Abstract
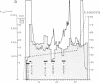
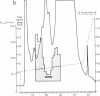
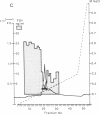
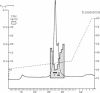
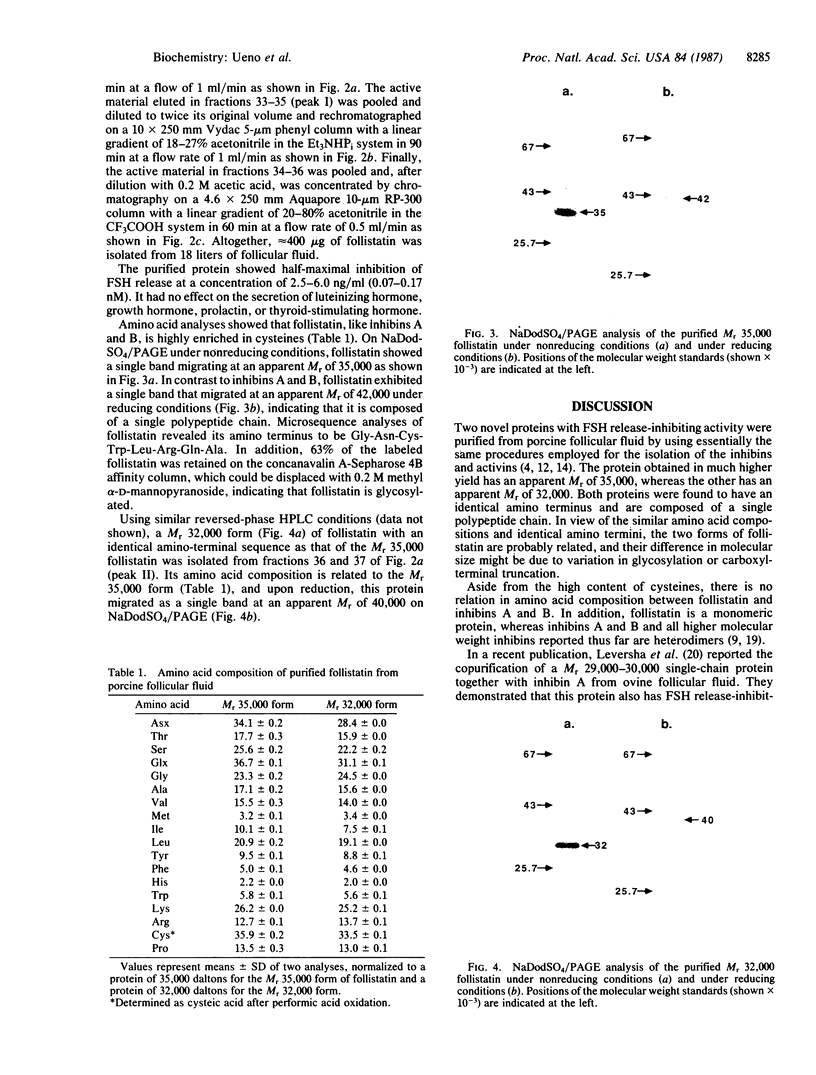
A Mr 35,000 protein with follicle-stimulating hormone release-inhibitory activity was isolated from porcine ovarian follicular fluid by heparin-Sepharose affinity chromatography, gel filtration on Sephacryl S-200, and multiple steps of high-performance liquid chromatography. The isolated molecule is highly enriched in cysteines and is composed of a single polypeptide chain. In addition, it has no sequence homology with the previously characterized follicular fluid inhibins, which are heterodimeric proteins of Mr 32,000 with follicle-stimulating hormone release-inhibiting activity. This protein specifically inhibits the basal secretion of follicle-stimulating hormone, but not that of luteinizing hormone, in the rat anterior pituitary monolayer culture system with a half-maximal effective dose of 2.5-6.0 ng/ml. Another form of the molecule of Mr 32,000 present in much lower concentration in follicular fluid was also isolated. It may differ from the Mr 35,000 form in glycosylation or carboxyl-terminal truncation. We suggest that this compound be called "follistatin" to signify its structural difference from inhibin.
Full text
PDF
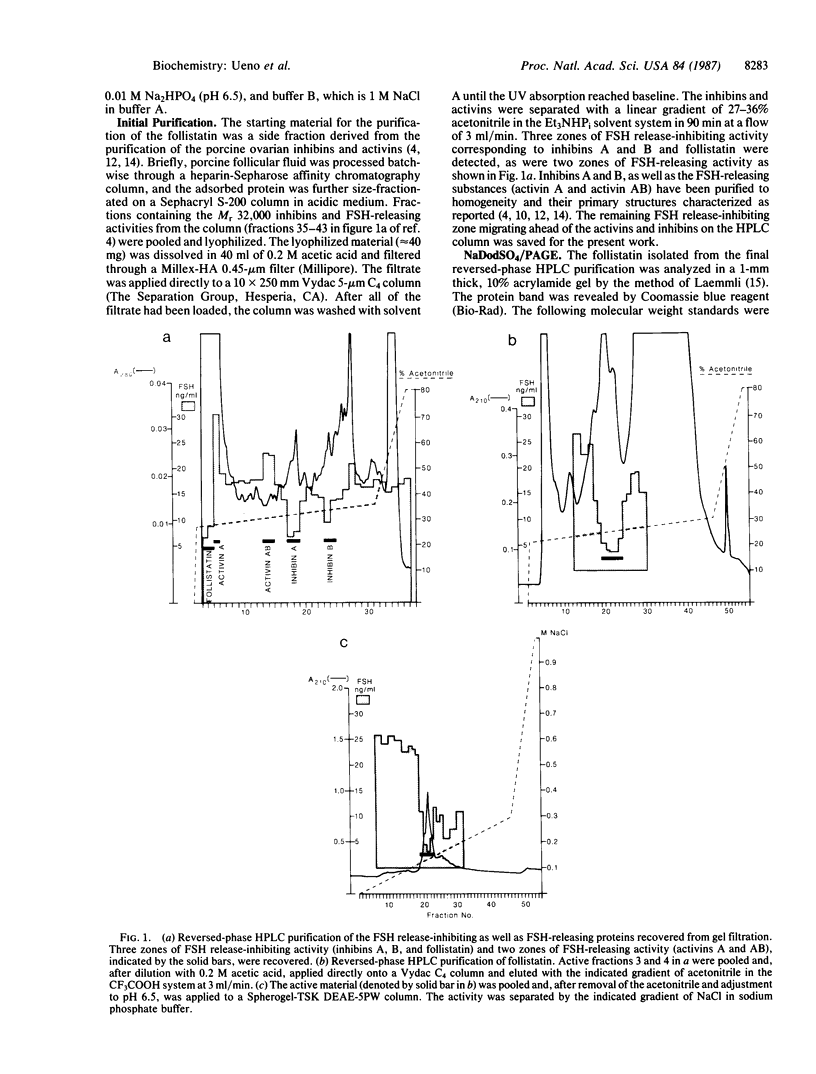
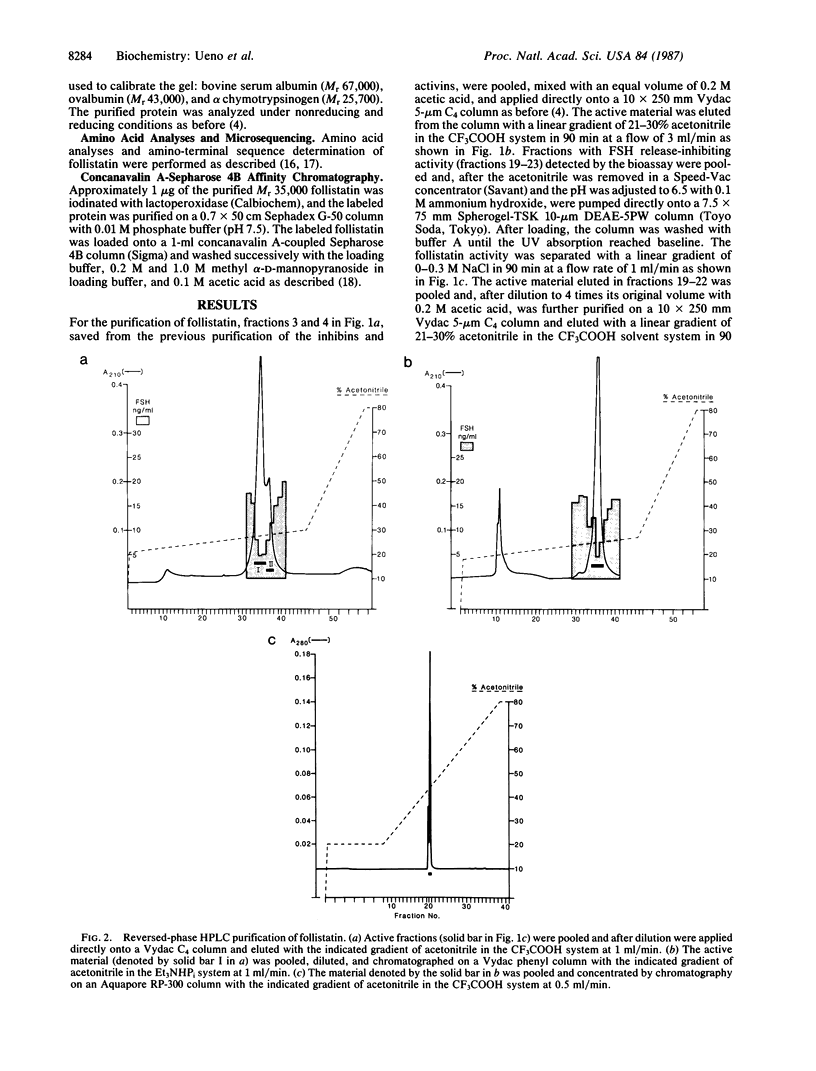

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böhlen P., Schroeder R. High-sensitivity amino acid analysis: methodology for the determination of amino acid compositions with less than 100 picomoles of peptides. Anal Biochem. 1982 Oct;126(1):144–152. doi: 10.1016/0003-2697(82)90120-8. [DOI] [PubMed] [Google Scholar]
- Esch F. S. Polypeptide microsequence analysis with the commercially available gas-phase sequencer. Anal Biochem. 1984 Jan;136(1):39–47. doi: 10.1016/0003-2697(84)90305-1. [DOI] [PubMed] [Google Scholar]
- Forage R. G., Ring J. M., Brown R. W., McInerney B. V., Cobon G. S., Gregson R. P., Robertson D. M., Morgan F. J., Hearn M. T., Findlay J. K. Cloning and sequence analysis of cDNA species coding for the two subunits of inhibin from bovine follicular fluid. Proc Natl Acad Sci U S A. 1986 May;83(10):3091–3095. doi: 10.1073/pnas.83.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchimont P., Verstraelen-Proyard J., Hazee-Hagelstein M. T., Renard C., Demoulin A., Bourguignon J. P., Hustin J. Inhibin: from concept to reality. Vitam Horm. 1979;37:243–302. doi: 10.1016/s0083-6729(08)61071-7. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Miyamoto K., Hasegawa Y., Nomura M., Igarashi M., Kangawa K., Matsuo H. Isolation of bovine follicular fluid inhibin of about 32 kDa. Mol Cell Endocrinol. 1986 Jan;44(1):55–60. doi: 10.1016/0303-7207(86)90105-x. [DOI] [PubMed] [Google Scholar]
- Leversha L. J., Robertson D. M., de Vos F. L., Morgan F. J., Hearn M. T., Wettenhall R. E., Findlay J. K., Burger H. G., de Kretser D. M. Isolation of inhibin from ovine follicular fluid. J Endocrinol. 1987 May;113(2):213–221. doi: 10.1677/joe.0.1130213. [DOI] [PubMed] [Google Scholar]
- Ling N., Ying S. Y., Ueno N., Esch F., Denoroy L., Guillemin R. Isolation and partial characterization of a Mr 32,000 protein with inhibin activity from porcine follicular fluid. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7217–7221. doi: 10.1073/pnas.82.21.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling N., Ying S. Y., Ueno N., Shimasaki S., Esch F., Hotta M., Guillemin R. A homodimer of the beta-subunits of inhibin A stimulates the secretion of pituitary follicle stimulating hormone. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1129–1137. doi: 10.1016/s0006-291x(86)80400-4. [DOI] [PubMed] [Google Scholar]
- Ling N., Ying S. Y., Ueno N., Shimasaki S., Esch F., Hotta M., Guillemin R. Pituitary FSH is released by a heterodimer of the beta-subunits from the two forms of inhibin. Nature. 1986 Jun 19;321(6072):779–782. doi: 10.1038/321779a0. [DOI] [PubMed] [Google Scholar]
- Mason A. J., Hayflick J. S., Ling N., Esch F., Ueno N., Ying S. Y., Guillemin R., Niall H., Seeburg P. H. Complementary DNA sequences of ovarian follicular fluid inhibin show precursor structure and homology with transforming growth factor-beta. Nature. 1985 Dec 19;318(6047):659–663. doi: 10.1038/318659a0. [DOI] [PubMed] [Google Scholar]
- McCullagh D. R. DUAL ENDOCRINE ACTIVITY OF THE TESTES. Science. 1932 Jul 1;76(1957):19–20. doi: 10.1126/science.76.1957.19. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Hasegawa Y., Fukuda M., Igarashi M. Demonstration of high molecular weight forms of inhibin in bovine follicular fluid (bFF) by using monoclonal antibodies to bFF 32K inhibin. Biochem Biophys Res Commun. 1986 May 14;136(3):1103–1109. doi: 10.1016/0006-291x(86)90447-x. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Hasegawa Y., Fukuda M., Nomura M., Igarashi M., Kangawa K., Matsuo H. Isolation of porcine follicular fluid inhibin of 32K daltons. Biochem Biophys Res Commun. 1985 Jun 14;129(2):396–403. doi: 10.1016/0006-291x(85)90164-0. [DOI] [PubMed] [Google Scholar]
- Rivier J., Spiess J., McClintock R., Vaughan J., Vale W. Purification and partial characterization of inhibin from porcine follicular fluid. Biochem Biophys Res Commun. 1985 Nov 27;133(1):120–127. doi: 10.1016/0006-291x(85)91849-2. [DOI] [PubMed] [Google Scholar]
- Robertson D. M., Foulds L. M., Leversha L., Morgan F. J., Hearn M. T., Burger H. G., Wettenhall R. E., de Kretser D. M. Isolation of inhibin from bovine follicular fluid. Biochem Biophys Res Commun. 1985 Jan 16;126(1):220–226. doi: 10.1016/0006-291x(85)90594-7. [DOI] [PubMed] [Google Scholar]
- Robertson D. M., de Vos F. L., Foulds L. M., McLachlan R. I., Burger H. G., Morgan F. J., Hearn M. T., de Kretser D. M. Isolation of a 31 kDa form of inhibin from bovine follicular fluid. Mol Cell Endocrinol. 1986 Mar;44(3):271–277. doi: 10.1016/0303-7207(86)90133-4. [DOI] [PubMed] [Google Scholar]
- Shibasaki T., Ling N., Guillemin R. Pituitary immunoreactive gamma-melanotropins are glycosylated oligopeptides. Nature. 1980 Jun 5;285(5764):416–417. doi: 10.1038/285416a0. [DOI] [PubMed] [Google Scholar]
- Vale W., Rivier J., Vaughan J., McClintock R., Corrigan A., Woo W., Karr D., Spiess J. Purification and characterization of an FSH releasing protein from porcine ovarian follicular fluid. Nature. 1986 Jun 19;321(6072):776–779. doi: 10.1038/321776a0. [DOI] [PubMed] [Google Scholar]