Abstract
Obese individuals are less able to oxidize fat than non-obese individuals. Caloric reduction or fasting can detect ketonuria. We investigated the differences of metabolic parameters in the presence of ketonuria after a minimum 8 hr fast in a cross-sectional analysis of 16,523 Koreans (6,512 women and 10,011 men). The relationship between the presence of ketonuria of all subjects and prevalence of obesity, central obesity, metabolic syndrome, and obesity-related metabolic parameters were assessed. The ketonuria group had lower prevalence of obesity, central obesity, and metabolic syndrome than the non-ketonuria group. In addition, all metabolic parameters (including body weight, waist circumference, blood glucose, high-density lipoprotein, triglyceride, blood pressure, and insulin) were favorable in the ketonuria group than in the non-ketonuria group, even after adjustment for age, tobacco use, and alcohol consumption. The odds ratios of having obesity (odds ratio [OR]=1.427 in women, OR=1.582 in men, P<0.05), central obesity (OR=1.675 in women, OR=1.889 in men, P<0.05), and metabolic syndrome (OR=3.505 in women, OR=1.356 in men, P<0.05) were increased in the non-ketonuria group compared to the ketonuria group. The presence of ketonuria after at least an 8 hr fast may be indicative of metabolic superiority.
Keywords: Urine Ketone, Lipolysis, Metabolic Syndrome, Korean
INTRODUCTION
Obesity triggers a variety of metabolic derangements (1-6) that include type 2 diabetes, hypertension, dyslipidemia, and cardiovascular disease (7). In addition, obesity causes chronic inflammation (8), which, in turn, results in insulin resistance (9) and mitochondrial dysfunction (10).
Ketone bodies (acetone, acetoacetate, and b-hydroxybutyrate) increase appreciably in the blood of people on very low calorie diet (VLCD) (11, 12). Acetone is a water-soluble volatile product of metabolism that can be detected upon exhalation and which is excreted in the urine. After a few days of VLCD, fat becomes the main source of energy, and VLCD regimens are consequently ketogenic (13). One study conducted during a Muslim fasting period observed that fasting in excess of 10 hr was associated with development of ketosis in normal controls after 3-6 days and presence of β-hydroxy butyric and diacetic acids in the urine, whereas obese individuals did not show ketosis even after 20 days of a ketogenic diet (14). According to this study, obese people did not readily develop ketosis and ketonuria, however, it was clear that the level of ketone bodies in the blood depended on the balance between their rate of production by the liver and their rate of utilization and excretion. Likewise, fat metabolism in obese individuals was different from those who were non-obese, as the level of free fatty acid in blood after 2 hr fasting was, on average, higher in obese people than in normal subjects, with the higher levels perhaps reflecting a priority for fat metabolism rather than carbohydrate metabolism in the obese (15, 16). From these basic metabolic aspects, a low carbohydrate ketogenic diet shows the effective results of weight reduction (17) and so remains in wide clinical use. While numerous studies have been concerned with the effect of a low carbohydrate ketogenic diet on body weight reduction of obese subjects, little is known of the metabolic differences after fasting in the healthy general population. For example, little information exists concerning the metabolic differences, if any, related to fasting in those with ketonuria and those without, and concerning the relationship between ketonuria and metabolic parameters and the prevalence of obesity, central obesity, and metabolic syndrome. The present cross-sectional study of over 16,000 Koreans addressed these shortcomings.
MATERIALS AND METHODS
Study design
The study was cross-sectional in design and used the data of a Health Promotion Center in Suwon, South Korea. The prevalence of obesity, central obesity, and metabolic syndrome according to the presence of ketonuria at least 8 hr after fasting was evaluated before an annual health check-up.
Study data
Data from self-administered questionnaires completed by 16,523 Korean (6,512 women and 10,011 men) prior to a routine health check-up in a Health Promotion Center in Suwon, Korea, during 2008 was used. We analyzed all adult subjects from twenties to fifties in their ages. We excluded the data of over sixties in their ages, because of inappropriate sample size and there were no pregnant women enrolled in this study. Also, the subsequent health test results of urine analysis, anthropometry (body weight, waist circumference, and body mass index; BMI), metabolic markers such as blood pressure, fasting sugar, triglyceride, high-density lipoprotein (HDL), and lifestyle information including history of tobacco use and alcohol consumption were included.
Anthropometry and laboratory test
Routine health examination was done after an overnight fast (minimum 8 hr). The height and body weight of the participants were measured while they wore light clothing without shoes. Weight was measured to the nearest 0.1 kg, and height was measured to the nearest centimeter. BMI was calculated as the weight divided by height squared (kg/m2). Trained nurses in the Health Promotion Center measured the waist circumference between the lower rib and the iliac crest (18) and electrically measured blood pressure after the participants had been at rest for at least 15 min using a model TM-2655P apparatus (PMS Instruments, Tokyo, Japan). The body composition of each participant was analyzed using the Body Impedance Analysis, In body 4.0 software (Biospace, Seoul, Korea). Additionally, all of the subjects underwent automated blood testing using a TBA-200FR apparatus (Toshiba, Tokyo, Japan); measurements included standard enzymatic measurements of total cholesterol (TC), high-density lipoprotein cholesterol (HDLC), triglycerides (TG), and fasting glucose (FG) in fresh serum samples.
Urine ketone body detection
The presence of ketonuria was assessed in urine using Uropaper (US-3100R; Eiken Chemical, Tokyo, Japan). Acetoacetic acid, acetone, and 3-hydroxy butyrate are three ketone bodies found in normal individuals. Under certain circumstances, these ketone bodies are found by lipolysis, which can be detected in urine by the appearance of a purple color upon addition of sodium nitroprusside to the collected urine, due to the presence of ketone bodies (a positive result). Ketonuria results are typically ranked on a three-point scale with respect to ketone body concentration: 1+ (10 mg/dL), 2+ (30 mg/dL), and 3+ (80 mg/dL). Presently, only a qualitative positive result was determined.
Definitions of obesity, central obesity and metabolic syndrome
Obesity was defined as a BMI ≥25 kg/m2 and central obesity was defined as a waist circumference ≥90 cm for men and ≥85 cm for women. The definitions were consistent with the 2006 Korean Society of Study of Obesity guidelines (19). The NCEP-ATP III guidelines (20) were used to define metabolic syndrome, which consisted of central obesity (waist circumference ≥90 cm for men and ≥85 cm for women), blood pressure ≥130/80 mmHg, TG ≥150 mg/dL, FG ≥110 mg/dL, and low HDLC (men <40 mg/dL, women <50 mg/dL). According to the NCEP-AP III guidelines, individuals with more than three of the aforementioned values that were abnormal were defined as having metabolic syndrome.
Statistical analyses
Baseline characteristics were descriptively compared to those of the entire subjects. Chi-square testing was done to compare the prevalence of obesity, central obesity, and metabolic syndrome. Independent t test was used to compare the metabolic parameters in the two groups and also ANCOVA test was done to evaluate metabolic difference after adjustment for age, tobacco use, and alcohol consumption. Finally, logistic regression analysis was done to determined the odds ratios (ORs) of obesity, central obesity, and metabolic syndrome of non-ketonuria individuals compared to those with ketonuria. Statistical analyses were done using SPSS version 11.5 software (SPSS, Chicago, IL, USA). All significant P values were <0.05.
Ethics statement
All examinees agreed to the use of their health check-up results and the Institutional Review Board of Ajou University Hospital approved the study (AJIRB-MED-OBS-09-147).
RESULTS
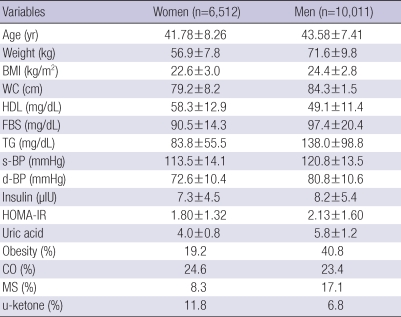
The study involved 16,523 Koreans (6,512 women and 10,011 men) whose mean age was 40-49-yr. Mean anthropometry and metabolic parameters in this study data were not seriously deteriorated. The prevalence of obesity, central obesity, and metabolic syndrome in women was 19.2%, 24.6%, 8.3%, respectively and the respective percentages in men were 40.8%, 23.4%, 17.1%. The rate of ketonuria in women was 11.8% and 6.8% in men (Table 1).
Table 1.
Baseline characteristics of study subjects
All values are mean±standard deviation.
BMI, body mass index; WC, waist circumference; HDL, high-density lipoprotein; FBS, fasting blood sugar; TG, triglyceride; s-BP, systolic blood pressure; d-BP, diastolic blood pressure; HOMA-IR, homeostasis model assessment-insulin resistance; Obesity, prevalence of obesity in this study subjects; CO, prevalence of central obesity in this study subjects; MS, prevalence of metabolic syndrome by NCEP-ATP III in this study subjects; u-ketone, prevalence of keteonuria after overnight fasting at least eight hours.
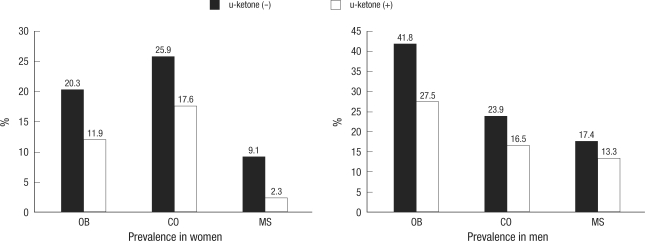
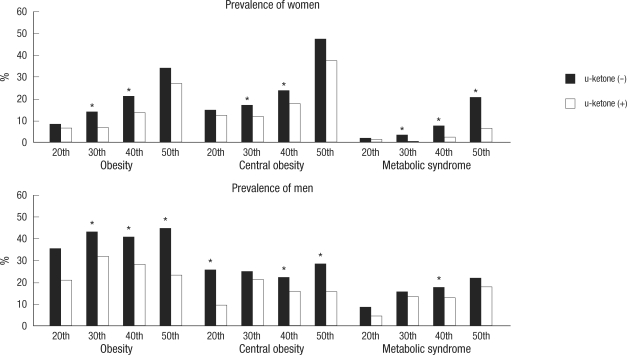
Comparisons of the prevalence of obesity, central obesity, and metabolic syndrome revealed lower rates of all parameters in subjects with ketonuria than in the non-ketonuria group (Fig. 1). The prevalence of obesity, central obesity, and metabolic syndrome was also compared in those subjects in the age range of 20-59-yr; results were lower in the ketonuria group than in the non-ketonuria group. In some generation, significant differences were detected in both genders by the presence of fasting ketonuria (Fig. 2).
Fig. 1.
Prevalence of obesity, central obesity, and metabolic syndrome according to the presence of ketonuria in all subjects. This figure is representing the prevalence of obesity, central obesity, and metabolic syndrome according to the presence of ketonuria and all showed statistically significant difference (P<0.05) in both genders.
OB, obesity (body mass index ≥25.0 kg/m2); CO, central obesity (men ≥90 cm, women ≥85 cm); MS, metabolic syndrome followed by NCEP-ATP III.
Fig. 2.
Prevalence of obesity, central obesity, and metabolic syndrome in each generation. This figure shows the prevalence of obesity, central obesity, and metabolic syndrome from the 20th to the 50th in both genders according to ketonuria under overnight fasting condition, which may represent that all subjects with ketonuria under fasting condition could be more metabolic benefit compared to the non-ketonuria.
*P<0.05 in each generation in both genders.
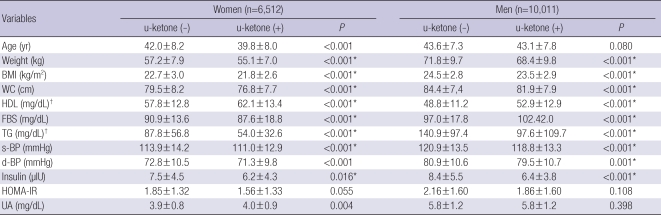
Comparisons of metabolic parameters in both genders also showed that almost all metabolic parameters including body weight, BMI, waist circumference, HDL, glucose, TG, blood pressure, and insulin level were significantly better in the ketonuria group than in non-ketonuria group before and after adjustment for age, tobacco use, and alcohol consumption (Table 2).
Table 2.
Comparisons of metabolic parameters according to urinary ketone in both genders
All values are mean±standard deviation.
P values were from independent t test in both genders.
*were marked on the P values were <0.05 after ANCOVA test after age, cigarette smoking, alcohol drinking adjustment; †was from non-parametric test (Mann-Whitney U test).
BMI, body mass index, calculated from body weight (kg)/height (m2); WC, waist circumference; HDL, high-density lipoprotein; FBS, fasting blood sugar; TG, triglyceride; s-BP, systolic blood pressure; d-BP, diastolic blood pressure; HOMA-IR, homeostasis of measurement assessment-insulin resistance; UA, uric acid.
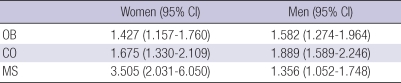
ORs of obesity, central obesity, and metabolic syndrome in the non-ketonuria group compared to the ketonuria group were evaluated after age adjustment. ORs in the non-ketonuria group were elevated in obesity (OR=1.427 in women, OR=1.582 in men), central obesity (OR=1.675 in women, OR=1.889 in men), and metabolic syndrome (OR=3.505 in women, OR=1.356 in men) compared to the ketonuria group (Table 3).
Table 3.
Odds ratios of having obesity, central obesity, and metabolic syndrome in the non-detection of urinary ketone group compared to the detection of urinary ketone group
This table shows odds ratios of having obesity, central obesity, and metabolic syndrome in the non-detection of urinary ketone group compared to the detection of urinary keteone group in both genders using logistic regression analysis was done after age-adjustment. All P values of odds ratios were <0.05.
OB, obesity (body mass index ≥25.0 kg/m2); CO, central obesity (waist circumference; men ≥90 cm, women ≥85 cm); MS, metabolic syndrome followed by NCEP-ATP III.
DISCUSSION
In the present study, individuals with ketonuria fared better metabolically than those in the non-ketonuria group regardless of gender or age. There was lower prevalence of obesity, central obesity, and metabolic syndrome in the ketonuria group than in the non-ketonuria group. In addition, various metabolic parameters such as HDL, TG, FG, insulin including body weight, BMI, and waist circumference showed better values. Moreover, the ORs of having obesity, central obesity, and metabolic syndrome were lower in those with ketonuria than in the non-ketonuria group.
Many studies have focused on the ketogenic effect of a low carbohydrate diet on weight reduction and metabolism. A meta-analysis of five trials with 447 participants and a 1-yr trial involving 311 obese women suggested that a low-carbohydrate diet is a feasible alternative to a low-fat diet for weight loss, and may also have favorable metabolic effects (21, 22). Another study that evaluated a low-carbohydrate, high-protein, high-fat diet (Atkins diet) and a low-calorie, high-carbohydrate, low-fat diet (conventional diet) found that the low-carbohydrate diet resulted in more weight loss than the conventional diet after the first six months, but the differences were not significant after one year (23).
Concerning the effect of a low-carbohydrate, ketogenic diet on nonalcoholic fatty liver disease, a small pilot study reported that four of five post-treatment liver biopsies showed histologic improvements in steatosis, inflammatory grade, and fibrosis after 3 months (24). Another study reported that Mediterranean and low-carbohydrate diets may be effective alternatives to a low-fat diet, with more favorable effects on lipids and glycemic control (25). One possible explanation for reduced body weight with a low-carbohydrate diet might be activated fatty acid oxidation (26).
The relationship between ketonuria and metabolic parameters in the general population after an overnight fast of at least 8 hr is ill-understood. The present cross-sectional observational study indicates that, similar to a low-carbohydrate diet for obese subjects, some (but not all) individuals display ketonuria after fasting. This may be a consequence of the oxidation of fat (lipolysis) in those with a healthy fat metabolism. Presently, only 8.8% of the total subjects (11.8% in women, 6.8% in men) showed ketonuria after simple fasting. This may mean that ketonuria is not a common phenomenon in modern people, and that the capability to oxidize fat decreases upon fasting, resulting in an abnormal fat metabolism. In our results, metabolic parameters such as BMI, waist circumference, HDL, glucose, TG, blood pressure, and insulin level were significantly lower in the ketonuria group than in the non-ketonuria group. Also, the OR of obesity, central obesity, and metabolic syndrome were higher in the non-ketonuria group than in the ketonuria group, even though the study data was cross-sectional. From this, we deduce that individuals with ketonuria have metabolic benefits under simple fasting, which may mean that ketonuria is associated with a higher fat oxidation ability than in the absence of ketonuria. This suggestion remains speculative, however, since fat oxidation capacity or ability was not directly measured.
This study has several limitations. The first is its cross-sectional observational nature. Causality could not be drawn from this study. Our study subjects represented one ethnic group and were a small number of people from one region, making generalized of the results impossible. We could not adjust many possible confounding factors such as regular exercise time and frequency, use of some medications, total fasting time and other metabolic parameter such as free fatty acid. Of these confounding factors, individual fasting time for the examination could be the most important, because ketonuria could be detected with increasing fasting time. Despite the shortcomings, the present study demonstrates that the presence of ketonuria can have the relationship of metabolic superiority in some sense.
ACKNOWLEDGMENTS
We thank Ms. Joo-Hee Song, Department of Family Practice and Community Health, for data collection and editing.
References
- 1.Paek KW, Chun KH, Lee KW. Relationship between metabolic syndrome and familial history of hypertension/stroke, diabetes, and cardiovascular disease. J Korean Med Sci. 2006;21:701–708. doi: 10.3346/jkms.2006.21.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snijder MB, Zimmet PZ, Visser M, Dekker JM, Seidell JC, Shaw JE. Independent and opposite associations of waist and hip circumferences with diabetes, hypertension and dyslipidemia: the AusDiab Study. Int J Obes Relat Metab Disord. 2004;28:402–409. doi: 10.1038/sj.ijo.0802567. [DOI] [PubMed] [Google Scholar]
- 3.Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Kostense PJ, Yudkin JS, Heine RJ, Nijpels G, Seidell JC. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: the Hoorn Study. Am J Clin Nutr. 2003;77:1192–1197. doi: 10.1093/ajcn/77.5.1192. [DOI] [PubMed] [Google Scholar]
- 4.Seidell JC, Pérusse L, Després JP, Bouchard C. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: the Quebec Family Study. Am J Clin Nutr. 2001;74:315–321. doi: 10.1093/ajcn/74.3.315. [DOI] [PubMed] [Google Scholar]
- 5.Jeong SK, Kim YK, Park JW, Shin YJ, Kim DS. Impact of visceral fat on metabolic syndrome and nonalcoholic liver diseases. J Korean Med Sci. 2008;23:789–795. doi: 10.3346/jkms.2008.23.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bigaard J, Frederiksen K, Tjønneland A, Thomsen BL, Overvad K, Heitmann BL, Sørensen TI. Waist and hip circumferences and all-cause mortality: usefulness of the waist-to-hip ratio? Int J Obes Relat Metab Disord. 2004;28:741–747. doi: 10.1038/sj.ijo.0802635. [DOI] [PubMed] [Google Scholar]
- 7.Jee SH, Sull JW, Park J, Lee SY, Ohrr H, Guallar E, Samet JM. Body mass index and mortality in Korean men and women. N Engl J Med. 2006;355:779–787. doi: 10.1056/NEJMoa054017. [DOI] [PubMed] [Google Scholar]
- 8.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh CH, Hung YJ, Wu DA, Kuo SW, Lee CH, Sheu WH, Li JC, Yeh KH, Chen CY, Pei D. Impact of Clinical Characteristics of Individual Metabolic Syndrome on the Severity of Insulin Resistance in Chinese Adults. J Korean Med Sci. 2007;22:74–80. doi: 10.3346/jkms.2007.22.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gianotti TF, Sookoian S, Dieuzeide G, García SI, Gemma C, González CD, Pirola CJ. A decreased mitochondrial DNA content is related to insulin resistance in adolescents. Obesity (Silver Spring) 2008;16:1591–1595. doi: 10.1038/oby.2008.253. [DOI] [PubMed] [Google Scholar]
- 11.Beisswenger BG, Delucia EM, Lapoint N, Sanford RJ, Beisswenger PJ. Ketosis leads to increased methylglyoxal production on the Atkins diet. Ann N Y Acad Sci. 2005;1043:201–210. doi: 10.1196/annals.1333.025. [DOI] [PubMed] [Google Scholar]
- 12.Musa-Veloso K, Likhodii SS, Cunnane SC. Breath acetone is a reliable indicator of ketosis in adults consuming ketogenic meals. Am J Clin Nutr. 2002;76:65–70. doi: 10.1093/ajcn/76.1.65. [DOI] [PubMed] [Google Scholar]
- 13.Shah P, Isley WL. Ketoacidosis during a low-carbohydrate diet. N Engl J Med. 2006;354:97–98. doi: 10.1056/NEJMc052709. [DOI] [PubMed] [Google Scholar]
- 14.Mohammadiha H. Resistance to ketonuria and ketosis in obese subjects. Am J Clin Nutr. 1974;27:1212–1213. doi: 10.1093/ajcn/27.11.1212. [DOI] [PubMed] [Google Scholar]
- 15.Opie LH, Walfish PG. Plasma free fatty acid concentrations in obesity. N Engl J Med. 1963;268:757–760. doi: 10.1056/NEJM196304042681404. [DOI] [PubMed] [Google Scholar]
- 16.Gordon JE, Chitkara ID, Wyon JB. Weanling diarrhea. Am J Med Sci. 1963;245:345–377. [PubMed] [Google Scholar]
- 17.Astrup A, Meinert Larsen T, Harper A. Atkins and other low-carbohydrate diets: hoax or an effective tool for weight loss? Lancet. 2004;364:897–899. doi: 10.1016/S0140-6736(04)16986-9. [DOI] [PubMed] [Google Scholar]
- 18.National Institutes of Health; National Heart, Lung, and Blood Institute and the North American Association of the Study of Obesity. The practical guide: identification, evaluation and treatment of overweight and obesity in adults. 2000. NIH publication number 00-4084. [Google Scholar]
- 19.Lee SY, Park HS, Kim DJ, Han JH, Kim SM, Cho GJ, Kim DY, Kwon HS, Kim SR, Lee CB, Oh SJ, Park CY, Yoo HJ. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007;75:72–80. doi: 10.1016/j.diabres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 21.Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS, Jr, Brehm BJ, Bucher HC. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:285–293. doi: 10.1001/archinte.166.3.285. [DOI] [PubMed] [Google Scholar]
- 22.Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, Kraemer HC, King AC. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969–977. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 23.Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 24.Tendler D, Lin S, Yancy WS, Jr, Mavropoulos J, Sylvestre P, Rockey DC, Westman EC. The effect of a low-carbohydrate, ketogenic diet on nonalcoholic fatty liver disease: a pilot study. Dig Dis Sci. 2007;52:589–593. doi: 10.1007/s10620-006-9433-5. [DOI] [PubMed] [Google Scholar]
- 25.Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H, Tangi-Rozental O, Zuk-Ramot R, Sarusi B, Brickner D, Schwartz Z, Sheiner E, Marko R, Katorza E, Thiery J, Fiedler GM, Blüher M, Stumvoll M, Stampfer MJ Dietary Intervention Randomized Controlled Trial (DIRECT) Group. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 26.Erlanson-Albertsson C, Mei J. The effect of low carbohydrate on energy metabolism. Int J Obes (Lond) 2005;29(Suppl 2):S26–S30. doi: 10.1038/sj.ijo.0803086. [DOI] [PubMed] [Google Scholar]