegeneration” [40–42].
Aging-related up-regulation of MHCII occurs also at the mRNA level [43]. Importantly, MHCII is expressed at very low levels on microglia in younger animals under basal conditions [44], which provides a clear baseline to detect aging-related changes in microglia immunophenotype. A key question is how changes in microglia immunophenotype (up-regulated MHCII) relate to changes in microglia immune function with normal brain aging. Interestingly, age-related increases in MHCII do not reflect an increase in antigen presentation [44]. Of relevance here is the question of whether these aging-related increases in microglia antigens represent an increase in microglia number with age (microgliosis) or increased antigen expression on a per cell basis. An aging-related microgliosis could argue against the notion that aging sensitizes microglia. That is to say, the exaggerated neuroinflammatory responses observed in older animals, which will be reviewed in greater detail in the next section, may be due to increased microglia numbers rather than an aging-induced sensitization of microglia. It should be noted that microgliosis and microglia sensitization are not mutually exclusive conditions.
Although there are not a large number of studies, those that have been done favor the idea that there is microglial sensitization. A stereological assessment of microglia numbers in hippocampal sub-regions indicated that microglia numbers appear to remain stable across the life span [45]. Moreover, flow cytometry on microglia isolated from young and aged animals conclusively showed that microglia MHCII expression increases on a per cell basis in aged animals compared to young [46]. Further, we recently conducted a study in which hippocampal microglia were rapidly isolated from younger (3 mo) and older (24 mo) animals and several microglia antigens were analyzed using real time PCR. We found that microglia MHCII, CD11b, and Iba-1 gene expression were highly up-regulated in older compared to younger animals, while controlling for microglia cell number [43]. A critical point to bear in mind with regard to the use of isolated microglia is the effect of the isolation procedure on antigen expression. The cellular stresses induced by the isolation procedure may function to elicit an up-regulation of activation antigens on microglia from aged animals. Despite these caveats, the preponderance of evidence suggests that aging results in the progressive up-regulation of microglia “activation” antigens such as MHCII.
Aging–related sensitization of the neuroinflammatory response to challenge
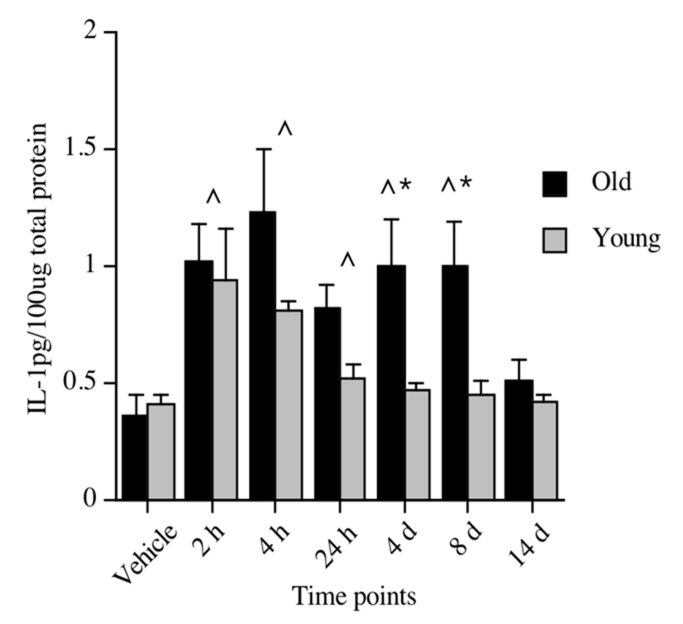
As mentioned in the Introduction, significantly elevated levels of pro-inflammatory cytokines, such as IL-1β, in key brain regions responsible for mediating memory, such as the hippocampus, has been shown to impair memory [47–57]. Thus, an obvious question is whether the neuroinflammatory response to challenge is sensitized in normal aging. A shift in microglia activation state does not necessarily reflect a primed or sensitized state. Macrophage populations show a high degree of functional plasticity depending on macrophage immunophenotype and the cytokine microenvironment, which underscores the difficulty in relating immunophenotype to functional outcomes [58]. To examine whether the age-related changes in microglia immunophenotype reflects an age-related change in microglia immune responsiveness (priming), our laboratory along with several others, began by examining the neuroinflammatory response of aged and young animals following a challenge with a bacteria or virus [59–64], a stressor [65, 66], or a surgical intervention [67]. Regardless of the type of challenge, aged animals exhibited a clear and exaggerated neuroinflammatory response compared to young adult animals; that is, a sensitized response. Our laboratory, for example, showed that adult and aged F344xBN rats had dramatically different cytokine responses to a live, replicating Escherichia coli (E. coli) infection. IL-1β protein was measured 2 hours, 4 hours, 24 hours, 4 days, 8 days, and 14 days following the injection. Both adult (3 months) and aging (24 months) rats had elevated levels of IL-1β in the hippocampus at 2 hours post-E. coli, with IL-1β in adult rats returning to vehicle control levels at 24 hours. However, aged rats showed significant elevations through day 8 post-infection, with a return to vehicle control levels at day 14 (See Fig. 2)[61]. A study by Godbout et al. [59] found similar results in the BALB/c mouse. A peripheral injection of lipopolysaccharide (LPS), a cell wall component of gram-negative bacteria, resulted in both exaggerated and prolonged elevations in IL-1β and IL-6 (both protein and mRNA) in aged, relative to adult mice. In both of these studies [59, 61], the exaggerated cytokine response was restricted to the brain and did not occur in the periphery. In fact, our laboratory found this amplified response to be particular to the hippocampus. We did not observe exaggerated or protracted responses in either hypothalamus, parietal cortex, pre-frontal cortex, spleen or serum [61]. Similarly, Buchanan et al. [65] reported that 30 min of restraint stress repeated over four days resulted in IL-1β mRNA expression that was exaggerated in the hippocampus of aged mice compared to non-stressed age-matched controls. This augmented response was not observed in hypothalamus or in peripheral plasma samples, implicating a hippocampus-specific response. Along the same lines, Rosczyk et al. [67] found IL-1β mRNA expression in the hippocampus to be amplified 24 hours after abdominal surgery in aged mice compared to age-matched sham controls. Young adult mice that underwent the same surgical procedure showed no differences in IL-1β mRNA compared to their age-matched sham controls. As a group, these studies provide an abundance of evidence indicating that a challenging event indeed results in a sensitized neuroinflammatory response in the aged organism, and in many cases, this response was specific to the hippocampal formation, which is important for memory function.
Figure 2.
IL-1β protein levels in hippocampus of young and old rats following a peripheral vehicle or E. coli injection at 2 hours, 4 hours, 24 hours, 4 days, 8 days, and 14 days. Note that vehicle samples were collected at 3 hours and 24 hours and because they did not differ, were pooled to form just one vehicle control group for each age group. ^Significant difference between E. coli-treated and vehicle-treated groups. *Significant difference between age groups. Error bars indicate ± SEM. Modified with permission from Barrientos, et al., 2009 [61].
While the studies reviewed indicate a sensitized neuroinflammatory response, they do not implicate microglia as the source of the inflammatory mediators within the CNS, or as the site of sensitization. In the above studies, even if microglia were the sole source of pro-inflammatory mediators in the CNS, it could be that the immune challenge acted at some other cell type, whether in a central or peripheral compartment, to deliver an exaggerated signal to the microglia in the aging subjects. To address the question of whether aging sensitizes microglia to a pro-inflammatory stimulus, we have recently demonstrated that hippocampal microglia isolated from aged animals exhibit a potentiated pro-inflammatory cytokine response (i.e. IL-1β, TNFα) to LPS ex vivo [68]. It is important to note that this study does not exclude the possibility that other CNS immunocompetent cells (i.e., perivascular macrophages) are sensitized in aged animals. While it would provide valuable insights to study age-related sensitization of other CNS immunocompetent cells under ex vivo conditions, microglia are particularly amenable to study ex vivo compared to other CNS cell types.
Aging and loss of CNS immunoregulatory control of microglia
The evidence presented thus far suggests that a progressive shift in the activation state of microglia occurs with aging resulting in chronically sensitized microglia. Microglia from the aged CNS could be described as hyper-vigilant or hyper-alert to disruptions in central homeostasis. It is as if with aging, microglia are in a refractory state of immunologic activation and thus cannot revert to a ground state of quiescent central housekeeping function. In other words, the functional plasticity of microglia may be compromised with aging. The immunologically primed state of microglia may thus reflect a loss of plasticity, suggesting that with aging, microglia are less capable of shifting among functional states necessary for maintaining homeostasis in the CNS microenvironment. Clearly, if microglia functional plasticity is compromised with aging, the implications for understanding aging-related cognitive impairments are considerable.
At issue here is understanding the mechanism(s) of how microglia become chronically sensitized during normal brain aging. There is now considerable evidence that the CNS microenvironment expresses proteins that inhibit microglia activation as well as pro-inflammatory immune responses [69]. Our focus here will be on the age-related decreases in two of these proteins, fractalkine (CX3CL1) and CD200, which are expressed preferentially on neurons and function to inhibit microglia through their cognate receptor expressed predominately on myelomonocytic cell types. We found that in aged animals CD200 gene expression was reduced in hippocampus compared to young animals [43]. Lyons et al. [70] subsequently showed that CD200 protein was also reduced in the hippocampus of aged animals. Interestingly, CD200 knockout mice constitutively display a chronically activated microglia phenotype characterized by a less ramified morphology and increased expression of the activation marker CD11b [71], a phenotype remarkably similar to microglia phenotypes observed in aged animals. Likewise, CX3CL1 also shows an age-related decrease in the CNS [15, 72]. Further, in addition to a decrease in CX3CL1, aged animals fail to up-regulate the microglia fractalkine receptor (CX3CR1) 24 hours after a peripheral inflammatory immune challenge. The failure to up-regulate microglia CX3CR1 in aged animals coincided with a potentiated neuroinflammatory response as well as reductions in social behavior, which are labile to pro-inflammatory cytokine changes [72]. The inability of aged microglia to up-regulate CX3CR1 in the face of an immune challenge again suggests a loss of microglia functional plasticity with age. In support of this idea, Bachstetter et al. [15] demonstrated that treatment with exogenous CX3CL1 reversed the age-related decrease in hippocampal neurogenesis and reduced the number of MHCII+ cells in the hippocampus. Of note, Bachstetter et al. [15] also showed that infusion of a CX3CR1 blocking antibody resulted in a considerable increase in the number of MHCII+ cells in the hippocampus as well as levels of IL-1β protein. Co-treatment with the IL-1 receptor antagonist (IL-1RA) abrogated the effects of CX3CR1 blockade. These studies provide compelling evidence that neuronal control over central pro-inflammatory processes may be compromised with normal aging, thereby predisposing the aged brain to exaggerated pro-inflammatory response in the face of immune challenges.
Neuroinflammatory effects on synaptic plasticity and hippocampus-dependent memory in aged subjects
It has been well documented that expression of pro-inflammatory cytokines above basal levels, particularly in the hippocampus, impairs both synaptic plasticity, as assessed by long-term potentiation (LTP) [73–77], and hippocampus-dependent memory in adult rodents [47–56]. Thus, populations or individuals that have either increased peripheral inflammatory responses to immune activating agents such as bacteria or viruses, or exaggerated brain inflammatory responses to signaling events within the brain, such as psychological stressors, are likely to be more susceptible to infection-induced memory impairments. Healthy, yet aging individuals fall into that category.
Long-term potentiation
Long-term potentiation (LTP) was defined when researchers [78] demonstrated that brief high-frequency electrical stimulation to any one of the three afferent pathways to the hippocampus produced an increase in synaptic efficacy lasting from hours to days, or even weeks. As we shall explore in the paragraphs ahead, LTP has become a biological model of hippocampal-dependent learning and memory. Once LTP is induced, a fixed amount of pre-synaptic stimulation induces increased excitatory post-synaptic potentials. LTP may be induced in the dentate gyrus granule cells by stimulation of the perforant pathway [78], CA3 pyramidal cells by stimulation of the mossy fiber pathway [79], CA1 pyramidal cells by stimulation of Schaffer collateral fiber pathway [79, 80], and in the piriform [81], entorhinal [82], and prefrontal cortices [83]. One defining feature of LTP is its dependence on high levels of post-synaptic calcium [84]. It is widely believed that the primary source of calcium influx during the induction of LTP occurs through an ion channel that is coupled to the N-methyl-D-aspartate (NMDA) subtype of the glutamate receptor [85, 86]. This receptor is unique in that stimulation of the channel ionophore requires glutamate binding as well as a moderate level of depolarization. It should be noted, however, that LTP may be induced in the hippocampus without the participation of NMDA receptors, provided that titanic stimulation is of sufficient intensity to activate voltage-dependent calcium channels and thus, increase post-synaptic intracellular calcium concentrations [87].
As mentioned earlier, LTP has been widely accepted as an electrophysiological model of hippocampal learning and memory. Hippocampal-dependent memory can be divided into two distinct forms: short-term and long-term memory. These forms of memory differ temporally and mechanistically. The facilitated synaptic transmission involved in short-term memory formation may last from a few seconds to several hours, whereas in long-term memory formation, facilitated synaptic transmission can last from many hours to several weeks and requires protein synthesis. Ultimately, structural changes encode permanent memory. In similar fashion, researchers have distinguished between early-phase LTP (E-LTP) and late-phase LTP (L-LTP) (reviewed in [88]). E-LTP requires calcium influx through NMDA receptors, as has been described above. In the longer lasting form of synaptic facilitation, L-LTP requires activation of cAMP-dependent protein kinase (PKA) and the transcription factor CREB [18, 89]. Moreover, L-LTP is dependent on new protein synthesis, and is thought to play a key role in producing the structural rearrangements that allow permanent storage of the memory [90, 91]. It should be noted that L-LTP has been shown to be dependent on brain-derived neurotrophic factor (BDNF) [92, 93], a growth factor extensively studied for its role in facilitating learning and memory (reviewed in [88]). Much evidence shows that LTP is involved in spatial learning and memory. For example, blocking E-LTP with NMDA receptor antagonists [94–98], or by a mutation in one of the NMDA receptor subunits [99], impairs rats’ spatial, but not non-spatial learning, spatial learning being mediated by the hippocampus. Moreover, blocking L-LTP impairs long-term memory formation (reviewed in [88, 100]).
The pro-inflammatory cytokine IL-1β was first shown to completely block LTP in the CA1 region [101] and the dentate gyrus [102–104] in rat hippocampal slices, and the CA3 region of murine hippocampal slices [105], nearly 20 years ago. In the years since, several studies have focused on examining IL-1β-induced synaptic plasticity impairments in aging animals, as measured by LTP.
Given that LTP is widely believed to facilitate spatial learning and memory, and pro-inflammatory cytokines effectively inhibit LTP, populations who exhibit exaggerated pro-inflammatory responses, as is the case with normal aging following a challenge, would likely be more vulnerable to memory impairments. Much of the evidence showing that aging rodents exhibit a decreased ability to sustain LTP compared to their younger counterparts, show that this behavioral profile is often accompanied by increases in pro-inflammatory cytokines [106–110].
Our laboratory, in collaboration with the Patterson laboratory, recently reported that LTP induced in area CA1 of hippocampal slices was not significantly different between healthy young (3 months) and aged but not senescent (24 months) F344xBN rats [111]. However, several days (4–5 days) following a peripheral E. coli infection, L-LTP induced by theta-burst stimulation was reduced in young rats, and nearly obliterated in aged animals. Theta-burst stimulation (12 bursts of 4 pulses at 100 Hz, delivered 200 ms apart), is a naturalistic stimulation protocol designed to mimic the burst firing of CA1 pyramidal cells at the theta frequency recorded in vivo from awake behaving animals during spatial exploration. These results were specific to late-phase LTP, the form of LTP implicated in long-term memory formation, as described above. Importantly, infected aged animals were not impaired on basal synaptic transmission, or measures of short-term synaptic plasticity (E-LTP; 1 x 1s train, at 100 Hz). Furthermore, high-frequency four-train L-LTP (4 x 1s trains, at 100 Hz, delivered 5 min apart), which produces a robust activation of many plasticity-related signaling cascades, was also not impaired in aged and infected rats. Interestingly, this pattern of electrophysiological data mirrors the behavioral results to be described in the next section.
Hippocampus-dependent contextual and spatial memory
Morris Water Maze
The hippocampus has long been recognized as a critical brain structure for mediating contextual and spatial memory. In the following section, we review studies that have shown a neuroinflammatory-induced exacerbation of hippocampus-dependent memory deficits in aged subjects. Two of the most commonly used behavioral paradigms to study hippocampus-dependent memory are the Morris water maze [112], and contextual fear conditioning [113, 114].
In the Morris water maze (MWM), an animal must swim in a tank of cool water to find a platform hidden just beneath the surface of the water that allows escape from the water. The animal receives multiple training trials per day for several days and, as the trials start from different locations and there are no intra-maze cues to indicate platform location, must use various visual cues located outside the tank to guide its way to the escape platform. At the end of training, a probe trial is often employed, in which the platform is removed from the tank and the animal’s search pattern recorded and analyzed for latency to target quadrant, time spent in target quadrant, and platform crossings (swimming over the location where the platform had been). A probe trial can be given immediately after the last training trial, serving as a measure of short-term memory (STM). Another probe trial can be given a day or more after the last training trial as a measure of long-term memory (LTM). Good performance on this task depends on intact motivational states and motor skills, as well as intact hippocampal function. Therefore, caution needs to be exercised to assure that animals are not actively sick or motorically impaired during training and/or testing of this task.
In our studies, 3 and 24 month old F344xBN rats were administered a peripheral injection of E. coli or vehicle 7 days prior to acquisition training. Thus, by the time training began, sickness behaviors had subsided in both young and aged animals, but IL-1β levels were still elevated in hippocampus of aged animals. STM and LTM for platform location were tested 1 and 48 hours following the last training trial, respectively. Neither STM nor LTM were affected by age alone. That is, healthy aged rats performed as well as did younger rats on both memory tests. Given that we do not observe differences in pro-inflammatory cytokines under basal conditions in these two age groups, this was not surprising. Peripheral infection did not produce a STM deficit in either young or aged rats, indicating that these animals were capable of learning well despite having had a peripheral infection a week prior to training and testing. Thus, it cannot be argued that the aging animals were motorically, motivationally, or otherwise unable to complete the task. In contrast, the LTM test revealed a large deficit in aged infected rats compared to their young counterparts. They showed no preference for the target quadrant, while all other groups continued to show selectivity to the target quadrant relative to the other quadrants [62]. Similar findings have been reported by Buchanan et al. [65]. Here, repeated mild stress (30 minutes of restraint stress over four days), which was shown to elevate hippocampal IL-1β mRNA in an exaggerated manner in aged mice, induced MWM memory impairments in old, but not young mice.
A seemingly contradictory set of findings was reported by Rosczyk et al. [67]. Aged mice showed impaired performance on basic acquisition parameters (they swam further and longer to locate the platform) compared to young animals, but these deficits were not exacerbated by abdominal surgery. The same was found in the memory test. Memory was affected by old age, but was not made worse by the surgical procedure. Although surgery sensitized IL-1β expression, it may have been insufficient to produce a cognitive impairment. Support for this possibility is provided by the finding that surgery-treated animals showed no suppression of locomotor activity despite being tested only 24 hours following the procedure, at the peak of elevated IL-1β expression. These findings lend support to the idea that challenge-induced hippocampus memory impairments in aged animals require exaggerated and prolonged pro-inflammatory cytokine expression.
Contextual Fear Conditioning
Contextual fear conditioning is another behavioral task that is perfectly suited to studying hippocampus-dependent memory in animals as hippocampal and non-hippocampal memory impairments can be dissociated. In this task, an animal is placed in a novel context where it is allowed to freely explore and learn the many features that become conjoined to make up a contextual representation [115]. After a few minutes, one or more electric shocks are delivered to the feet of the subject through a metal grid floor. This is clearly an aversive, albeit short-lived, stimulus that becomes strongly associated with the context. To test memory, the animal is placed back in the conditioning context either a few hours after conditioning (to measure STM), or a day(s) later (to measure LTM), and the amount of time spent freezing in the context is measured. Freezing is the rat’s dominant defensive fear response, and is used as an index of memory for fear of the conditioning context. An auditory cue, such as a tone, is often paired with the shock at the time of conditioning. The animal’s freezing to the tone in a novel context can be tested independently from the test of the context. Memory for the context depends on the hippocampus, while memory for the tone does not [113, 114], thus allowing distinctions between an intervention that impairs hippocampus-dependent memory or hippocampus-independent memory. Because this paradigm requires only a single session for learning, it is ideal for the study of either retrograde and/or anterograde effects of an immune challenge on memory consolidation.
A recent study from our laboratory examined both retrograde and anterograde effects of peripheral infection on memory formation. In the retrograde experiment, young and aged animals were challenged with an E. coli infection immediately after conditioning. With this procedure animals are unaffected at the time of conditioning, and thus if an impairment ensues it can be attributed to the infection’s affect on memory consolidation, which occurs after the training experiences, rather than on processes that occur during the training trial (learning). In this experiment, we found no effect of aging per se. That is, vehicle-treated young and aged rats froze to the conditioning context equally. E. coli-challenged aged animals however, showed a reduction in freezing compared to every other group (young infected rats were no different than vehicle-treated young rats), indicating impaired memory for the context. Freezing to the tone in a novel context, however, was not impaired in these animals, pointing to specificity of the memory impairment to the hippocampus. In the anterograde experiment, young and aged rats were administered E. coli 4 days prior to conditioning. In this experiment, we measured both STM and LTM. Again, no effect of age per se was observed. Memory impairments in E. coli-treated aged rats were restricted to LTM. E. coli had no effect on the STM test given 1h after training. This result indicates that the memory deficits could not be attributed to a failure of the aged E. coli-treated rats to properly sample and encode the environment. It also indicates that the effects were not caused by an inability to express what was learned (to freeze to the conditioning context). These are important observations because they eliminate the possible interpretation that overt sickness at the time of acquisition or testing account for the deficits in LTM.
Strengthening these conclusions, sickness behaviors, including fever, subside by day 4 post-E. coli infection [116]. Additionally, memory for the tone in a novel context was not impaired in E. coli-treated aged rats (or in any group), again confirming that the E. coli-induced memory impairment is restricted to hippocampus-dependent processes. These memory deficits are concordant with the exaggerated pro-inflammatory response found in the hippocampus of aging animals [62]. In a separate study, we have shown that the duration of contextual fear conditioning memory deficits parallels the duration of IL-1β increases in hippocampus of aged rats [61]. That is, IL-1β protein levels are elevated 8 days following E. coli challenge, returning to vehicle-treated control levels at 14 days. Mirroring these results, we found that if rats were conditioned either 4 or 8 days following infection, hippocampus-dependent memory was significantly impaired. However, when 14 days were allowed to elapse between E. coli and learning, there was no impairment, implicating amplified pro-inflammatory cytokines in the hippocampus as the critical element in challenge-induced memory deficits in otherwise healthy aged animals. However, it should be noted that from this evidence alone, the relationship remains correlational, and not causal (see below).
Putative mechanisms of pro-inflammatory cytokine-induced hippocampal dysfunction
As reviewed above, pro-inflammatory cytokines such as IL-1β, when elevated above basal levels in the hippocampus impairs hippocampal-dependent memory processes. How? Many mechanisms have been implicated in the actions of IL-1β on learning and memory processes, and only some can be reviewed here.
IL-1β inhibits LTP
As discussed in an earlier section, IL-1β has been shown to completely block LTP in hippocampus [101, 102, 105, 117]. The cellular mechanisms by which this inhibition occurs, is not fully understood, though IL-1 has been shown to inhibit release of acetylcholine [118] and glutamate [74, 119] in hippocampal synaptosomes, reduce calcium influx in hippocampal synaptosomes [119], and inhibit calcium channel currents in hippocampal neurons [120]. It should be recognized however, that a broader literature claims a neuroexcitatory role for IL-1β (e.g., enhancement of NMDA receptor-mediated calcium increase and increases in glutamate) [121–124], and this apparent contradiction remains to be elucidated.
Elevations in IL-1β have been demonstrated to increase reactive oxygen species (ROS) formation in the hippocampus [74], and in turn, these activate certain members of the mitogen-activated protein (MAP) kinase family, such as c-jun N-terminal kinase (JNK) [74, 125] and p38 [74, 126]. While activation of other members of the MAP kinase family, such as extracellular signal-regulated protein kinase (ERK) results in neurite outgrowth, cell proliferation, or differentiation [127], activation of JNK and p38 has been shown to induce cell damage or cell death [128]. Inhibiting JNK in vivo with D-JNKI1 [125] or in vitro with SP600125 [129], blocked IL-1β-induced LTP inhibition in hippocampus. Moreover, in vivo treatment with the p38 inhibitor, SB203580, attenuated pro-inflammatory-induced inhibition of hippocampal LTP [126]. Furthermore, inhibiting ROS formation, through an antioxidant diet, reversed IL-1β-induced LTP inhibition as well as IL-1β-induced JNK and p38 activation [74]. Together, these findings confirm that IL-1β plays a prominent role in inhibiting important cellular processes critical to memory formation such as LTP, through activation of these stress-activated protein kinases.
IL-1β down-regulates BDNF
Another possibility is that IL-1β works indirectly by modulating downstream mediators that in turn cause the memory impairment. Brain-derived neurotrophic factor (BDNF) is a plausible downstream mediator capable of affecting memory processes due to its critical role in late synaptic plasticity processes [92, 93, 100, 130–132] and long-term memory [133–135]. Furthermore, BDNF is rapidly and selectively induced in the hippocampus following contextual fear conditioning [133], and the systemic administration of either IL-1 or LPS [136, 137] or stress-induced elevations of IL-1β in hippocampus [20, 55] decreases BDNF mRNA in the hippocampus [48, 136, 137]. Moreover, intra-hippocampal administration of the IL-1 receptor antagonist, IL-1RA, prevents both the BDNF downregulation, and the memory impairments produced by the challenge [48]. Taken together, these studies provide strong evidence for the idea that pro-inflammatory cytokine-induced memory impairments may involve the downregulation of BDNF.
IL-1β down-regulates Arc
The immediate early gene activity-dependent cytoskeletal-associated protein (Arc) is another mediator downstream of IL-1β that has been identified as a key modulator of hippocampal memory consolidation[138]. Arc mRNA was found to be rapidly and specifically distributed throughout the dendritic arbor of the hippocampus after neuronal activity [139, 140] and localized to regions receiving direct synaptic activation [141]. Inhibiting Arc protein expression with antisense oligodeoxynucleotides resulted in impaired long-term memory consolidation and L-LTP [142]. Importantly, short-term memory and E-LTP were not impaired by Arc inhibition. This is noted because this is a pattern we have observed in E. coli-challenged aged rats who exhibit long-lasting neuroinflammatory responses [62, 111]. That is, infected aged animals exhibited LTM, but not STM impairments and L-LTP, but not E-LTP deficits, and these alterations correlated with elevated levels of hippocampal IL-1β. In a separate study, our laboratory reported [143] that a peripheral E. coli challenge in young and aged rats resulted in a profound suppression of hippocampal Arc expression. This suppression correlated with LTM deficits, and an elevation in hippocampal IL-6 protein. Furthermore, administering the IL-1 receptor antagonist IL-1RA, blocked all of these effects, underscoring the potential role of Arc in neuroinflammatory-induced memory impairments.
Potential Therapies
Pharmacologic interventions: IL-1RA and minocycline
The evidence reviewed thus far indicates that normal aging results in potentiated neuroinflammatory responses to immune challenge. The preponderance of studies show that an immune challenge in aged animals induces exaggerated brain cytokine responses in parallel with behavioral changes such as impairments in LTM. Here we will review a handful of studies showing that pharmacologic modulation of cytokines prior to immune challenge in aged animals blocks the behavioral effects of immune challenge. Some of these studies were touched upon in an earlier section, and are expanded upon here. In order to understand whether the neuroinflammatory sequelae of peripheral infection is causal rather than merely correlated with impaired LTM in older animals, we administered the IL-1 receptor antagonist IL-1RA intra-cisterna magna (ICM) immediately prior to a peripheral E. coli infection [143]. We assessed the effects of IL-1RA on E. coli-induced changes in hippocampal IL-6 as a measure of neuroinflammation and the immediate early gene Arc, as well as LTM. Several key findings emerged from this study. As noted above, we found that E. coli treatment results in a profound reduction in basal hippocampal Arc expression. Importantly, prior treatment with IL-1RA blocked the E. coli-induced suppression of Arc in parallel with blocking E. coli-induced IL-6. Next we found that E. coli suppressed conditioning-induced Arc and impaired LTM. IL-1RA treatment abrogated the E. coli-induced impairment of LTM. These effects of E. coli and IL-1RA were found only in aged, but not young animals. Notably, IL-1RA treatment exerted its effects up to 8 days post-treatment, which is remarkable given the reported half-life (1–2 hours) of IL-1RA, suggesting that preventing the cascade of neuroinflammatory sequelae in the aged brain may protect against the deleterious effects of the otherwise unrestrained inflammatory response following a challenge.
As discussed earlier, aged animals show deficits in LTP, which are accompanied by increases in pro-inflammatory cytokines [110, 111], and these effects were further amplified by an immune challenge [111]. Chapman et al. [111] also examined the effects of IL-1RA and found that a single central administration of the anti-inflammatory cytokine, at the time of a peripheral E. coli injection, abrogated the aging-associated, infection-induced impairment of theta-burst L-LTP. Consistent with these findings, Abraham and Johnson [144] reported that central administration of IL-1RA blocks LPS-induced sickness behaviors in aged mice.
These studies provide evidence that an exaggerated pro-inflammatory cytokine response in aged animals plays a pivotal role in the behavioral phenotype of aged animals exposed to an immune challenge. Additional studies have shown that age-related changes in microglia may underlie the potentiated neuroinflammatory and behavioral responses to immune challenge. The microglia inhibitor, minocycline, was found to attenuate the age-related increase in hippocampal IL-1β, while partially restoring deficits in LTP [145]. It is important to note this study was conducted in immunologically unchallenged animals. However, a study by Henry et al. [146] showed that pre-treating aged animals with minocycline reduced the hippocampal pro-inflammatory response to LPS.
Dietary treatments
Reactive oxygen species are part of the neuroinflammatory cascade [147–154] and can play a prominent role in the development of age-related declines in LTP [148–150], cognitive and sickness behaviors [153, 155–157], and other neuronal functions [152]. Several studies have reported age-related decreases in vitamins C and E (alpha-tocopherol), and polyunsaturated fatty acids, such as arachidonic acid in hippocampus, and increases in hippocampal superoxide dismutase activity, a profile indicative of compromised antioxidative defenses [148–151, 158]. This profile has also correlated with impaired LTP maintenance and increased hippocampal IL-1. Thus, a variety of studies have examined the effects of antioxidant treatments on these age-related changes. An 8-week regimen of an alpha-lipoic acid-enriched diet reversed increases in superoxide dismutase activity and decreases in alpha-tocopherol levels in aged rats, as well as restored LTP deficits to levels comparable to those observed in young animals. In addition, this dietary supplement also reduced elevated hippocampal IL-1β levels [149]. In a separate study, supplementing either omega 6 or omega 3 fatty-acids to the diets of aged rats reversed levels of key polyunsaturated fatty acids (arachidonic and docosahexanoic acids) and restored the ability of aged rats to sustain LTP [148]. Supplementing the diets of aged animals with alpha-tocopherol ameliorated age-related [158, 159] and LPS-induced sensitized [155] responses such as LTP deficits, decreases in membrane arachidonic acid levels, increases in IL-1, IL-6, and lipid peroxidation, and deficits in social exploration behavior. Vitamin D3, when incorporated into the diets of young and aged rats for 2 weeks, was shown to have significant anti-inflammatory effects. Increased phenotypic (MHCII and CD11b expression) and functional markers of glial activation (IL-1β elevations) found in hippocampus of aged rats, were abrogated with the vitamin D3-supplemented diet, while causing no change in young rats [151].
Strawberries, blueberries, and blackberries have distinguished themselves as being potent antioxidant fruits. As such, several laboratories have incorporated them into the diets of aged animals to examine their ability to reverse age-related changes. Shukitt-Hale et al. [160] found that a 2% blackberry-enriched diet improved STM of aged rats in the MWM, compared to control rats. Similarly, Joseph et al. [161], found that feeding aged rats for 8 weeks with diets supplemented with spinach, strawberry, or blueberry extracts effectively reversed age-related deficits in neuronal function as well as STM on the MWM. Along the same lines, a four-week diet supplemented with reseveratrol, a polyphenol found in red grapes, reduced LPS-induced sensitized IL-1 mRNA expression in the hippocampus of aged mice. Furthermore, this antioxidant treatment abrogated the LPS-induced deficits in spatial working memory in aged animals [162]. Taken together, these studies suggest that dietary supplements high in antioxidants may be an effective therapy in buffering against age-related neuroinflammatory-induced cognitive impairments, though it should be noted that the effectiveness of these supplements have not been tested on LTM paradigms.
Exercise
Physical exercise has been extensively shown to profoundly increase BDNF mRNA and protein levels in the hippocampus (reviewed in [163]). Given the prominent role of BDNF in learning and memory processes, the effects of exercise-induced BDNF on learning and memory performance is of great interest here. Several studies [164, 165] have reported exercise-induced learning and memory enhancements on tasks dependent on the hippocampus, such as the MWM and contextual fear conditioning. Moreover, this exercise-induced memory enhancement was shown to be dependent on hippocampal BDNF induction because inhibiting BDNF activity in the hippocampus blocked the cognitive benefits of exercise [164].
As has been discussed earlier, aging animals exhibit a potentiated pro-inflammatory response to a peripheral challenge, and elevated pro-inflammatory cytokine levels down-regulate BDNF expression. Therefore, it stands to reason that exercise may be an effective therapeutic intervention to elevate BDNF levels in aged animals, such that in the event of a challenge, BDNF levels are not profoundly depleted, thus protecting memory functions. This line of research, with an emphasis on aging, remains largely unexplored, and thus further study is needed to understand the role of exercise-induced BDNF expression in buffering against the challenge-induced neuroinflammatory effects on memory in aged subjects.
Summary
Taken together, the evidence suggests that in the aged brain, the neuroinflammatory response to a peripheral challenge is dysregulated, resulting in a potentiated pro-inflammatory cytokine response, whose source appears to be sensitized microglia. This exaggerated response appears to be most prominent in the hippocampal formation, the critical brain region mediating contextual and spatial memory consolidation, and may be the cause of hippocampal memory impairments in aged individuals. Pro-inflammatory cytokines such as IL-1β may affect cognitive processes by impairing synaptic plasticity through activation of MAP kinases JNK and p38, and/or by inhibiting downstream mediators essential to hippocampal-dependent memory processes such as BDNF and Arc. Blocking this exaggerated brain cytokine response pharmacologically, or through diet and exercise modifications may effectively block the deleterious behavioral effects, not only suggesting that these may be useful therapeutic interventions, but also supporting the view that pro-inflammatory cytokines have a causal, rather than merely correlational relationship with impaired long-term memory in older individuals.
Finally, it should be noted that the present perspective has broader implications than those involving contextual and spatial memory in the aged population. Brain cytokines and other inflammatory molecules modulate many processes other than memory. These have often been labeled “sickness behaviors” and include fever, decreased food/water intake, decreased motor activity and social interaction, increased slow-wave sleep, hyperalgesia, and hypothalamic-pituitary-adrenal axis activation [166]. In addition, changes in mood have often been viewed as part of this complex. At least some of these alterations function to facilitate the efficiency of fever, conserving vital energy resources for use in elevating core body temperature, to more effectively kill off the offending agent [166, 167] and these responses are widely recognized as adaptive. However, when this response is prolonged or exaggerated, these behaviors take on pathophysiological characteristics, and the mood components resemble major depression [168]. As such, aging may be a vulnerability factor for these outcomes to challenge. In support of this idea, our laboratory reported a delayed, yet prolonged fever response in aged rats following an E. coli infection compared to younger cohorts [116]. In addition, the Godbout and Johnson laboratories have found many of these sickness responses to be exaggerated and/or prolonged in aging mice following an LPS challenge, compared to non-challenged age-matched controls and younger cohorts [46, 59, 137, 144, 146, 155, 169]. Together, these findings make a strong argument for the idea that aging individuals faced with a challenging life event are more vulnerable than are younger individuals to numerous endpoints that are modulated or caused by neuroinflammatory products such as pro-inflammatory cytokines. These may range from memory deficits, the focus of this review, to clinical depression.
References
- [1].Vincent GK, Velkoff VA. In: The Next Four Decades: The older population in the United States: 2010 to 2050. CB, editor. United States Department of Commerce; Washington, DC: 2010. [Google Scholar]
- [2].Laursen P. The impact of aging on cognitive functions. An 11 year follow-up study of four age cohorts. Acta Neurol Scand Suppl. 1997;172:7–86. [PubMed] [Google Scholar]
- [3].Wofford JL, Loehr LR, Schwartz E. Acute cognitive impairment in elderly ED patients: etiologies and outcomes. Am J Emerg Med. 1996;14:649–653. doi: 10.1016/S0735-6757(96)90080-7. [DOI] [PubMed] [Google Scholar]
- [4].Bekker AY, Weeks EJ. Cognitive function after anaesthesia in the elderly. Best Pract Res Clin Anaesthesiol. 2003;17:259–272. doi: 10.1016/s1521-6896(03)00005-3. [DOI] [PubMed] [Google Scholar]
- [5].VonDras DD, Powless MR, Olson AK, Wheeler D, Snudden AL. Differential effects of everyday stress on the episodic memory test performances of young, mid-life, and older adults. Aging Ment Health. 2005;9:60–70. doi: 10.1080/13607860412331323782. [DOI] [PubMed] [Google Scholar]
- [6].Tsolaki M, Drevelegas A, Karachristianou S, Kapinas K, Divanoglou D, Routsonis K. Correlation of dementia, neuropsychological and MRI findings in multiple sclerosis. Dementia. 1994;5:48–52. doi: 10.1159/000106694. [DOI] [PubMed] [Google Scholar]
- [7].Unverzagt FW, Gao S, Baiyewu O, Ogunniyi AO, Gureje O, Perkins A, Emsley CL, Dickens J, Evans R, Musick B, Hall KS, Hui SL, Hendrie HC. Prevalence of cognitive impairment: data from the Indianapolis Study of Health and Aging. Neurology. 2001;57:1655–1662. doi: 10.1212/wnl.57.9.1655. [DOI] [PubMed] [Google Scholar]
- [8].Foster TC. Biological markers of age-related memory deficits: treatment of senescent physiology. CNS Drugs. 2006;20:153–166. doi: 10.2165/00023210-200620020-00006. [DOI] [PubMed] [Google Scholar]
- [9].Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to- brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- [10].Banks WA, Kastin AJ, Brennan JM, Vallance KL. Adsorptive endocytosis of HIV-1gp120 by blood-brain barrier is enhanced by lipopolysaccharide. Exp Neurol. 1999;156:165–171. doi: 10.1006/exnr.1998.7011. [DOI] [PubMed] [Google Scholar]
- [11].Dinarello CA, Cannon JG, Wolff SM. New concepts on the pathogenesis of fever. Rev Infect Dis. 1988;10:168–189. doi: 10.1093/clinids/10.1.168. [DOI] [PubMed] [Google Scholar]
- [12].Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gaykema RPA, Goehler LE, Tilders FJH, Bol JGJM, McGorry M, Fleshner M, Maier SF, Watkins LR. Bacterial endotoxin induces fos immunoreactivity in primary afferent neurons of the vagus nerve. Neuroimmunomodulation. 1998;5:234–240. doi: 10.1159/000026343. [DOI] [PubMed] [Google Scholar]
- [14].Goehler LE, Relton JK, Dripps D, Kiechle R, Tartaglia N, Maier SF, Watkins LR. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain Res Bull. 1997;43:357–364. doi: 10.1016/s0361-9230(97)00020-8. [DOI] [PubMed] [Google Scholar]
- [15].Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, Gemma C. Fractalkine and CX(3)CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Banks WA, Kastin AJ, Gutierrez EG. Interleukin-1 alpha in blood has direct access to cortical brain cells. Neurosci Lett. 1993;163:41–44. doi: 10.1016/0304-3940(93)90224-9. [DOI] [PubMed] [Google Scholar]
- [17].Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain-barrier. Neuroimmunomodulation. 1995;2:241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- [18].Cao C, Matsumura K, Yamagata K, Watanabe Y. Endothelial cells of the rat brain vasculature express cyclooxygenase-2 mRNA in response to systemic interleukin-1 beta: a possible site of prostaglandin synthesis responsible for fever. Brain Res. 1996;733:263–272. doi: 10.1016/0006-8993(96)00575-6. [DOI] [PubMed] [Google Scholar]
- [19].Laye S, Goujon E, Combe C, VanHoy R, Kelley KW, Parnet P, Dantzer R. Effects of lipopolysaccharide and glucocorticoids on expression of interleukin-1 beta converting enzyme in the pituitary and brain of mice. J Neuroimmunol. 1996;68:61–66. doi: 10.1016/0165-5728(96)00066-5. [DOI] [PubMed] [Google Scholar]
- [20].Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neurosci. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Turrin NP, Gayle D, Ilyin SE, Flynn MC, Langhans W, Schwartz GJ, Plata-Salaman CR. Pro-inflammatory and anti-inflammatory cytokine mRNA induction in the periphery and brain following intraperitoneal administration of bacterial lipopolysaccharide. Brain Res Bull. 2001;54:443–453. doi: 10.1016/s0361-9230(01)00445-2. [DOI] [PubMed] [Google Scholar]
- [22].Van Dam AM, Bauer J, Tilders FJ, Berkenbosch F. Endotoxin-induced appearance of immunoreactive interleukin-1 beta in ramified microglia in rat brain: a light and electron microscopic study. Neuroscience. 1995;65:815–826. doi: 10.1016/0306-4522(94)00549-k. [DOI] [PubMed] [Google Scholar]
- [23].Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- [24].Schiltz JC, Sawchenko PE. Signaling the brain in systemic inflammation: the role of perivascular cells. Front Biosci. 2003;8:s1321–1329. doi: 10.2741/1211. [DOI] [PubMed] [Google Scholar]
- [25].Rogers J, Luber-Narod J, Styren SD, Civin WH. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer's disease. Neurobiol Aging. 1988;9:339–349. doi: 10.1016/s0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- [26].Sobel RA, Ames MB. Major histocompatibility complex molecule expression in the human central nervous system: immunohistochemical analysis of 40 patients. J Neuropathol Exp Neurol. 1988;47:19–28. doi: 10.1097/00005072-198801000-00003. [DOI] [PubMed] [Google Scholar]
- [27].Mattiace LA, Davies P, Dickson DW. Detection of HLA-DR on microglia in the human brain is a function of both clinical and technical factors. Am J Pathol. 1990;136:1101–1114. [PMC free article] [PubMed] [Google Scholar]
- [28].Tafti M, Nishino S, Aldrich MS, Liao W, Dement WC, Mignot E. Major histocompatibility class II molecules in the CNS: increased microglial expression at the onset of narcolepsy in canine model. J Neurosci. 1996;16:4588–4595. doi: 10.1523/JNEUROSCI.16-15-04588.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Streit WJ, Sparks DL. Activation of microglia in the brains of humans with heart disease and hypercholesterolemic rabbits. J Mol Med. 1997;75:130–138. doi: 10.1007/s001090050097. [DOI] [PubMed] [Google Scholar]
- [30].Perry VH, Matyszak MK, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7:60–67. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- [31].Ogura K, Ogawa M, Yoshida M. Effects of ageing on microglia in the normal rat brain: immunohistochemical observations. Neuroreport. 1994;5:1224–1226. doi: 10.1097/00001756-199406020-00016. [DOI] [PubMed] [Google Scholar]
- [32].Morgan TE, Rozovsky I, Goldsmith SK, Stone DJ, Yoshida T, Finch CE. Increased transcription of the astrocyte gene GFAP during middle-age is attenuated by food restriction: implications for the role of oxidative stress. Free Radic Biol Med. 1997;23:524–528. doi: 10.1016/s0891-5849(97)00120-2. [DOI] [PubMed] [Google Scholar]
- [33].Rozovsky I, Finch CE, Morgan TE. Age-related activation of microglia and astrocytes: in vitro studies show persistent phenotypes of aging, increased proliferation, and resistance to down-regulation. Neurobiol Aging. 1998;19:97–103. doi: 10.1016/s0197-4580(97)00169-3. [DOI] [PubMed] [Google Scholar]
- [34].Morgan TE, Xie Z, Goldsmith S, Yoshida T, Lanzrein AS, Stone D, Rozovsky I, Perry G, Smith MA, Finch CE. The mosaic of brain glial hyperactivity during normal ageing and its attenuation by food restriction. Neuroscience. 1999;89:687–699. doi: 10.1016/s0306-4522(98)00334-0. [DOI] [PubMed] [Google Scholar]
- [35].Nicolle MM, Gonzalez J, Sugaya K, Baskerville KA, Bryan D, Lund K, Gallagher M, McKinney M. Signatures of hippocampal oxidative stress in aged spatial learning-impaired rodents. Neuroscience. 2001;107:415–431. doi: 10.1016/s0306-4522(01)00374-8. [DOI] [PubMed] [Google Scholar]
- [36].Sheffield LG, Berman NE. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol Aging. 1998;19:47–55. doi: 10.1016/s0197-4580(97)00168-1. [DOI] [PubMed] [Google Scholar]
- [37].Sloane JA, Hollander W, Moss MB, Rosene DL, Abraham CR. Increased microglial activation and protein nitration in white matter of the aging monkey. Neurobiol Aging. 1999;20:395–405. doi: 10.1016/s0197-4580(99)00066-4. [DOI] [PubMed] [Google Scholar]
- [38].Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- [39].Cotrina ML, Nedergaard M. Astrocytes in the aging brain. J Neurosci Res. 2002;67:1–10. doi: 10.1002/jnr.10121. [DOI] [PubMed] [Google Scholar]
- [40].Cacabelos R, Alvarez XA, Fernandez-Novoa L, Franco A, Mangues R, Pellicer A, Nishimura T. Brain interleukin-1 beta in Alzheimer's disease and vascular dementia. Methods Find Exp Clin Pharmacol. 1994;16:141–151. [PubMed] [Google Scholar]
- [41].Luterman JD, Haroutunian V, Yemul S, Ho L, Purohit D, Aisen PS, Mohs R, Pasinetti GM. Cytokine gene expression as a function of the clinical progression of Alzheimer disease dementia. Arch Neurol. 2000;57:1153–1160. doi: 10.1001/archneur.57.8.1153. [DOI] [PubMed] [Google Scholar]
- [42].Remarque EJ, Bollen EL, Weverling-Rijnsburger AW, Laterveer JC, Blauw GJ, Westendorp RG. Patients with Alzheimer's disease display a pro-inflammatory phenotype. Exp Gerontol. 2001;36:171–176. doi: 10.1016/s0531-5565(00)00176-5. [DOI] [PubMed] [Google Scholar]
- [43].Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- [44].Perry VH. A revised view of the central nervous system microenvironment and major histocompatibility complex class II antigen presentation. J Neuroimmunol. 1998;90:113–121. doi: 10.1016/s0165-5728(98)00145-3. [DOI] [PubMed] [Google Scholar]
- [45].Long JM, Kalehua AN, Muth NJ, Calhoun ME, Jucker M, Hengemihle JM, Ingram DK, Mouton PR. Stereological analysis of astrocyte and microglia in aging mouse hippocampus. Neurobiol Aging. 1998;19:497–503. doi: 10.1016/s0197-4580(98)00088-8. [DOI] [PubMed] [Google Scholar]
- [46].Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Barrientos RM, Higgins EA, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 2002;134:291–298. doi: 10.1016/s0166-4328(02)00043-8. [DOI] [PubMed] [Google Scholar]
- [48].Barrientos RM, Sprunger DB, Campeau S, Higgins EA, Watkins LR, Rudy JW, Maier SF. Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience. 2003;121:847–853. doi: 10.1016/s0306-4522(03)00564-5. [DOI] [PubMed] [Google Scholar]
- [49].Barrientos RM, Sprunger DB, Campeau S, Watkins LR, Rudy JW, Maier SF. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1beta administration. J Neuroimmunol. 2004;155:119–126. doi: 10.1016/j.jneuroim.2004.06.009. [DOI] [PubMed] [Google Scholar]
- [50].Gibertini M, Newton C, Friedman H, Klein TW. Spatial learning impairment in mice infected with Legionella pneumophila or administered exogenous interleukin-1-beta. Brain Behav Immun. 1995;9:113–128. doi: 10.1006/brbi.1995.1012. [DOI] [PubMed] [Google Scholar]
- [51].Hauss-Wegrzyniak B, Dobrzanski P, Stoehr JD, Wenk GL. Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer's disease. Brain Res. 1998;780:294–303. doi: 10.1016/s0006-8993(97)01215-8. [DOI] [PubMed] [Google Scholar]
- [52].Hauss-Wegrzyniak B, Lynch MA, Vraniak PD, Wenk GL. Chronic brain inflammation results in cell loss in the entorhinal cortex and impaired LTP in perforant path-granule cell synapses. Exp Neurol. 2002;176:336–341. doi: 10.1006/exnr.2002.7966. [DOI] [PubMed] [Google Scholar]
- [53].Hauss-Wegrzyniak B, Willard LB, Del Soldato P, Pepeu G, Wenk GL. Peripheral administration of novel anti-inflammatories can attenuate the effects of chronic inflammation within the CNS. Brain Res. 1999;815:36–43. doi: 10.1016/s0006-8993(98)01081-6. [DOI] [PubMed] [Google Scholar]
- [54].Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav Immun. 1998;12:212–229. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- [55].Pugh CR, Nguyen KT, Gonyea JL, Fleshner M, Wakins LR, Maier SF, Rudy JW. Role of interleukin-1 beta in impairment of contextual fear conditioning caused by social isolation. Behav Brain Res. 1999;106:109–118. doi: 10.1016/s0166-4328(99)00098-4. [DOI] [PubMed] [Google Scholar]
- [56].Hein AM, Stasko MR, Matousek SB, Scott-McKean JJ, Maier SF, Olschowka JA, Costa AC, O'Banion MK. Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain Behav Immun. 2010;24:243–253. doi: 10.1016/j.bbi.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Akana SF, Strack AM, Hanson ES, Horsley CJ, Milligan ED, Bhatnagar S, Dallman MF. Interactions among chronic cold, corticosterone and puberty on energy intake and deposition. Stress. 1999;3:131–146. doi: 10.3109/10253899909001118. [DOI] [PubMed] [Google Scholar]
- [58].Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- [60].Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, Johnson RW. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiol Aging. 2008;29:614–621. doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun. 2009;23:46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- [63].Sparkman NL, Martin LA, Calvert WS, Boehm GW. Effects of intraperitoneal lipopolysaccharide on Morris maze performance in year-old and 2-month-old female C57BL/6J mice. Behav Brain Res. 2005;159:145–151. doi: 10.1016/j.bbr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- [64].Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, J, OC, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Buchanan JB, Sparkman NL, Chen J, Johnson RW. Cognitive and neuroinflammatory consequences of mild repeated stress are exacerbated in aged mice. Psychoneuroendocrinology. 2008;33:755–765. doi: 10.1016/j.psyneuen.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sparkman NL, Johnson RW. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 2008;15:323–330. doi: 10.1159/000156474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Rosczyk HA, Sparkman NL, Johnson RW. Neuroinflammation and cognitive function in aged mice following minor surgery. Exp Gerontol. 2008;43:840–846. doi: 10.1016/j.exger.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Frank MG, Barrientos RM, Watkins LR, Maier SF. Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. J Neuroimmunol. 2010 doi: 10.1016/j.jneuroim.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal 'On' and 'Off' signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- [70].Lyons A, Downer EJ, Crotty S, Nolan YM, Mills KH, Lynch MA. CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. J Neurosci. 2007;27:8309–8313. doi: 10.1523/JNEUROSCI.1781-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- [72].Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP. Protracted downregulation of CX(3)CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav Immun. 2010 doi: 10.1016/j.bbi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Vereker E, Campbell V, Roche E, McEntee E, Lynch MA. Lipopolysaccharide inhibits long term potentiation in the rat dentate gyrus by activating caspase-1. J Biol Chem. 2000;275:26252–26258. doi: 10.1074/jbc.M002226200. [DOI] [PubMed] [Google Scholar]
- [74].Vereker E, O'Donnell E, Lynch MA. The inhibitory effect of interleukin-1beta on long-term potentiation is coupled with increased activity of stress-activated protein kinases. J Neurosci. 2000;20:6811–6819. doi: 10.1523/JNEUROSCI.20-18-06811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Cumiskey D, Curran BP, Herron CE, O'Connor JJ. A role for inflammatory mediators in the IL-18 mediated attenuation of LTP in the rat dentate gyrus. Neuropharmacology. 2007;52:1616–1623. doi: 10.1016/j.neuropharm.2007.03.006. [DOI] [PubMed] [Google Scholar]
- [76].Curran BP, O'Connor JJ. The inhibition of long-term potentiation in the rat dentate gyrus by pro-inflammatory cytokines is attenuated in the presence of nicotine. Neurosci Lett. 2003;344:103–106. doi: 10.1016/s0304-3940(03)00440-3. [DOI] [PubMed] [Google Scholar]
- [77].Tancredi V, D'Antuono M, Cafe C, Giovedi S, Bue MC, D'Arcangelo G, Onofri F, Benfenati F. The inhibitory effects of interleukin-6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen-activated protein kinase ERK. J Neurochem. 2000;75:634–643. doi: 10.1046/j.1471-4159.2000.0750634.x. [DOI] [PubMed] [Google Scholar]
- [78].Bliss TVP, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. Journal of Physiology (London) 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Alger BE, Teyler TJ. Long-term and short-term plasticity in the CA1, CA3, and dentate regions of the rat hippocampal slice. Brain Res. 1976;110:463–480. doi: 10.1016/0006-8993(76)90858-1. [DOI] [PubMed] [Google Scholar]
- [80].Andersen P, Sundberg SH, Sveen O, Wigstrom H. Specific long-lasting potentiation of synaptic transmission in hippocampal slices. Nature. 1977;266:736–737. doi: 10.1038/266736a0. [DOI] [PubMed] [Google Scholar]
- [81].Stripling JS, Patneau DK, Gramlich CA. Selective long-term potentiation in the pyriform cortex. Brain Res. 1988;441:281–291. doi: 10.1016/0006-8993(88)91406-0. [DOI] [PubMed] [Google Scholar]
- [82].Wilhite BL, Teyler TJ, Hendricks C. Functional relations of the rodent claustral-entorhinal-hippocampal system. Brain Res. 1986;365:54–60. doi: 10.1016/0006-8993(86)90721-3. [DOI] [PubMed] [Google Scholar]
- [83].Laroche S, Doyere V, Bloch V. Linear relation between the magnitude of long-term potentiation in the dentate gyrus and associative learning in the rat. A demonstration using commissural inhibition and local infusion of an N-methyl-D- aspartate receptor antagonist. Neuroscience. 1989;28:375–386. doi: 10.1016/0306-4522(89)90184-x. [DOI] [PubMed] [Google Scholar]
- [84].Malenka RC, Kauer JA, Zucker RS, Nicoll RA. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science. 1988;242:81–84. doi: 10.1126/science.2845577. [DOI] [PubMed] [Google Scholar]
- [85].Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol (Lond) 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Collingridge GL, Kehl SJ, McLennan H. The antagonism of amino acid-induced excitations of rat hippocampal CA1 neurones in vitro. J Physiol (Lond) 1983;334:19–31. doi: 10.1113/jphysiol.1983.sp014477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Grover LM, Teyler TJ. Two components of long-term potentiation induced by different patterns of afferent activation. Nature. 1990;347:477–479. doi: 10.1038/347477a0. [DOI] [PubMed] [Google Scholar]
- [88].Pang PT, Lu B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res Rev. 2004;3:407–430. doi: 10.1016/j.arr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- [89].Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- [90].Alvarez P, Squire LR. Memory consolidation and the medial temporal lobe: a simple network model. Proc Natl Acad Sci U S A. 1994;91:7041–7045. doi: 10.1073/pnas.91.15.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Nguyen PV, Kandel ER. A macromolecular synthesis-dependent late phase of long-term potentiation requiring cAMP in the medial perforant pathway of rat hippocampal slices. J Neurosci. 1996;16:3189–3198. doi: 10.1523/JNEUROSCI.16-10-03189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Barco A, Patterson S, Alarcon JM, Gromova P, Mata-Roig M, Morozov A, Kandel ER. Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron. 2005;48:123–137. doi: 10.1016/j.neuron.2005.09.005. [DOI] [PubMed] [Google Scholar]
- [93].Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- [94].Davis S, Butcher SP, Morris RG. The NMDA receptor antagonist D-2-amino-5-phosphonopentanoate (D-AP5) impairs spatial learning and LTP in vivo at intracerebral concentrations comparable to those that block LTP in vitro. J Neurosci. 1992;12:21–34. doi: 10.1523/JNEUROSCI.12-01-00021.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Morris RG. Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D- aspartate receptor antagonist AP5. J Neurosci. 1989;9:3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Morris RG. Further studies of the role of hippocampal synaptic plasticity in spatial learning: is hippocampal LTP a mechanism for automatically recording attended experience? J Physiol Paris. 1996;90:333–334. doi: 10.1016/s0928-4257(97)87912-0. [DOI] [PubMed] [Google Scholar]
- [97].Morris RGM, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- [98].Richter-Levin G, Canevari L, Bliss TV. Long-term potentiation and glutamate release in the dentate gyrus: links to spatial learning. Behav Brain Res. 1995;66:37–40. doi: 10.1016/0166-4328(94)00121-u. [DOI] [PubMed] [Google Scholar]
- [99].Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, et al. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature. 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- [100].Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem. 2002;9:224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Bellinger FP, Madamba S, Siggins GR. Interleukin-1 Beta inhibits synaptic stregnth and long-term potentiation in the rat CA1 hippocampus. Brain Res. 1993;628:227–234. doi: 10.1016/0006-8993(93)90959-q. [DOI] [PubMed] [Google Scholar]
- [102].Coogan A, O'Connor JJ. Inhibition of NMDA receptor-mediated synaptic transmission in the rat dentate gyrus in vitro by IL-1 beta. Neuroreport. 1997;8:2107–2110. doi: 10.1097/00001756-199707070-00004. [DOI] [PubMed] [Google Scholar]
- [103].Coogan AN, O'Connor JJ. Interleukin-1beta inhibits a tetraethylammonium-induced synaptic potentiation in the rat dentate gyrus in vitro. Eur J Pharmacol. 1999;374:197–206. doi: 10.1016/s0014-2999(99)00320-9. [DOI] [PubMed] [Google Scholar]
- [104].Cunningham AJ, Murray CA, O'Neil LAJ, Lynch MA, O'Connor JJ. Interleukin-1 (IL-1) and tumor necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci Lett. 1996;203:17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- [105].Katsuki H, Nakai S, Hirai Y, Akaji K, Kiso Y, Satoh M. Interleukin-1 B inhibits long-term potentiation in the CA3 region of mouse hippocampal slices. Eur J Pharmacol. 1990;181:323–326. doi: 10.1016/0014-2999(90)90099-r. [DOI] [PubMed] [Google Scholar]
- [106].Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- [107].Diana G, Scotti de Carolis A, Frank C, Domenici MR, Sagratella S. Selective reduction of hippocampal dentate frequency potentiation in aged rats with impaired place learning. Brain Res Bull. 1994;35:107–111. doi: 10.1016/0361-9230(94)90089-2. [DOI] [PubMed] [Google Scholar]
- [108].Landfield PW, McGaugh JL, Lynch G. Impaired synaptic potentiation processes in the hippocampus of aged, memory-deficient rats. Brain Res. 1978;150:85–101. doi: 10.1016/0006-8993(78)90655-8. [DOI] [PubMed] [Google Scholar]
- [109].Lynch MA, Voss KL. Membrane arachidonic acid concentration correlates with age and induction of long-term potentiation in the dentate gyrus in the rat. Eur J Neurosci. 1994;6:1008–1014. doi: 10.1111/j.1460-9568.1994.tb00595.x. [DOI] [PubMed] [Google Scholar]
- [110].Lynch MA. Age-related impairment in long-term potentiation in hippocampus: a role for the cytokine, interleukin-1 beta? Prog Neurobiol. 1998;56:571–589. doi: 10.1016/s0301-0082(98)00054-9. [DOI] [PubMed] [Google Scholar]
- [111].Chapman TR, Barrientos RM, Ahrendsen JT, Maier SF, Patterson SL. Synaptic correlates of increased cognitive vulnerability with aging: peripheral immune challenge and aging interact to disrupt theta-burst late-phase long-term potentiation in hippocampal area CA1. J Neurosci. 2010;30:7598–7603. doi: 10.1523/JNEUROSCI.5172-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. The Journal of Neuroscience Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- [113].Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- [114].Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- [115].Rudy JW, O'Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cognit Affect & Behav Neurosci. 2001;1:66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- [116].Barrientos RM, Watkins LR, Rudy JW, Maier SF. Characterization of the sickness response in young and aging rats following E. coli infection. Brain Behav Immun. 2009;23:450–454. doi: 10.1016/j.bbi.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Coogan AN, O'Neill LA, O'Connor JJ. The P38 mitogen-activated protein kinase inhibitor SB203580 antagonizes the inhibitory effects of interleukin-1beta on long-term potentiation in the rat dentate gyrus in vitro. Neuroscience. 1999;93:57–69. doi: 10.1016/s0306-4522(99)00100-1. [DOI] [PubMed] [Google Scholar]
- [118].Rada P, Mark GP, Vitek MP, Mangano RM, Blume AJ, Beer B, Hoebel BG. Interleukin-1 beta decreases acetylcholine measured by microdialysis in the hippocampus of freely moving rats. Brain Res. 1991;550:287–290. doi: 10.1016/0006-8993(91)91330-4. [DOI] [PubMed] [Google Scholar]
- [119].Murray CA, McGahon B, McBennett S, Lynch MA. Interleukin-1 beta inhibits glutamate release in hippocampus of young, but not aged, rats. Neurobiol Aging. 1997;18:343–348. doi: 10.1016/s0197-4580(97)80317-x. [DOI] [PubMed] [Google Scholar]
- [120].Plata-Salaman CR, ffrench-Mullen JM. Interleukin-1 beta inhibits Ca2+ channel currents in hippocampal neurons through protein kinase C. Eur J Pharmacol. 1994;266:1–10. doi: 10.1016/0922-4106(94)90202-x. [DOI] [PubMed] [Google Scholar]
- [121].Casamenti F, Prosperi C, Scali C, Giovannelli L, Colivicchi MA, Faussone-Pellegrini MS, Pepeu G. Interleukin-1 beta activates forebrain glial cells and increases nitric oxide production and cortical glutamate and GABA release in vivo: implications for Alzheimer's disease. Neuroscience. 1999;91:831–842. doi: 10.1016/s0306-4522(98)00680-0. [DOI] [PubMed] [Google Scholar]
- [122].Li Y, Liu L, Kang J, Sheng JG, Barger SW, Mrak RE, Griffin WS. Neuronal-glial interactions mediated by interleukin-1 enhance neuronal acetylcholinesterase activity and mRNA expression. J Neurosci. 2000;20:149–155. doi: 10.1523/JNEUROSCI.20-01-00149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Viviani B, Gardoni F, Bartesaghi S, Corsini E, Facchi A, Galli CL, Di Luca M, Marinovich M. Interleukin-1 beta released by gp120 drives neural death through tyrosine phosphorylation and trafficking of NMDA receptors. J Biol Chem. 2006;281:30212–30222. doi: 10.1074/jbc.M602156200. [DOI] [PubMed] [Google Scholar]
- [125].Minogue AM, Schmid AW, Fogarty MP, Moore AC, Campbell VA, Herron CE, Lynch MA. Activation of the c-Jun N-terminal kinase signaling cascade mediates the effect of amyloid-beta on long term potentiation and cell death in hippocampus: a role for interleukin-1beta? J Biol Chem. 2003;278:27971–27980. doi: 10.1074/jbc.M302530200. [DOI] [PubMed] [Google Scholar]
- [126].Kelly A, Vereker E, Nolan Y, Brady M, Barry C, Loscher CE, Mills KH, Lynch MA. Activation of p38 plays a pivotal role in the inhibitory effect of lipopolysaccharide and interleukin-1 beta on long term potentiation in rat dentate gyrus. J Biol Chem. 2003;278:19453–19462. doi: 10.1074/jbc.M301938200. [DOI] [PubMed] [Google Scholar]
- [127].Seger R, Krebs EG. The MAPK signaling cascade. Faseb J. 1995;9:726–735. [PubMed] [Google Scholar]
- [128].Maroney AC, Glicksman MA, Basma AN, Walton KM, Knight E, Jr, Murphy CA, Bartlett BA, Finn JP, Angeles T, Matsuda Y, Neff NT, Dionne CA. Motoneuron apoptosis is blocked by CEP-1347 (KT 7515), a novel inhibitor of the JNK signaling pathway. J Neurosci. 1998;18:104–111. doi: 10.1523/JNEUROSCI.18-01-00104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Curran BP, Murray HJ, O'Connor JJ. A role for c-Jun N-terminal kinase in the inhibition of long-term potentiation by interleukin-1beta and long-term depression in the rat dentate gyrus in vitro. Neuroscience. 2003;118:347–357. doi: 10.1016/s0306-4522(02)00941-7. [DOI] [PubMed] [Google Scholar]
- [130].Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain- derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Messaoudi E, Ying SW, Kanhema T, Croll SD, Bramham CR. Brain-derived neurotrophic factor triggers transcription-dependent, late phase long-term potentiation in vivo. J Neurosci. 2002;22:7453–7461. doi: 10.1523/JNEUROSCI.22-17-07453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Patterson SL, Pittenger C, Morozov A, Martin KC, Scanlin H, Drake C, Kandel ER. Some forms of cAMP-mediated long-lasting potentiation are associated with release of BDNF and nuclear translocation of phospho-MAP kinase. Neuron. 2001;32:123–140. doi: 10.1016/s0896-6273(01)00443-3. [DOI] [PubMed] [Google Scholar]
- [133].Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- [134].Ma YL, Wang HL, Wu HC, Wei CL, Lee EH. Brain-derived neurotrophic factor antisense oligonucleotide impairs memory retention and inhibits long-term potentiation in rats. Neuroscience. 1998;82:957–967. doi: 10.1016/s0306-4522(97)00325-4. [DOI] [PubMed] [Google Scholar]
- [135].Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Lapchak PA, Araujo DM, Hefti F. Systemic interleukin-1 beta decreases brain-derived neurotrophic factor messenger RNA expression in the rat hippocampal formation. Neuroscience. 1993;53:297–301. doi: 10.1016/0306-4522(93)90196-m. [DOI] [PubMed] [Google Scholar]
- [137].Richwine AF, Parkin AO, Buchanan JB, Chen J, Markham JA, Juraska JM, Johnson RW. Architectural changes to CA1 pyramidal neurons in adult and aged mice after peripheral immune stimulation. Psychoneuroendocrinology. 2008;33:1369–1377. doi: 10.1016/j.psyneuen.2008.08.003. [DOI] [PubMed] [Google Scholar]
- [138].Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci. 2008;28:11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci U S A. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- [141].Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- [142].Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Frank MG, Barrientos RM, Hein AM, Biedenkapp JC, Watkins LR, Maier SF. IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344xBN F1 rats. Brain Behav Immun. 2010;24:254–262. doi: 10.1016/j.bbi.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Abraham J, Johnson RW. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain Behav Immun. 2009;23:396–401. doi: 10.1016/j.bbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006;99:1263–1272. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- [146].Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Godbout JP, Berg BM, Kelley KW, Johnson RW. alpha-Tocopherol reduces lipopolysaccharide-induced peroxide radical formation and interleukin-6 secretion in primary murine microglia and in brain. J Neuroimmunol. 2004;149:101–109. doi: 10.1016/j.jneuroim.2003.12.017. [DOI] [PubMed] [Google Scholar]
- [148].Martin DS, Spencer P, Horrobin DF, Lynch MA. Long-term potentiation in aged rats is restored when the age-related decrease in polyunsaturated fatty acid concentration is reversed. Prostaglandins Leukot Essent Fatty Acids. 2002;67:121–130. doi: 10.1054/plef.2002.0408. [DOI] [PubMed] [Google Scholar]
- [149].McGahon BM, Martin DS, Horrobin DF, Lynch MA. Age-related changes in LTP and antioxidant defenses are reversed by an alpha-lipoic acid-enriched diet. Neurobiol Aging. 1999;20:655–664. doi: 10.1016/s0197-4580(99)00050-0. [DOI] [PubMed] [Google Scholar]
- [150].McGahon BM, Murray CA, Horrobin DF, Lynch Age-related changes in oxidative mechanisms and LTP are reversed by dietary manipulation. Neurobiol Aging. 1999;20:643–653. doi: 10.1016/s0197-4580(99)00027-5. [DOI] [PubMed] [Google Scholar]
- [151].Moore ME, Piazza A, McCartney Y, Lynch MA. Evidence that vitamin D3 reverses age-related inflammatory changes in the rat hippocampus. Biochem Soc Trans. 2005;33:573–577. doi: 10.1042/BST0330573. [DOI] [PubMed] [Google Scholar]
- [152].Gemma C, Mesches MH, Sepesi B, Choo K, Holmes DB, Bickford PC. Diets enriched in foods with high antioxidant activity reverse age-induced decreases in cerebellar beta-adrenergic function and increases in proinflammatory cytokines. J Neurosci. 2002;22:6114–6120. doi: 10.1523/JNEUROSCI.22-14-06114.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Cartford MC, Gemma C, Bickford PC. Eighteen-month-old Fischer 344 rats fed a spinach-enriched diet show improved delay classical eyeblink conditioning and reduced expression of tumor necrosis factor alpha (TNFalpha) and TNFbeta in the cerebellum. J Neurosci. 2002;22:5813–5816. doi: 10.1523/JNEUROSCI.22-14-05813.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Wenk GL, McGann-Gramling K, Hauss-Wegrzyniak B, Ronchetti D, Maucci R, Rosi S, Gasparini L, Ongini E. Attenuation of chronic neuroinflammation by a nitric oxide-releasing derivative of the antioxidant ferulic acid. J Neurochem. 2004;89:484–493. doi: 10.1111/j.1471-4159.2004.02359.x. [DOI] [PubMed] [Google Scholar]
- [155].Berg BM, Godbout JP, Chen J, Kelley KW, Johnson RW. alpha-Tocopherol and selenium facilitate recovery from lipopolysaccharide-induced sickness in aged mice. J Nutr. 2005;135:1157–1163. doi: 10.1093/jn/135.5.1157. [DOI] [PubMed] [Google Scholar]
- [156].Perkins AJ, Hendrie HC, Callahan CM, Gao S, Unverzagt FW, Xu Y, Hall KS, Hui SL. Association of antioxidants with memory in a multiethnic elderly sample using the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 1999;150:37–44. doi: 10.1093/oxfordjournals.aje.a009915. [DOI] [PubMed] [Google Scholar]
- [157].Perrig WJ, Perrig P, Stahelin HB. The relation between antioxidants and memory performance in the old and very old. J Am Geriatr Soc. 1997;45:718–724. doi: 10.1111/j.1532-5415.1997.tb01476.x. [DOI] [PubMed] [Google Scholar]
- [158].Murray CA, Lynch MA. Dietary supplementation with vitamin E reverses the age-related deficit in long term potentiation in dentate gyrus. J Biol Chem. 1998;273:12161–12168. doi: 10.1074/jbc.273.20.12161. [DOI] [PubMed] [Google Scholar]
- [159].Murray CA, Clements MP, Lynch MA. Interleukin-1 induces lipid peroxidation and membrane changes in rat hippocampus: An age-related study. Gerontology. 1999;45:136–142. doi: 10.1159/000022076. [DOI] [PubMed] [Google Scholar]
- [160].Shukitt-Hale B, Cheng V, Joseph JA. Effects of blackberries on motor and cognitive function in aged rats. Nutr Neurosci. 2009;12:135–140. doi: 10.1179/147683009X423292. [DOI] [PubMed] [Google Scholar]
- [161].Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Abraham J, Johnson RW. Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuvenation Res. 2009;12:445–453. doi: 10.1089/rej.2009.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- [164].Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- [165].Greenwood BN, Strong PV, Foley TE, Fleshner M. A behavioral analysis of the impact of voluntary physical activity on hippocampus-dependent contextual conditioning. Hippocampus. 2009;19:988–1001. doi: 10.1002/hipo.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Dantzer R, Bluthe RM, Gheusi G, Cremona S, Laye S, Parnet P, Kelley KW. Molecular basis of sickness behavior. Ann N Y Acad Sci. 1998;856:132–138. doi: 10.1111/j.1749-6632.1998.tb08321.x. [DOI] [PubMed] [Google Scholar]
- [167].Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- [168].Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am. 2009;29:247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging. 2008;29:1744–1753. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]