Abstract
The purpose of this study was to examine the urban-rural differences in the prevalence and associated factors with type 2 diabetes mellitus (T2DM) in Korean adults. A total of 1,060 adults >30 yr of age from urban (189 males and 331 females) and rural districts (219 males and 321 females) were recruited. Anthropometric measures, blood pressure, lipid profiles, and fasting and 2-hr after 75-g oral glucose load blood glucose were obtained. The crude- and age-standardized prevalence of T2DM was 15.4% and 14.5%, and 11.7% and 8.6% in urban and rural districts, respectively. Diabetic subjects were older and obese, and had a higher triglyceride level, and systolic blood pressure compared to non-diabetes in both population. Multivariate regression analysis revealed that older age, high triglyceride levels, central obesity, and hypertension were significantly associated with T2DM in both areas. Low monthly incomes were significantly associated with T2DM in urban population, while a family history of T2DM was significantly associated with T2DM in rural area. T2DM is more prevalent in urban than in rural population, and low economic status or genetic factor is differently associated with T2DM in both population, respectively.
Keywords: Diabetes, Prevalence, Urban, Rural
INTRODUCTION
Type 2 diabetes mellitus (T2DM) and related complications are major emerging health problems worldwide, including Korea (1-3). The World Health Organization (WHO) reports on the prevalence of T2DM warned that T2DM poses a serious threat to developing countries with respect to the existing health care system because T2DM is predicted to increase dramatically over the next 2 decades, reaching 300 million, with a tendency to increase even more in developing countries (1, 2, 4). Previous reports have suggested that the increase in T2DM in Asia differs from the increase in T2DM reported in other parts of the world; specifically, T2DM has developed in a younger age group, occurs more frequently in urban populations, and develops in a much shorter period of time (5-7). Environmental factors, such as urbanization and subsequent westernization of lifestyle, in addition to genetic susceptibility, are considered as possible etiologies for the T2DM epidemic in Asia (5-8). In this regard, the International Diabetes Federation (IDF) has recommended that interventions to prevent or delay the progression of T2DM differ in high-risk individuals based on ethnic or cultural heterogeneity (9).
In Korea, recent epidemiologic studies have revealed that the prevalence of T2DM varies (7, 10-14). Although it should be considered that there are differences in terms of the estimation time, method of diagnosis, and diagnostic criteria, environmental factors could affect the development or progression of T2DM differently between urban and rural populations, even with ethnic homogeneity (15, 16). However, little research has been conducted to identify the contemporary prevalence and associated factors of diabetes in urban and rural populations in Korea. In the present study, our aims were to elucidate the differences in prevalence and associated factors with T2DM between urban and rural populations. Our findings may serve as a basis to design necessary population-based intervention programs for disease prevention and prevention of complications of T2DM in Korea.
MATERIALS AND METHODS
Gyeongsangnam-do is a southeastern region of Korea with a temperate climate. Gyeongsangnam-do is comprised of 10 cities, 10 districts, and 314 small towns. The urban population was selected from a community located in the southeastern area of Gyeongsangnam-do, Korea, called "Gimhae-si". The rural population was chosen from a community called "Haman-gun" located in the center of Gyeongsangnam-do. The characteristics of rural life were defined to represent a livelihood related to agriculture or agrarian activities, while the livelihood of urban dwellers was primarily office work.
Selection of the study population was done with the same measures for both urban and rural areas. Two hundred fifty families were initially selected and the cube root proportional allotment was applied to minimize the standard error. From this, 1,260 and 840 families were selected from the urban and rural populations, respectively. Subjects from selected families were randomly extracted in an equal ratio of males and females and an equal distribution of the age group. The urban group was comprised of 1,105 individuals and the rural group was comprised of 858 individuals. Of those who initially participated in the study, 189 and 219 males, and 331 and 321 females from urban and rural areas, respectively, finally completed the planned survey procedures.
Only one person was selected in each family to minimize errors originating from clustering of some risk factors related to genetic predisposition, food habits, and environmental factors.
Ten investigation sites from urban and rural areas, respectively, were selected by multistage stratified cluster random sampling method in 2005. Exclusion criteria were pregnant women, and subjects with infectious diseases, malignancies, or steroid use, those who had undergone surgery within 1 month, and physically or mentally disabled subjects who were unable to understand the questionnaires and examinations.
The study team consisted of 12 survey personnel, including doctors, nurses, nursing students, and nursing aids. To standardize survey measurements and procedures, they were trained in the use of a specially prepared manual protocol. All field workers were trained and certified to administer the questionnaire, to take anthropometric and blood pressure measurements, and to draw blood. This study was performed between July and August 2006.
After informed consent was obtained, participants were administered a questionnaire which requested age, gender, monthly income, educational status, marital status, status of medical insurance, physical activity, smoking and alcohol status, and family history of diabetes and hypertension. Physical activity during work and leisure time was defined as activity that causes sweating or deep breathing, performed at least 30 min a day with a frequency of 3 times per week. A family history of diabetes was defined as having blood relatives, alive or deceased, with T2DM.
Two measurements for height, weight, and waist and hip circumference were taken with light clothing and without shoes before breakfast. Weight was measured by modern digital bathroom scales placed on a flat surface. For height, the subject stood erect, touching the occiput, back, buttocks, and heels on the wall and gazing horizontally while keeping the tragus and lateral orbital margin in the same horizontal plane. The waist circumference was measured by placing a plastic tape horizontally, midway between the 12th rib and iliac crest on the mid-axillary line. The hip circumference was measured to the nearest centimeter at the greatest protrusion of the buttocks just below the iliac crest. The body mass index (BMI) and waist-to-hip ratio (WHR) were subsequently computed. The BMI was calculated as the weight in kilograms divided by the square of the height in meters (kg/m2).
Ten minute rests before blood pressure measurement were ensured to minimize variation in blood pressure. Left arm systolic and diastolic blood pressure measurements were taken twice using a standard mercury sphygmomanometer and the stethoscope bell was placed lightly over the brachial artery, and averaged for analyses. A third measure measurement was taken only when the difference between the two measurements was >5 mmHg.
A fasting blood sample was collected from each individual for determination of metabolic profiles. All subjects maintained an overnight fast >12 hr prior to blood collection. Estimation of the fasting plasma glucose, total cholesterol, triglycerides, HDL-C, and low density lipoprotein-cholesterol was carried out. The plasma glucose level was assayed using the hexokinase enzymatic method and serum JDL-C and triglyceride levels were measured by the homogeneous assay and enzymatic method, respectively (Sigma Co., St. Louis, MO, USA). A 2-hr post-glucose measurement after a 75-g oral glucose load was taken.
Subjects with a fasting plasma glucose level ≥126 mg/dL or a 2-hr post-glucose level after a 75-g oral glucose tolerance test ≥200 mg/dL were diagnosed as T2DM. Patients who had a history of T2DM diagnosed by a physician, or patients who were under treatment with an oral hypoglycemic agent or insulin were also categorized as T2DM. With this definition, T1DM cannot be excluded. However, in Korea, as in the rest of the world, the overwhelming proportion of cases identified through this definition would have T2DM.
Statistical analysis
Data were reported as the mean and standard deviation for continuous variables and as percentages for categorical variables. For testing of significance, continuous variables were compared using an independent t-test, and categorical data were compared using a chi-squared square test. Statistical comparisons were performed using SPSS, version 12.0 software (SPSS Inc., Chicago, IL, USA). The multivariate-adjusted odd ratios (ORs) and 95% confidence intervals (CIs) are presented. All significant tests were 2-sided, and the results were considered statistically significant at a P<0.05. We used logistic regression to quantify the individual effect of predictor variables, with T2DM as a dependent variable.
Ethics statement
The protocol was approved by the Institutional Review Board of Busan Paik Hospital, Inje University (IJUBPH #05-031 and IJUBPH #06-033). Verbal consent was obtained from subjects prior to inclusion if they were illiterate and written consent was obtained from literate subjects. All subjects were informed of their right to withdraw from the study at any stage or to restrict their data from the analysis.
RESULTS
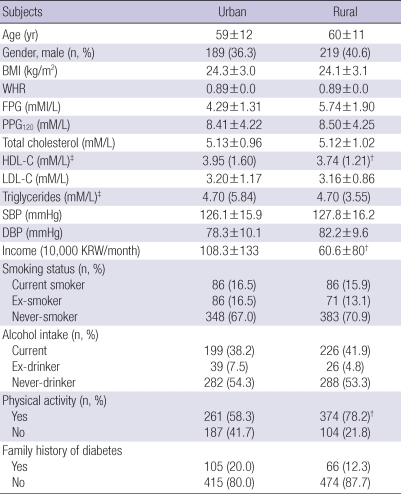
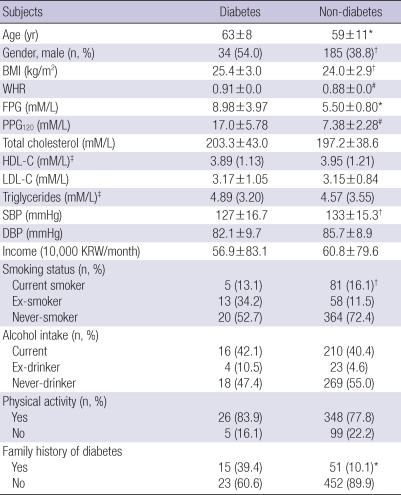
Of 1,963 subjects selected, 960 subjects participated in and completed the planned study protocol by a rate of 47% and 62% for urban and rural populations, respectively. Table 1 shows a description of the anthropometric, metabolic, and lifestyle characteristics of the 960 subjects according to the residential area. The mean ages for urban and rural subjects were 59±12 and 60±11 yr, respectively. Neither an age nor gender difference was observed in urban and rural subjects. The BMI, WHR, total cholesterol, systolic blood pressure, and diastolic blood pressure were not different between the urban and rural populations. Smoking, alcohol intake, and family history of T2DM were also similar between the two groups. Rural populations were more physically active. The serum HDL-C level and monthly income were significantly higher in the urban than rural population (3.95 vs 3.74 mM/L, P<0.01; and 108.3±133 vs 60.6±80 1,000 KRW/month, P<0.05, respectively; Table 1).
Table 1.
Characteristics of the subjects studied according to gender and urban and rural location
*P<0.01; †P<0.05. Data represent the means±SD (for normal distribution) or median (range); ‡Logarithmic transformation performed before analysis.
BMI, body mass index; WHR, waist-to-hip ratio; FPG, fasting plasma glucose; PPG120, 120-min post-challenge plasma glucose; HDL-C, high density lipoprotein-cholesterol; LDL-C, low density lipoprotein-cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; KRW, Korean Won.
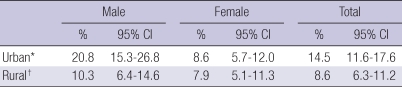
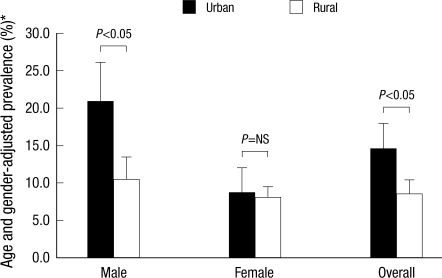
The age and gender-standardized overall prevalence of T2DM in the Korean population (in the year, 2005) was significantly higher in urban (14.5%) than in rural (8.6%) residents. There was a higher prevalence of T2DM in male subjects compared to female subjects in both populations (male vs female; 20.8% vs 8.6%, P<0.01; and 10.3% vs 7.9%, P<0.05, respectively; Table 2, Fig. 1).
Table 2.
Age- and gender-standardized prevalence of type 2 diabetes
*P<0.01; †P<0.05.
Fig. 1.
Age- and gender-standardized prevalence of type 2 diabetes.
*Prevalence rates were standardized to the age- and sex-distribution of the Korean population in 2005.
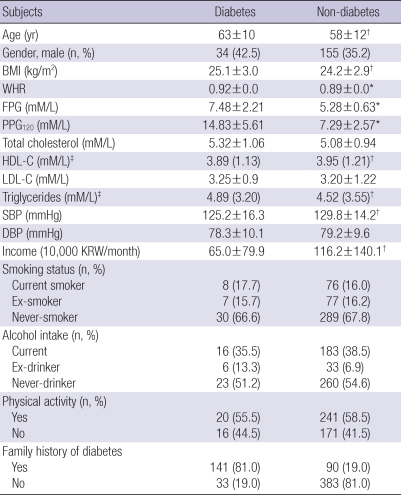
Comparisons of factors according to the presence or absence of T2DM in urban and rural populations are listed in Tables 3, 4, respectively. Diabetic subjects were older (P<0.05) and had a higher BMI (P<0.05), WHR (P<0.01), triglyceride level, and systolic BP (P<0.05) compared to non-diabetic subjects in both population. The monthly income was smaller in subjects with T2DM than those without T2DM in the urban population, which was not observed in the rural population (Table 3). In addition, HDL-C level was significantly lower among subjects with T2DM in urban compared to those without T2DM, but not in rural districts (Table 3). However, subjects with a family history of T2DM in the rural region had a higher risk of T2DM compared with those in the urban region (Table 4).
Table 3.
Comparison of selected factors according to the presence or absence of diabetes in urban population
*P<0.01; †P<0.05. Data represent the means±SD (for normal distribution) or median (range); ‡Logarithmic transformation performed before analysis.
BMI, body mass index; WHR, waist-to-hip ratio; FPG, fasting plasma glucose; PPG120, 120-min post-challenge plasma glucose; HDL-C, high density lipoprotein-cholesterol; LDL-C, low density lipoprotein-cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; KRW, Korean Won.
Table 4.
Comparison of selected factors according to the presence or absence of diabetes in rural population
*P<0.01; †P<0.05. Data represent the means±SD (for normal distribution) or median (range); ‡Logarithmic transformation performed before analysis.
BMI, body mass index; WHR, waist-to-hip ratio; FPG, fasting plasma glucose; PPG120, 120-min post-challenge plasma glucose; HDL-C, high density lipoprotein-cholesterol; LDL-C, low density lipoprotein-cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; KRW, Korean Won.
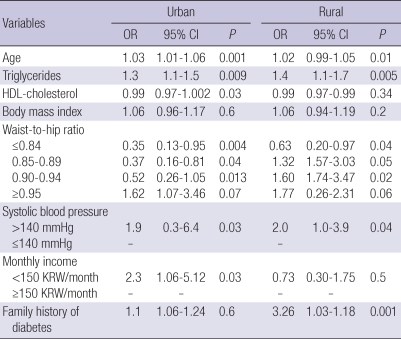
The co-variables included in logistic multivariate regression were as follows: age, WHR, BMI, triglycerides, HDL-C, monthly income, hypertension, and family history of T2DM. Age, triglycerides, WHR (i.e., visceral obesity), and hypertension were significantly associated with T2DM in both urban and rural populations. Interestingly, the risk of T2DM was 2.3-fold higher in subjects with a lower monthly income in the urban population (95% CI=1.06-5.12, P=0.03), while this association was not observed in the rural population (OR 0.73, 95% CI=0.303-1.75, P=0.5). In contrast, the prevalence of T2DM was notably higher (3.3-fold) in subjects with a family history of T2DM in the rural population (95% CI=1.03-1.18, P<0.01), an association which was not observed in the urban population (OR 1.1, 95% CI=1.06-1.24, P=0.6; Table 5).
Table 5.
Logistic regression analysis using diabetes as the dependent variables according to urban/rural location
OR, odds ratio; CI, confidence intervals.
DISCUSSION
In this study, we found a higher prevalence of T2DM in an urban population compared to a rural population in Korea. These marked urban and rural differences in the prevalence of T2DM have been reported in other developing countries (17). In agreement with a previous study, older age, hypertension, and central obesity were associated with an increasing prevalence of T2DM in Korea (7, 10-14).
Our study also confirmed that traditional risk factors for T2DM, including central obesity, were associated with T2DM in both urban and rural populations (18). However, there were some urban and rural differences in risk factors associated with T2DM. While the genetic predisposition (i.e., family history) was significantly associated with T2DM in the rural population, the factor associated with lifestyle (i.e., socioeconomic status) was significantly associated with T2DM in the urban population. Although generalized obesity appeared to be associated with T2DM, we did not observe an association between increased BMI and diabetes. It may be postulated that the obesity-related risk in our population is not similar to that found in Western population, as mentioned earlier (6). A further study is needed to clarify this finding in view of the ethnic differences and genetic predisposition in developing T2DM.
The prevalence of T2DM has increased substantially in Korea. Indeed, a previous study reported that the age- and gender-adjusted prevalence of diabetes increased approximately 71% over a 6 yr period (from 6.9% in 1997 to 11.8% in 2003) (13). Several previous studies, which were based on rural populations, reported that prevalence of T2DM in Korea was 7.2% in 1993 and 7.9% in 1997 (19, 20). In agreement with this increasing trend, the prevalence of T2DM was estimated to be 8.4% in 2005 in the current study. The prevalence of T2DM in the rural population in this study was similar to that in Japan (9.1% for men and 10.8% for women in 2000) (21). Along with a surge in economic growth, a westernized lifestyle and a change in the socio-cultural environment have contributed to this epidemic. However, these factors interact with other risk factors, such as genetic predisposition, obesity, and increase in the aging population, differently between ethnic or racial groups, or even within the same groups with different environments (15, 16, 22). In a recent study in Korea (7), a family history of diabetes, low socioeconomic status, older age, sedentary lifestyle, and central obesity were identified as risk factors for diabetes. However, the study did not compare the risk factors between urban and rural populations to identify contributing factors associated with urbanization.
This urban and rural comparison study indicates the consequences of accumulating conventional risk factors for T2DM. This effect has been attributed to a sedentary lifestyle and dietary changes that occur with rapid westernization (23). Since most urban populations lead a sedentary lifestyle compared to rural populations, this difference in lifestyle could be one reason why the urban population had a lower HDL-C than the rural population. In support of this notion, the prevalence of T2DM was much higher among males than females (20.8% vs 8.6%) in the urban population. It seems reasonable to argue that differences in lifestyle habits play a crucial role in the increased prevalence of T2DM, even in the urban population. In the current study, the presence of a family history of T2DM did not reach statistical significance as a risk factor in the urban population. A change in lifestyle may interfere with genetic susceptibility for T2DM. However, in recent studies involving the rural populations in developing countries, the risk factors for T2DM varied from those for the urban population (24, 25). Therefore, the validity of each risk factor for the urban or rural population needs to be assessed. Our study has focused solely on risk factors concerning urban and rural populations in Korea. Slight differences among the prevalence of T2DM reported in Korea are probably accounted for by differences in population characteristics, such as age, regions and dates studied, urbanization, and diagnostic criteria of T2DM (7, 10-13). Thus, reliable nationwide data about the prevalence of T2DM and the differences between urban and rural populations are not available.
In developing countries, the prevalence of T2DM is higher in urban than rural populations, while in developed countries the prevalence of T2DM is higher in rural that urban populations (26, 27). In developing countries, T2DM is associated with high socioeconomic status (28); however, low socioeconomic status has been reported to be an independent risk factor for T2DM in another study (29). Conventional risk factors are also higher or more frequent among subjects with low socioeconomic status who have more limited access to the health care system (29). Thus, the urban and rural differences in the prevalence of T2DM are not universal. Indeed, data from previous epidemiologic studies in Korea support this diversity, as mentioned above (7, 10-14). Clearly, population-based effective public health measures are needed to enhance the primary prevention, detection, and treatment of T2DM in urban and rural populations.
Another interesting observation in this study was that low socioeconomic status had a significant association with T2DM only in the urban population, whereas a positive family history of T2DM was independently associated with T2DM only in the rural population. However, we could not identify other risk factors, such as physical inactivity or educational status, associated with T2DM, which are consequences of economic transition and urbanization.
The present study provides the first population-based estimates of the prevalence of T2DM and difference in associated factors between urban and rural populations in Korea. However, caution should be taken to generalize the results because of the limitation of the small sample size, inter-regional and intra-regional heterogeneity of socio-economic status and intensity of labor, and cross-sectional nature of this study, by which this study is not representative of the entire Korean population. Thus, through this study design, it is not possible to identify the risk factors affecting the urban and rural difference in the prevalence of T2DM. Longitudinal studies involving rural and urban cohorts would be useful to examine the association between conventional risk factors for T2DM as a function of time.
In conclusion, T2DM is more prevalent in urban than rural population, and low economic and genetic factors are urbanrural differences of risk factors associated with T2DM between both populations. These findings have important implications for targeting efforts to develop and implement prevention intervention programs for T2DM with different measures between urban and rural populations in Korea. To combat the diabetic epidemic in Korea, interventions on lifestyle modification should be addressed in the urban populations with low economic status and rural populations with a family history of T2DM. This report is the first study to identify the differences in risk factors for T2DM across the urban and rural districts in Korea.
ACKNOWLEDGMENTS
We acknowledge the survey team members involved in facilitating the work reported herein through fieldwork and technical assistance. We express our gratitude to Gye Oh Lee, Professor of Department of Statistics at Hannam University and Consultant to the Korean Gallup Organization, for his help in sampling subjects. Most importantly we thank all of the participants of this study for their active cooperation.
Footnotes
This work was supported by Health Promotion Funds from the Korean Ministry for Health, Welfare, and Family Affairs, 2005 and Grant from Inje University, 2010.
References
- 1.Ko M, Kim MT, Nam JJ. Assessing risk factors of coronary heart disease and its risk prediction among Korean adults: the 2001 Korea National Health and Nutrition Examination Survey. Int J Cardiol. 2006;110:184–190. doi: 10.1016/j.ijcard.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 2.Roglic G, Unwin N, Bennett PH, Mathers C, Tuomilehto J, Nag S, Connolly V, King H. The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care. 2005;28:2130–2135. doi: 10.2337/diacare.28.9.2130. [DOI] [PubMed] [Google Scholar]
- 3.Cho M, Park JS, Nam J, Kim CS, Nam JH, Kim HJ, Ahn CW, Cha BS, Lim SK, Kim KR, Lee HC, Huh KB. Association of Abdominal Obesity with Atherosclerosis in Type 2 Diabetes Mellitus (T2DM) in Korea. J Korean Med Sci. 2008;23:781–788. doi: 10.3346/jkms.2008.23.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King H, Rewers M WHO Ad Hoc Diabetes Reporting Group. Global estimates for prevalence of diabetes mellitus and impaired glucose tolerance in adults. Diabetes Care. 1993;16:157–177. doi: 10.2337/diacare.16.1.157. [DOI] [PubMed] [Google Scholar]
- 5.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 6.Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, Zimmet P, Son HY. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 7.Kim SM, Lee JS, Lee J, Na JK, Han JH, Yoon DK, Baik SH, Choi DS, Choi KM. Prevalence of diabetes and impaired fasting glucose in Korea: Korean National Health and Nutrition Survey 2001. Diabetes Care. 2006;29:226–231. doi: 10.2337/diacare.29.02.06.dc05-0481. [DOI] [PubMed] [Google Scholar]
- 8.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 9.IDF Clinical Guidelines Task Force. Global Guideline for Type 2 Diabetes: recommendations for standard, comprehensive, and minimal care. Diabet Med. 2006;23:579–593. doi: 10.1111/j.1464-5491.2006.01918.x. [DOI] [PubMed] [Google Scholar]
- 10.Choi YJ, Kim HC, Kim HM, Park SW, Kim J, Kim DJ. Prevalence and management of diabetes in Korean adults: Korea National Health and Nutrition Examination Surveys 1998-2005. Diabetes Care. 2009;32:2016–2020. doi: 10.2337/dc08-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi YJ, Cho YM, Park CK, Jang HC, Park KS, Kim SY, Lee HK. Rapidly increasing diabetes-related mortality with socio-environmental changes in South Korea during the last two decades. Diabetes Res Clin Pract. 2006;74:295–300. doi: 10.1016/j.diabres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 12.Park KS. Prevention of type 2 diabetes mellitus from the viewpoint of genetics. Diabetes Res Clin Pract. 2004;66(Suppl 1):S33–S35. doi: 10.1016/j.diabres.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Song KH, Nam-Goomg IS, Han SM, Kim MS, Lee EJ, Lee YS, Lee MS, Yoon S, Lee KU, Park JY. Change in prevalence and 6-year incidence of diabetes and impaired fasting glucose in Korean subjects living in a rural area. Diabetes Res Clin Pract. 2007;78:378–384. doi: 10.1016/j.diabres.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Qiao Q, Hu G, Tuomilehto J, Nakagami T, Balkau B, Borch-Johnsen K, Ramachandran A, Mohan V, Iyer SR, Tominaga M, Kiyohara Y, Kato I, Okubo K, Nagai M, Shibazaki S, Yang Z, Tong Z, Fan Q, Wang B, Chew SK, Tan BY, Heng D, Emmanuel S, Tajima N, Iwamoto Y, Snehalatha C, Vijay V, Kapur A, Dong Y, Nan H, Gao W, Shi H, Fu F DECODA Study Group. Age- and sex-specific prevalence of diabetes and impaired glucose regulation in 11 Asian cohorts. Diabetes Care. 2003;26:1770–1780. doi: 10.2337/diacare.26.6.1770. [DOI] [PubMed] [Google Scholar]
- 15.McNeely MJ, Boyko EJ. Type 2 diabetes prevalence in Asian Americans: results of a national health survey. Diabetes Care. 2004;27:66–69. doi: 10.2337/diacare.27.1.66. [DOI] [PubMed] [Google Scholar]
- 16.Singh GK, Hiatt RA. Trends and disparities in socioeconomic and behavioural characteristics, life expectancy, and cause-specific mortality of native-born and foreign-born populations in the United States, 1979-2003. Int J Epidemiol. 2006;35:903–919. doi: 10.1093/ije/dyl089. [DOI] [PubMed] [Google Scholar]
- 17.Ramachandran A, Snehalatha C, Dharmaraj D, Viswanathan M. Prevalence of glucose intolerance in Asian Indians. Urban-rural difference and significance of upper body adiposity. Diabetes Care. 1992;15:1348–1355. doi: 10.2337/diacare.15.10.1348. [DOI] [PubMed] [Google Scholar]
- 18.Heikes KE, Eddy DM, Arondekar B, Schlessinger L. Diabetes Risk Calculator: a simple tool for detecting undiagnosed diabetes and pre-diabetes. Diabetes Care. 2008;31:1040–1045. doi: 10.2337/dc07-1150. [DOI] [PubMed] [Google Scholar]
- 19.Park Y, Lee H, Koh CS, Min H, Yoo K, Kim Y, Shin Y. Prevalence of diabetes and IGT in Yonchon County, South Korea. Diabetes Care. 1995;18:545–548. doi: 10.2337/diacare.18.4.545. [DOI] [PubMed] [Google Scholar]
- 20.Park JY, Kim YI, Choi CS, Chung YE, Kim SW, Lee MS, Lee SI, Hong SK, Lee KU. Prevalence of diabetes, impaired glucose tolerance, and impaired fasting glucose in a rural population of Korea, according to 1997 American Diabetes Association and 1985 World Health Organization criteria. Diabetes Care. 2000;23:707–708. doi: 10.2337/diacare.23.5.707. [DOI] [PubMed] [Google Scholar]
- 21.Sekikawa A, Eguchi H, Tominaga M, Igarashi K, Abe T, Manaka H, Sasaki H, Fukuyama H, Kato T, Kiyohara Y, Fujishima M. Prevalence of type 2 diabetes mellitus and impaired glucose tolerance in a rural area of Japan. The Funagata diabetes study. J Diabetes Complications. 2000;14:78–83. doi: 10.1016/s1056-8727(00)00074-x. [DOI] [PubMed] [Google Scholar]
- 22.Sone H, Mizuno S, Ohashi Y, Yamada N. Type 2 diabetes prevalence in Asian subjects. Diabetes Care. 2004;27:1251–1252. doi: 10.2337/diacare.27.5.1251. [DOI] [PubMed] [Google Scholar]
- 23.McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. 1991;337:382–386. doi: 10.1016/0140-6736(91)91164-p. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt MI, Duncan BB, Bang H, Pankow JS, Ballantyne CM, Golden SH, Folsom AR, Chambless LE Atherosclerosis Risk in Communities Investigators. Identifying individuals at high risk for diabetes: The Atherosclerosis Risk in Communities study. Diabetes Care. 2005;28:2013–2018. doi: 10.2337/diacare.28.8.2013. [DOI] [PubMed] [Google Scholar]
- 25.Ramachandran A, Snehalatha C, Baskar AD, Mary S, Kumar CK, Selvam S, Catherine S, Vijay V. Temporal changes in prevalence of diabetes and impaired glucose tolerance associated with lifestyle transition occurring in the rural population in India. Diabetologia. 2004;47:860–865. doi: 10.1007/s00125-004-1387-6. [DOI] [PubMed] [Google Scholar]
- 26.McDermott R, Rowley KG, Lee AJ, Knight S, O'Dea K. Increase in prevalence of obesity and diabetes and decrease in plasma cholesterol in a central Australian aboriginal community. Med J Aust. 2000;172:480–484. doi: 10.5694/j.1326-5377.2000.tb124071.x. [DOI] [PubMed] [Google Scholar]
- 27.Dunstan DW, Zimmet PZ, Welborn TA, De Courten MP, Cameron AJ, Sicree RA, Dwyer T, Colagiuri S, Jolley D, Knuiman M, Atkins R, Shaw JE. The rising prevalence of diabetes and impaired glucose tolerance: the Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care. 2002;25:829–834. doi: 10.2337/diacare.25.5.829. [DOI] [PubMed] [Google Scholar]
- 28.Vijayakumar G, Arun R, Kutty VR. High prevalence of type 2 diabetes mellitus and other metabolic disorders in rural Central Kerala. J Assoc Physicians India. 2009;57:563–567. [PubMed] [Google Scholar]
- 29.Guize L, Jaffiol C, Guéniot M, Bringer J, Giudicelli C, Tramoni M, Thomas F, Pannier B, Bean K, Jego B. Diabetes and socio-economic deprivation. A study in a large French population. Bull Acad Natl Med. 2008;192:1707–1723. [PubMed] [Google Scholar]