Abstract
Antibiotic-associated diarrhea (AAD) is a common complication of antibiotic use. There is growing interest in probiotics for the treatment of AAD and Clostridium difficile infection because of the wide availability of probiotics. The aim of this multicenter, randomized, placebo-controlled, double-blind trial was to assess the efficacy of probiotic Lactobacillus (Lacidofil® cap) for the prevention of AAD in adults. From September 2008 to November 2009, a total of 214 patients with respiratory tract infection who had begun receiving antibiotics were randomized to receive Lactobacillus (Lacidofil® cap) or placebo for 14 days. Patients recorded bowel frequency and stool consistency daily for 14 days. The primary outcome was the proportion of patients who developed AAD within 14 days of enrollment. AAD developed in 4 (3.9%) of 103 patients in the Lactobacillus group and in 8 (7.2%) of 111 patients in the placebo group (P=0.44). However, the Lactobacillus group showed lower change in bowel frequency and consistency (50/103, 48.5%) than the placebo group (35/111, 31.5%) (P=0.01). Although the Lacidofil® cap does not reduce the rate of occurrence of AAD in adult patients with respiratory tract infection who have taken antibiotics, the Lactobacillus group maintains their bowel habits to a greater extent than the placebo group.
Keywords: Probiotics, Lactobacillus, Antibiotic-associated Diarrhea
INTRODUCTION
Antibiotic-associated diarrhea (AAD) is emerging as a common complication of antibiotic use. The frequency of AAD can be high (26-60%) during hospital outbreaks or intermediate (13-29%) during endemic periods and is relatively low in outpatient settings (usually less than 0.1%) (1, 2). Risk factors for AAD include the use of broad-spectrum antibiotics, various host factors (old age, poor general condition), a longer hospitalization period and exposure to nosocomial pathogens (1-3). AAD can occur 2 to 8 weeks after exposure to antibiotics as a result of disruption of intestinal microflora. One of the main roles of normal gut microflora is to protect against colonization by intestinal pathogens (4). Once this protective barrier is broken, patients are more susceptible to infection with opportunistic pathogens. Probiotics have been proposed to treat AAD and Clostridium difficile disease. Probiotics mainly assist in re-establishing the disrupted intestinal microflora, enhancing immune responses and clearing pathogens and their toxins from the host (5-7).
There is growing interest in probiotics for the treatment of AAD and C. difficile disease because of the wide availability of probiotics as dietary supplements and concern over recent outbreaks of severe C. difficile disease in Canada and the United Kingdom (8, 9). Research on probiotics has been reported for the past 28 yr (1977-2005), but the studies have been variable in trial design, type of probiotic and dosage and duration of treatment, and thus have often yielded contradictory results (10). Twenty-five randomized, controlled trials (RCTs) provided adequate data regarding the efficacy of probiotics for the prevention of AAD (10). Of the 25 RCTs, only 6 utilized Lactobacillus rhamnosus (11-16).
There were a few RCTs that proved the efficacy of Lactobacillus in preventing AAD, and debate arose regarding the study populations. A study on the use of L. rhamnosus (2×1010 colonforming units per day) for preventing AAD in 302 adults receiving antibiotics at the Mayo Clinic showed no efficacy of L. rhamnosus in decreasing the overall incidence of AAD because quite a few patients had stool cultures, additional tests for diarrhea, or a positive diagnosis of C. difficile (15). However, 89 patients in a recent RCT on the daily administration of a Lactobacillusfermented milk product showed that it was safe and effective in the prevention of AAD (17). A recent RCT involving 135 patients demonstrated that a probiotic Lactobacillus preparation prevented AAD and C. difficile-associated diarrhea (18).
Therefore, we conducted a multicenter, randomized, placebo-controlled, double-blind trial to assess the efficacy of probiotic Lactobacillus (Lacidofil® cap) for the prevention of AAD in Korean adults.
MATERIALS AND METHODS
Participants
Adult patients receiving antibiotic therapy for respiratory tract infection were screened at 10 tertiary hospitals. The inclusion criteria were adult inpatients aged >18 yr who received oral or injected antibiotics for respiratory tract infections within 48 hr of enrollment. The following patients were excluded from the study: 1) those who were diagnosed with C. difficile colitis within the previous 3 months, 2) those who were given tube feeding or who underwent an ileostomy or colostomy, 3) those with basal diarrheal disease (acute enteritis, inflammatory bowel disease, radiation enteritis, ischemic colitis and diarrhea caused by carcinoid), 4) those receiving other probiotics during the previous 15 days, 5) those treated with immunosuppressant drugs and those with immune deficiency, 6) those who underwent radiotherapy or chemotherapy treatment for cancer, 7) those treated with antidiarrheal, antispasmodic or motility agents for other diseases, 8) pregnant or lactating women, 9) those who underwent gastrointestinal surgery 3 months prior to the study, 10) those with a history of hypersensitivity to cephalosporins, penicillin or clavulanic acid, 11) those with verified diabetic autonomic neuropathy, 12) those who underwent organ transplantations, and 13) those with underlying conditions or diseases who, in the opinion of the investigator, were unsuitable for inclusion.
Study design
Between September 2008 and November 2009, a total of 214 patients with respiratory tract infection who had begun to receive antibiotics were randomly allocated to receive Lactobacillus (Lacidofil® cap) or a placebo for 14 days. This study was conducted at 10 investigational centers. Participants recorded their bowel frequency and stool consistency every day for 14 days. The primary outcome (AAD-1) was defined as loose or watery stools more than 3 times per day for at least 2 days within 14 days of enrollment. The secondary outcome (AAD-2) was defined as loose or watery stools more than 2 times per day for at least 2 days within 14 days of enrollment.
The admitting medical team identified eligible patients who had an antibiotic prescription for the treatment of respiratory tract infection. After the investigators obtained informed consent, they collected baseline data and prescribed the study drug on a randomized basis. The hospital pharmacy dispensed the drug. The treatment group received a probiotic preparation (Lacidofil® cap) containing L. rhamnosus R0011·L. acidophilus R0052 bacterial culture (2×109 colony-forming units), maltodextrin, Mg stearate, and ascorbic acid. The control group received a placebo drug composed of maltodextrin, Mg stearate and ascorbic acid. Participants began using the Lacidofil® cap or placebo drug within 48 hr of initiation of antibiotic therapy. The Lacidofil® cap and placebo were administrated at a dose of 1 capsule twice a day for 14 days. The administration of other drugs for accompanying diseases permitted subject to the investigator's judgment. The use of antidiarrheal (e.g., loperamide, polypcarbophil), antispasmodic (e.g., tiropramide, pinaverium, buscopan) or motility agents (e.g., domperidone, mosapride, itopride, levosulpride) that could affect the symptoms was prohibited. These drugs were withheld until the patient developed AAD, after which their use was allowed.
Investigators followed up participants for 14 days to check stool frequency, stool consistency and compliance. If participants had AAD, investigators collected further data on clinical symptoms and blood test results. As the safety of Lacidofil® cap has already been proven, its safety was not evaluated in this study.
Sample size
We estimated that the minimum sample size was 220 for each group. The sample size was calculated on the basis of an 18% difference in the proportion of AAD compared with the control group with an expected compliance rate of 80%, a statistical power (two sided) of 90%, a significance level of 5% and a dropout rate of 10%.
Randomization and double blinding
Among patients with respiratory tract infection, those who met the inclusion criteria were selected and allocated trial numbers. The subjects were allocated to the treatment (Lacidofil® cap treatment) or control groups (placebo) according to their trial numbers. To maintain double-blinding, the envelope containing the random trial number allocation code was sealed by the investigators, and the treatment allocations were not disclosed until the clinical trial was completed, except for cases for which access to the code was necessary. All investigators, participants, outcome assessors and data analysts were blinded throughout the study.
Statistical analysis
SPSS for Windows software (V. 13.0, SPSS, Chicago, IL, USA) was used for statistical analysis. The chi-square test was used to determine the difference in the incidence of AAD between the 2 groups, and the Student's t-test was used for analysis of continuous variables. Multiple logistic regression analysis was used to identify the risk factors for AAD, and effect estimates are presented as the odds ratio (OR) and 95% confidence interval (CI). For all analyses, a P value of 0.05 or less (two sided) was considered significant.
Ethics statement
The Institutional Review Board (IRB) of each hospital approved the study protocol (Ewha Womans University Mokdong Hospital, IRB No. 187-17). Written informed consent was obtained from each patient.
RESULTS
Participant flow and baseline characteristics of participants
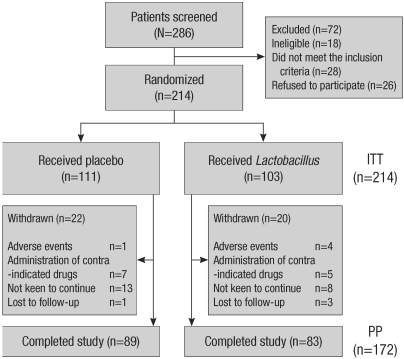
Patient flow is summarized in Fig. 1. Over the 14-month study period, 286 patients were screened as potential candidates for this study by nurses. Of the 286 patients screened, 18 met 1 or more of the exclusion criteria, 28 did not meet all of the inclusion criteria, and 26 refused to participate in the study. Of the 214 patients who met the inclusion criteria and agreed to participate in the study, 42 failed to complete the study. Finally, 172 patients completed the study. Of the 42 patients who failed to complete the study, 5 had adverse events, 12 received contraindicated drugs, 21 did not continue the treatment protocol, and 4 were lost to follow-up. We performed intention-to-treat (ITT) analysis of data from 214 patients and per-protocol (PP) analysis of data from 172 patients.
Fig. 1.
Patient flow diagram.
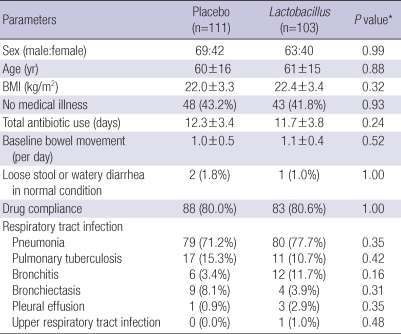
Baseline characteristics are summarized in Table 1. The placebo (n=111) and the Lactobacillus groups (n=103) were similar in terms of their demographics and medical profiles at enrollment. There were no significant differences in total dose of antibiotics, baseline bowel movement, drug compliance, or pulmonary infections between the 2 groups. Respiratory tract infections, in descending order of frequency, were pneumonia, pulmonary tuberculosis, bronchitis, bronchiectasis, pleural effusion and upper respiratory tract infection.
Table 1.
Baseline characteristics of study patients
*χ2 or Fisher exact test.
BMI, body mass index.
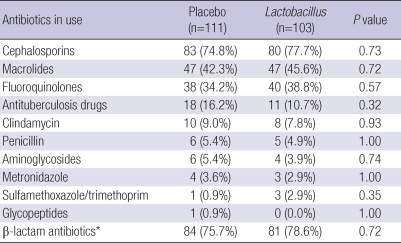
Antibiotic use for both groups is summarized in Table 2. The antibiotics used were, in descending order, cephalosporin, macrolides, fluoroquniolones, antituberculosis drugs, clindamycin and penicillin. β-lactam antibiotics, including cephalosporins and penicillin, were commonly used in both the placebo and the Lactobacillus groups (75.7% vs 78.6%) (P=0.72). There were no significant differences in antibiotic use between the 2 groups.
Table 2.
Antibiotics use in the placebo and the Lactobacillus groups
*Cephalosporins plus penicillin.
Analysis of outcome measures
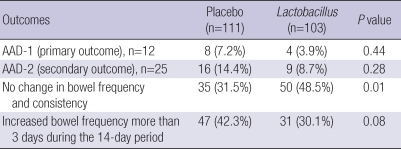
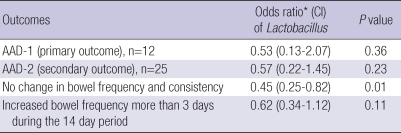
In ITT analysis (n=214), AAD-1 developed in 4 (3.9%) of 103 patients in the Lactobacillus group and in 8 (7.2%) of 111 patients in the placebo group (P=0.44). AAD-2 developed in 9 (8.7%) of 103 patients in the Lactobacillus group and in 16 (14.4%) of 111 patients in the placebo group (P=0.28). There was no significant difference in AAD occurrence between the 2 groups. However, no changes in bowel frequency or consistency were reported by 50 (48.5%) of 103 patients in the Lactobacillus group and by 35 (31.5%) of 111 patients in the placebo group (P=0.01). Increased bowel frequency over a period of more than 3 days during the 14-day period was observed in 47 (42.3%) of 111 patients in the placebo group and 31 (30.1%) of 103 patients in the Lactobacillus group (P=0.08) (Table 3). The results of multivariate analysis of the OR of Lactobacillus for AAD-1, AAD-2, and change in bowel frequency and consistency are shown in Table 4. The Lactobacillus group was less likely to experience changes in bowel frequency and consistency compared with the placebo group (OR 0.43, 95% CI 0.25-0.82), which means that although Lacidofil® cap does not reduce the rate of occurrence rate of AAD in adult patients with respiratory tract infection who have taken antibiotics, the Lactobacillus group maintained their bowel habits to a greater extent than the placebo group.
Table 3.
Comparison of AAD between the placebo and the Lactobacillus groups (ITT analysis, n=214)
Table 4.
Odds ratio of Lactobacillus (ITT analysis, n=214)
*Logistic analysis: adjusted for age, baseline bowel movement, medical illness, duration of total antibiotic use, and remnant drugs.
CI, confidence interval.
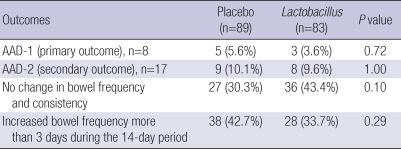
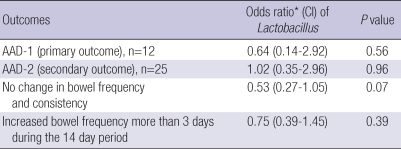
In PP analysis (n=172, excluding 42 patients who dropped out), AAD-1 developed in 3 (3.6%) of 83 patients in the Lactobacillus group and in 5 (5.6%) of 89 patients in the placebo group (P=0.72). AAD-2 developed in 8 (9.6%) of 103 patients in the Lactobacillus group and in 9 (10.1%) of 89 patients in the placebo group (P=1.00). However, normal bowel movement was reported by 36 (43.4%) of 83 patients in the Lactobacillus group and 27 (30.3%) of 89 patients in the placebo group (P=0.10) (Table 5). The results of multivariate analysis of the OR of Lactobacillus for AAD-1, AAD-2, and change in bowel frequency and consistency are shown in Table 6. The OR for no change in bowel frequency and consistency was 0.53 (95% CI 0.27-1.05, P=0.07).
Table 5.
Comparison of AAD between the placebo and the Lactobacillus groups (PP analysis, n=172)
Table 6.
Odds ratio of Lactobacillus (PP analysis, n=172)
*Logistic analysis: adjusted for age, baseline bowel movement, medical illness, and duration of total antibiotic use.
CI, confidence interval.
Risk factors for AAD
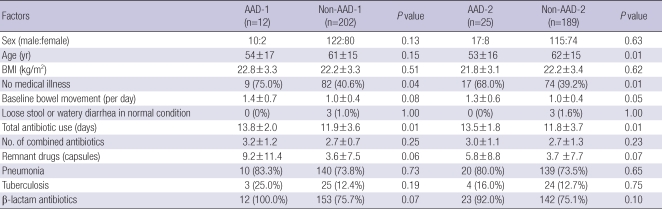
The results of ITT analysis (n=214) of risk factors for AAD-1 (n=12) and non-AAD-1 (n=202) are shown in Table 7. AAD-1 was more prevalent in patients with no medical illness (75.0% vs 40.6%) (P=0.04) and those with a longer duration of antibiotic use (13.8±2.0 vs 11.9±3.6 days) (P=0.01). There was also a tendency for AAD-1 patients to have greater baseline bowel movement (1.4±0.7 vs 1.0±0.4 per day) (P=0.08), remnant drugs (9.2±11.4 vs 3.6±7.5 capsules) (P=0.06) or to use β-lactam antibiotics (100% vs 75.7%) (P=0.07). AAD-2 was more prevalent in patients who were younger (53±16 vs 62 ±15 yr) (P=0.01), had no medical illness (68.0% vs 39.2%) (P=0.01) or had a longer duration of antibiotic use (13.5±1.8 vs 11.8±3.7 days) (P=0.01). In addition, patients who developed AAD-2 had a tendency to have greater baseline bowel movement (1.3±0.6 vs 1.0±0.4 per day) (P=0.05), remnant drugs (5.8±8.8 vs 3.7±7.7 capsules) (P=0.07), or to use β-lactam antibiotics (92.0% vs 75.1%) (P=0.10).
Table 7.
Risk factors for AAD (ITT analysis, n=214)
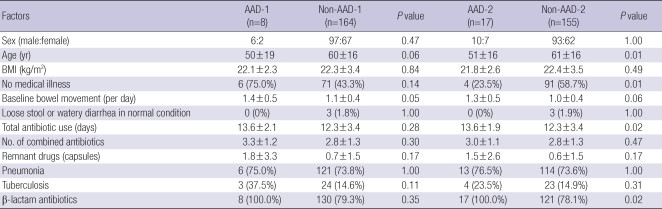
The results of PP analysis (n=172) of the risk factors for AAD-1 (n=8) and non-AAD-1 (n=164) are shown in Table 8. AAD-1 tended to occur in patients who were younger (50±19 vs 60±16 yr) (P=0.06), or had elevated baseline bowel movement (1.4±0.5 vs 1.0±0.4 per day) (P=0.05). AAD-2 was more prevalent in patients who were young (51±16 vs 61±16 yr) (P=0.01), had a medical illness (23.5% vs 58.7%) (P=0.01), had a longer duration of antibiotic use (13.6±1.9 vs 12.3±3.4 days) (P=0.02) or use β-lactam antibiotics (100% vs 78.1%) (P=0.02). In addition, patients with AAD-2 had a tendency to have an increased baseline bowel movement (1.3±0.5 vs 1.0±0.4 per day) (P=0.06).
Table 8.
Risk factors for AAD (PP analysis, n=172)
Adverse events
Mild abdominal discomfort was reported by 1 patient (0.9%) in the placebo group and by 3 patients (2.9%) in the Lactobacillus group (P=0.35). Skin eruption was reported by 1 patient (0.97%) in the Lactobacillus group. However, there were no significant adverse events associated with the use of Lacidofil® cap.
DISCUSSION
This study evaluated the efficacy of a Lactobacillus probiotic single-agent regimen for AAD. In our study, the number of AAD cases was not statistically different between the 2 groups and the prevalence of AAD was low (3.9-7.2%) compared with a previous report (2-25%) as assessed by ITT analysis (2). This result may be attributed to the short-term follow-up period because AAD may occur up to 2 months after stopping antibiotic treatment (1). Recent meta-analysis of 10 randomized, blinded, placebo-controlled trials showed that the combined risk ratio (RR) of developing AAD was significantly lower in the Lactobacillus groups than in the placebo group (RR 0.35, 95% CI 0.19-0.67) (19). Large differences exist among trials, including our study. The indications for antibiotic therapy differed among the studies and included respiratory tract infection, otitis media, urinary tract infection and Helicobacter pylori infection (19).
According to a previous report, doses used in Lactobacillus regimens range from 2×109 to 4×1010 colony-forming units per day (19). There was considerable variation in the probiotic regimens used. It has been suggested that doses of probiotics should exceed 1010 colony-forming units per day (10). In our study, the dose of the Lactobacillus regimen was 4×109 colony-forming units. This may be the reason why the Lactobacillus group preparation did not exhibit a protective effect for AAD. Thus, it is expected that if the does had been greater (>1010 colony-forming units per day), it may have had a preventive effect on AAD. The effects of increasing dosages of probiotics should be monitored, irrespective of whether adverse events are expected. Further evaluations using standardized Lactobacillus dosage forms and regimens are warranted.
AAD was defined as loose or watery stools more than 3 times per day for at least 2 days. This stringent definition enabled us to differentiate between clinically relevant and clinically unimportant changes in the consistency of stools. The secondary outcome, AAD-2, was defined as loose or watery stools more than 2 times per day for at least 2 days. However, definitions of AAD vary between published studies. For example, Vanderhoof et al. defined diarrhea as ≥2 liquid stools per 24 hr on ≥2 days (13). Bowel frequency and consistency reflects bowel habits. Tables 3 and 4 showed that there were significant differences in bowel frequency and consistency. These results suggest that maintenance of usual bowel habit was promoted by the probiotic. This result is consistent with that of a previous trial (20) performed on children. Although the overall incidence of diarrhea was surprisingly low and the administration of a combination of Bifidobacterium longum PL03, L. rhamnosus KL53A, and L. plantarum PL02 did not significantly alter the incidence of diarrhea, it reduced the daily frequency of stools (20).
In our study, ITT (n=214) analysis gave better results than PP (n=172) analysis. Forty-two patients did not complete the study. Thus, the number of patients enrolled was less than that required to achieve the target statistical power for the PP analysis. Studies involving larger numbers of patients are necessary. Regarding risk factors, patients who had elevated baseline bowel movements, more remnant drugs, and greater use of β-lactam antibiotics had higher incidences of AAD-1 and AAD-2. These results show that patients with elevated bowel movement and low drug compliance had a higher incidence of AAD. However, increased bowel movements may have affected the underlying risk for development of AAD or their response to the probiotic. Thus, for analysis of the OR of Lactobacillus (Tables 4, 6), we adjusted the data for baseline bowel movement and other confounding factors (age, medical illness, duration of total antibiotic use, and remnant drugs). β-lactam antibiotics, including penicillin and cephalosporins, are proven risk factors for AAD (1). In addition, AAD was more prevalent in subjects who were young, had no medical illness and had used antibiotics for a long period. Our result is not in accord with that of a previous report (1). The reason for this may be selection bias in our study.
L. rhamnosus has inhibitory activity against a wide range of bacteria, including C. difficile (21). In addition, L. rhamnosus is expected to be useful for the prevention of AAD because it survives the digestive process and is not killed by the acidic pH of the stomach or by bile (22). When administered exogenously, L. rhamnosus persists in the colon for at least a week and modifies the colonic environment with potential positive health effects (23, 24). There are 3 reports showing L. rhamnosus prevents AAD in children (12-14). For adults, 2 reports have been published concerning the synergistic effect of L. rhamnosus with anti-H. pylori eradication therapy (11, 16). The rationale for the use of probiotics is that the use of antibiotics may result in a disturbance of the normal intestinal microflora, which is one of the key factors in the pathogenesis of AAD and C. difficile infection (25). There are several possible mechanisms by which probiotics, including Lactobacillus, exert preventative effects on AAD. These include the synthesis of antimicrobial substances (17, 22, 26), competition for nutrients required for the growth of pathogens, competitive inhibition of adhesion of pathogens, and modification of toxins or toxin receptors (27).
Concerns about the safety of probiotics have been raised because probiotics are living organisms that, when given to ill patients, could elicit adverse reactions. As some intestinal bacteria have been shown to migrate from the intestine to other organs, antibiotic-resistant gene acquisition is also a potential concern (10). Although bacteremia and fungemia have been reported in the literatures (28, 29), our study showed only mild abdominal discomfort in 1 patient and skin eruption in 3 patients without any significant adverse events. Caution should be exercised for patients who are severely ill and are receiving nutrition or antibiotics through a potentially open portal catheter or a nasogastric tube.
Our study had some limitations. First, the incidence of AAD was much lower in our study than in previous studies. Our patients were followed up for only 2 weeks after antibiotic therapy. As AAD can occur up to 2 months after stopping antibiotic treatment (1), some cases might have been missed. Second, the patients were not normally distributed. Some centers recruited more patients than allocated, and others had fewer cases. This imbalance was caused by differences in the hospital size and location, and the incidence of antibiotic-naïve respiratory infections at the hospitals. Third, the difference between hospitals in the main antibiotic prescribed is a potential weakness because the incidence of AAD differs between groups of antibiotics. However, there was no significant difference in antibiotic use between the 2 groups. Finally, although the required sample size was 220, we performed the study using 214 patients. The most frequent limitation of previous studies may also have been insufficient power to detect significant differences (19). Few investigators have calculated required sample sizes, and 3 investigators reported that slow recruitment of study patients resulted in premature termination of the trial (10, 30). However, our study has value as the first prospective, randomized, double-blind, multicenter study on the effect of probiotic Lactobacillus for the prevention of AAD in Korea.
In conclusion, although Lacidofil® cap does not reduce the occurrence of AAD in adult patients with respiratory tract infection who have taken antibiotics, the Lactobacillus group maintains their bowel habits to a greater extent than the placebo group without any significant adverse events.
Footnotes
This study was supported by the research fund of the Korean Association for the Study of Intestinal Diseases. There was no conflict of interest between the Lacidofil® cap pharmaceutical company (Pharmbio Korea Co., Ltd.) and this study.
References
- 1.McFarland LV. Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Dig Dis. 1998;16:292–307. doi: 10.1159/000016879. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346:334–339. doi: 10.1056/NEJMcp011603. [DOI] [PubMed] [Google Scholar]
- 3.Ackermann G, Thomalla S, Ackermann F, Schaumann R, Rodloff AC, Ruf BR. Prevalence and characteristics of bacteria and host factors in an outbreak situation of antibiotic-associated diarrhoea. J Med Microbiol. 2005;54:149–153. doi: 10.1099/jmm.0.45812-0. [DOI] [PubMed] [Google Scholar]
- 4.McFarland LV. Normal flora: diversity and functions. Microb Ecol Health Dis. 2000;12:193–207. [Google Scholar]
- 5.McFarland LV. A review of the evidence of health claims for biotherapeutic agents. Microb Ecol Health Dis. 2000;12:65–76. [Google Scholar]
- 6.Qamar A, Aboudola S, Warny M, Michetti P, Pothoulakis C, LaMont JT, Kelly CP. Saccharomyces boulardii stimulates intestinal immunoglobulin A immune response to Clostridium difficile toxin A in mice. Infect Immun. 2001;69:2762–2765. doi: 10.1128/IAI.69.4.2762-2765.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elmer GW. Probiotics: "Living drugs". Am J Health Syst Pharm. 2001;58:1101–1109. doi: 10.1093/ajhp/58.12.1101. [DOI] [PubMed] [Google Scholar]
- 8.Pépin J, Valiquette L, Alary ME, Villemure P, Pelletier A, Forget K, Pépin K, Chouinard D. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease activity. CMAJ. 2004;171:466–472. doi: 10.1503/cmaj.1041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katikireddi V. UK launches inquired into Clostridium difficile outbreak. CMAJ. 2005;173:138. doi: 10.1503/cmaj.050771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101:812–822. doi: 10.1111/j.1572-0241.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 11.Cremonini F, Di Caro S, Covino M, Armuzzi A, Gabrielli M, Santarelli L, Nista EC, Cammarota G, Gasbarrini G, Gasbarrini A. Effect of different probiotic preparations on anti-Helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am J Gastroenterol. 2002;97:2744–2749. doi: 10.1111/j.1572-0241.2002.07063.x. [DOI] [PubMed] [Google Scholar]
- 12.Arvola T, Laiho K, Torkkeli S, Mykkänen H, Salminen S, Maunula L, Isolauri E. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999;104:e64. doi: 10.1542/peds.104.5.e64. [DOI] [PubMed] [Google Scholar]
- 13.Vanderhoof JA, Whitney DB, Antonson DL, Hanner TL, Lupo JV, Young RJ. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr. 1999;135:564–568. doi: 10.1016/s0022-3476(99)70053-3. [DOI] [PubMed] [Google Scholar]
- 14.Szajewska H, Kotowska M, Mrukowicz JZ, Armańska M, Mikołajczyk W. Efficacy of Lactobacillus GG in prevention of nosocomial diarrhea in infants. J Pediatr. 2001;138:361–365. doi: 10.1067/mpd.2001.111321. [DOI] [PubMed] [Google Scholar]
- 15.Thomas MR, Litin SC, Osmon DR, Corr AP, Weaver AL, Lohse CM. Lack of effect of Lactobacillus GG on antibiotic-associated diarrhea: a randomized, placebo-controlled trial. Mayo Clin Proc. 2001;76:883–889. doi: 10.4065/76.9.883. [DOI] [PubMed] [Google Scholar]
- 16.Armuzzi A, Cremonini F, Ojetti V, Bartolozzi F, Canducci F, Candelli M, Santarelli L, Cammarota G, De Lorenzo A, Pola P, Gasbarrini G, Gasbarrini A. Effect of Lactobacillus GG supplementation on antibiotic-associated gastrointestinal side effects during Helicobacter pylori eradication therapy: a pilot study. Digestion. 2001;63:1–7. doi: 10.1159/000051865. [DOI] [PubMed] [Google Scholar]
- 17.Beausoleil M, Fortier N, Guénette S, L'ecuyer A, Savoie M, Franco M, Lachaine J, Weiss K. Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: a randomized, double-blind, placebo-controlled trial. Can J Gastroenterol. 2007;21:732–736. doi: 10.1155/2007/720205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hickson M, D'Souza AL, Muthu N, Rogers TR, Want S, Rajkumar C, Bulpitt CJ. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80. doi: 10.1136/bmj.39231.599815.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kale-Pradhan PB, Jassal HK, Wilhelm SM. Role of Lactobacillus in the prevention of antibiotic-associated diarrhea: a meta-analysis. Pharmacotherapy. 2010;30:119–126. doi: 10.1592/phco.30.2.119. [DOI] [PubMed] [Google Scholar]
- 20.Szymański H, Armańska M, Kowalska-Duplaga K, Szajewska H. Bifidobacterium longum PL03, Lactobacillus rhamnosus KL53A, and Lactobacillus plantarum PL02 in the prevention of antibiotic-associated diarrhea in children: a randomized controlled pilot trial. Digestion. 2008;78:13–17. doi: 10.1159/000151300. [DOI] [PubMed] [Google Scholar]
- 21.Silva M, Jacobus NV, Deneke C, Gorbach SL. Antimicrobial substance from a human Lactobacillus strain. Antimicrob Agents Chemother. 1987;31:1231–1233. doi: 10.1128/aac.31.8.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alander M, Korpela R, Saxelin M, Vilpponen-Salmela T, Mattila-Sandholm T, von Wright A. Recovery of Lactobacillus rhamnosus GG from human colonic biopsies. Lett Appl Microbiol. 1997;24:361–364. doi: 10.1046/j.1472-765x.1997.00140.x. [DOI] [PubMed] [Google Scholar]
- 23.Goldin BR, Gorbach SL, Saxelin M, Barakat S, Gualtieri L, Salminen S. Survival of Lactobacillus species (strain GG) in human gastrointestinal tract. Dig Dis Sci. 1992;37:121–128. doi: 10.1007/BF01308354. [DOI] [PubMed] [Google Scholar]
- 24.Ling WH, Korpela R, Mykkänen H, Salminen S, Hänninen O. Lactobacillus strain GG supplementation decreases colonic hydrolytic and reductive enzyme activities in healthy female adults. J Nutr. 1994;124:18–23. doi: 10.1093/jn/124.1.18. [DOI] [PubMed] [Google Scholar]
- 25.Surawicz CM, Elmer GW, Speelman P, McFarland LV, Chinn J, van Belle G. Prevention of antibiotic-associated diarrhea by Saccharomyces boulardii: a prospective-study. Gastroenterology. 1989;96:981–988. doi: 10.1016/0016-5085(89)91613-2. [DOI] [PubMed] [Google Scholar]
- 26.Coconnier MH, Liévin V, Bernet-Camard MF, Hudault S, Servin AL. Antibacterial effect of the adhering human Lactobacillus acidophilus strain LB. Antimicrob Agents Chemother. 1997;41:1046–1052. doi: 10.1128/aac.41.5.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruszczyński M, Radzikowski A, Szajewska H. Clinical trial: effectiveness of Lactobacillus rhamnosus (strains E/N, Oxy and Pen) in the prevention of antibiotic-associated diarrhoea in children. Aliment Pharmacol Ther. 2008;28:154–161. doi: 10.1111/j.1365-2036.2008.03714.x. [DOI] [PubMed] [Google Scholar]
- 28.De Groote MA, Frank DN, Dowell E, Glode MP, Pace NR. Lactobacillus rhamnosus GG bacteremia associated with probiotic use in a child with short gut syndrome. Pediatr Infect Dis J. 2005;24:278–280. doi: 10.1097/01.inf.0000154588.79356.e6. [DOI] [PubMed] [Google Scholar]
- 29.Young RJ, Vanderhoof JA. Two cases of Lactobacillus bacteremia during probiotic treatment of short gut syndrome. J Pediatr Gastroenterol Nutr. 2004;39:436–437. doi: 10.1097/00005176-200410000-00027. [DOI] [PubMed] [Google Scholar]
- 30.Jirapinyo P, Densupsoontorn N, Thamonsiri N, Wongarn R. Prevention of antibiotic-associated diarrhea in infants by probiotics. J Med Assoc Thai. 2002;85(Suppl 2):S739–S742. [PubMed] [Google Scholar]