Abstract
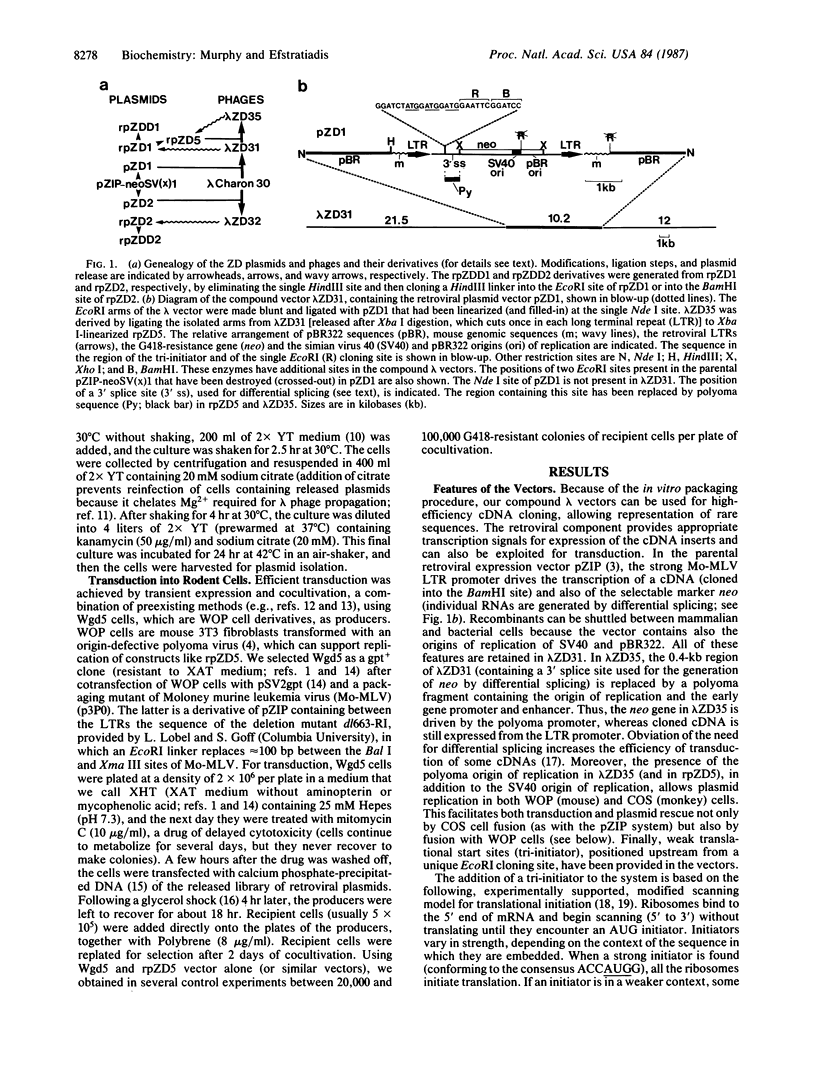
We have constructed a series of compound cloning vectors (lambda ZD vectors), each consisting of phage lambda arms carrying a modified version of the retroviral expression vector pZIP-neoSV (x)1. cDNA, inserted into a cloning site present in the retroviral vector component, is cloned with high efficiency using the lambda system. A cDNA library in plasmids is then released by homologous recombination between the retroviral long terminal repeats. Retroviral transduction is achieved by transient expression of the released library in a cell line containing a packaging mutant of Moloney murine leukemia virus, followed by cocultivation of these producers with recipient cells. Transcription of cDNAs in the recipient cells is driven by the strong long terminal repeat promoter, and the transcripts, even from truncated cDNAs, can be expressed because translational start sites have been provided in all three reading frames (tri-initiator). Sequences conferring a recognizable phenotype can be rescued by cell fusion. The functionality of the tri-initiator and the rescue of a rare cDNA have been successfully tested using model systems.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard H. U., Helinski D. R. Use of the lambda phage promoter PL to promote gene expression in hybrid plasmid cloning vehicles. Methods Enzymol. 1979;68:482–492. doi: 10.1016/0076-6879(79)68037-0. [DOI] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Dailey L., Basilico C. Sequences in the polyomavirus DNA regulatory region involved in viral DNA replication and early gene expression. J Virol. 1985 Jun;54(3):739–749. doi: 10.1128/jvi.54.3.739-749.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. L., O'Malley K. A., Wheeler T. B. Polyethylene glycol-induced mammalian cell hybridization: effect of polyethylene glycol molecular weight and concentration. Somatic Cell Genet. 1976 May;2(3):271–280. doi: 10.1007/BF01538965. [DOI] [PubMed] [Google Scholar]
- Episkopou V., Murphy A. J., Efstratiadis A. Cell-specified expression of a selectable hybrid gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4657–4661. doi: 10.1073/pnas.81.15.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost E., Williams J. Mapping temperature-sensitive and host-range mutations of adenovirus type 5 by marker rescue. Virology. 1978 Nov;91(1):39–50. doi: 10.1016/0042-6822(78)90353-7. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kavathas P., Sukhatme V. P., Herzenberg L. A., Parnes J. R. Isolation of the gene encoding the human T-lymphocyte differentiation antigen Leu-2 (T8) by gene transfer and cDNA subtraction. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7688–7692. doi: 10.1073/pnas.81.24.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Korman A. J., Frantz J. D., Strominger J. L., Mulligan R. C. Expression of human class II major histocompatibility complex antigens using retrovirus vectors. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2150–2154. doi: 10.1073/pnas.84.8.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Bifunctional messenger RNAs in eukaryotes. Cell. 1986 Nov 21;47(4):481–483. doi: 10.1016/0092-8674(86)90609-4. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. Translation of insulin-related polypeptides from messenger RNAs with tandemly reiterated copies of the ribosome binding site. Cell. 1983 Oct;34(3):971–978. doi: 10.1016/0092-8674(83)90554-8. [DOI] [PubMed] [Google Scholar]
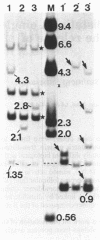
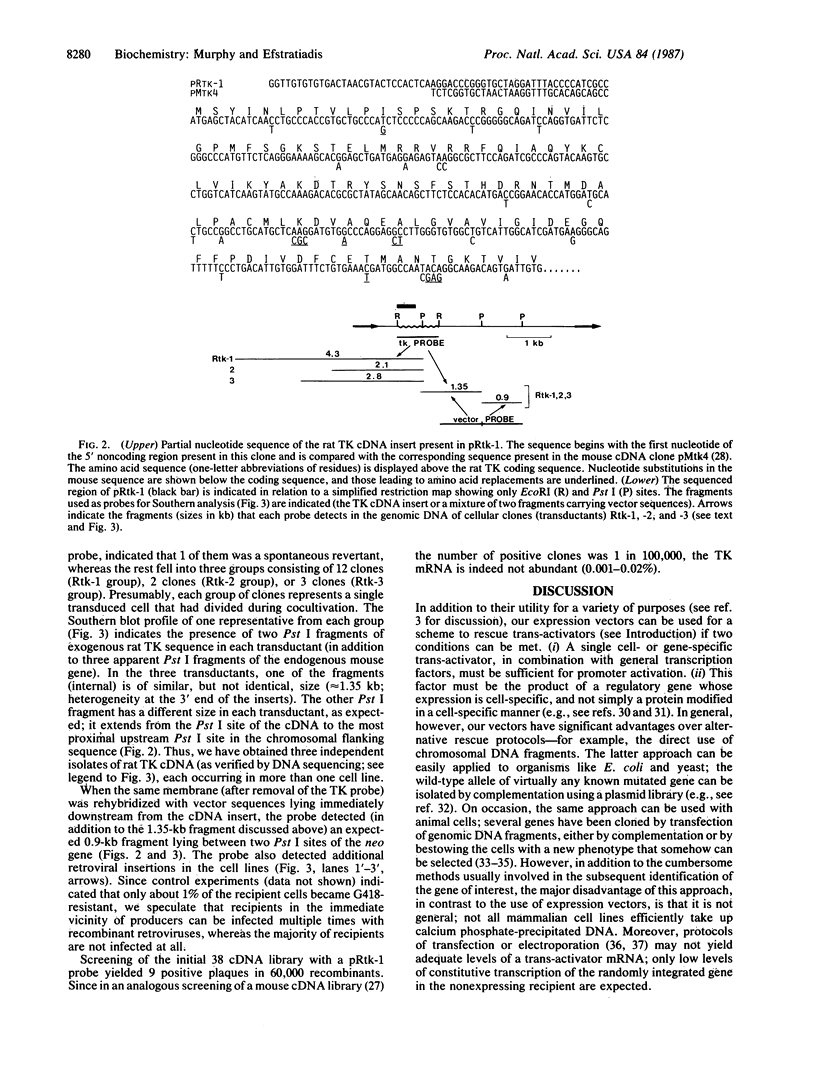
- Lin P. F., Lieberman H. B., Yeh D. B., Xu T., Zhao S. Y., Ruddle F. H. Molecular cloning and structural analysis of murine thymidine kinase genomic and cDNA sequences. Mol Cell Biol. 1985 Nov;5(11):3149–3156. doi: 10.1128/mcb.5.11.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman D. R., Thomas Y., Maddon P. J., Chess L., Axel R. The isolation and sequence of the gene encoding T8: a molecule defining functional classes of T lymphocytes. Cell. 1985 Feb;40(2):237–246. doi: 10.1016/0092-8674(85)90138-2. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann E., Schaefer-Ridder M., Wang Y., Hofschneider P. H. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1(7):841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. Bacteriophage lambda vector for transducing a cDNA clone library into mammalian cells. Mol Cell Biol. 1985 May;5(5):1136–1142. doi: 10.1128/mcb.5.5.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. S., Topol J. A Drosophila RNA polymerase II transcription factor binds to the regulatory site of an hsp 70 gene. Cell. 1984 May;37(1):273–283. doi: 10.1016/0092-8674(84)90323-4. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S., Huskey R. J. Deletion mutants of bacteriophage lambda. I. Isolation and initial characterization. J Mol Biol. 1971 Mar 14;56(2):369–384. doi: 10.1016/0022-2836(71)90471-2. [DOI] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Lipsich L., Wigler M. Isolation of the chicken thymidine kinase gene by plasmid rescue. Nature. 1980 May 22;285(5762):207–210. doi: 10.1038/285207a0. [DOI] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm D. L., Horness D., Kucera J., Blattner F. R. Construction of coliphage lambda Charon vectors with BamHI cloning sites. Gene. 1980 Dec;12(3-4):301–309. doi: 10.1016/0378-1119(80)90113-4. [DOI] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sugden B., Marsh K., Yates J. A vector that replicates as a plasmid and can be efficiently selected in B-lymphoblasts transformed by Epstein-Barr virus. Mol Cell Biol. 1985 Feb;5(2):410–413. doi: 10.1128/mcb.5.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman G. M., Lupton S., Kamen R. Polyomavirus enhancer contains multiple redundant sequence elements that activate both DNA replication and gene expression. Mol Cell Biol. 1985 Apr;5(4):649–658. doi: 10.1128/mcb.5.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]
- Yokota T., Arai N., Lee F., Rennick D., Mosmann T., Arai K. Use of a cDNA expression vector for isolation of mouse interleukin 2 cDNA clones: expression of T-cell growth-factor activity after transfection of monkey cells. Proc Natl Acad Sci U S A. 1985 Jan;82(1):68–72. doi: 10.1073/pnas.82.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]