Abstract
Background
Members of the tumor necrosis factor (TNF) superfamily, such as TNFα, potently promote atherogenesis in mice and humans. TNF receptor-associated factors (TRAFs) are cytoplasmic adaptor proteins for this group of cytokines.
Methods and Results
This study tested the hypothesis that TRAF1 modulates atherogenesis in vivo. TRAF1−/−/LDLR−/− mice consuming a high-cholesterol diet for 18 weeks developed significantly smaller atherosclerotic lesions compared with LDLR−/− (low density lipoprotein receptor) control animals. As the most prominent change in histologic composition, plaques of TRAF1-deficient animals contained significantly fewer macrophages. Bone marrow transplantations revealed that TRAF1 deficiency on both hematopoetic as well as vascular resident cells contributed to the reduction in atherogenesis observed. Mechanistic studies showed that deficiency of TRAF1 in endothelial cells and monocytes reduced adhesion of inflammatory cells to the endothelium in static and dynamic assays. Impaired adhesion coincided with reduced cell spreading, actin polymerization, and CD29 expression in macrophages, as well as decreased expression of the adhesion molecules ICAM-1 and VCAM-1 on endothelial cells. SiRNA studies on human cells verified these findings. Furthermore, TRAF1 mRNA levels were significantly elevated in blood of patients with acute coronary syndrome.
Conclusions
TRAF1 deficiency attenuates atherogenesis in mice, most likely due to impaired monocyte recruitment to the vessel wall. These data identify TRAF1 as a potential treatment target for atherosclerosis.
Keywords: TRAF1, inflammation, atherosclerosis, monocyte recruitment
Introduction
Atherosclerosis is a chronic inflammatory disease orchestrated by a network of inflammatory cytokines 1, 2. Substantial in vitro and in vivo evidence implicates members of the tumor necrosis factor (TNF) receptor/interleukin-1 (IL-1)/toll-like receptor superfamily, such as TNFα, CD40L, and IL-1β, in the development of atherosclerosis 3–5. TNF receptor-associated factors (TRAFs) function as intracellular adaptor proteins that mediate signaling for the TNF/IL-1/toll-like receptor superfamily by upstream interaction with the respective receptors and consequent activation of downstream signaling molecules 6, 7.
TRAF1, a 46 kD molecule, associates with several receptors including TNFR1, TNFR2, and CD40. According to several studies TRAF1 functions as an inhibitory protein 8, 9. In contrast to other TRAFs, most resting cells lack TRAF1, but rapidly express TRAF1 upon stimulation with TNFα, CD40L, LPS, or lymphocyte receptor ligands 10, 11. These data strongly suggest that TRAF1 participates in a negative feedback loop. Several reports revealed that TRAF1 interferes with TRAF2-dependent NFκB activation 12, 13. Tsitsikov et al. demonstrated enhanced TNFα-induced signaling in TRAF1-deficient lymphocytes coinciding with hypersensitivity of TRAF1-deficient mice to skin necrosis provoked by TNFα 14. Similarly, TRAF1-deficient mice proved more susceptible to TNFα-induced liver damage 15. However, reports suggesting an opposite, pro-inflammatory role for TRAF1 as activator of NFκB and/or JNK 16, 17 have hampered conclusive evaluation of the physiological role of TRAF1. Some of these controversies stem from differences in methodology as well as differential cell type-, cognate receptor-, and target gene-specific TRAF1-mediated functions, warranting a disease-based in vivo evaluation.
Although TRAFs likely modulate atherogenesis in vivo knowledge of the role of TRAFs in atherosclerosis remains rudimentary. Some reports identified TRAF6 as mediator of CD40L-induced pro-inflammatory signals in monocytes and implicated this molecule in neointima formation in mice 18, 19. Expression of TRAF2 and TRAF3 has been associated with shear stress in vitro and in vivo 20, 21. Luo et al. recently demonstrated that activation of TNFR2 mediates ischemia-induced arteriogenesis by inducing TRAF2-dependent survival pathways 22. Our group recently demonstrated overexpression of several TRAFs, particularly TRAF1, in human and mouse atheromata 23. Based on these data, this study tested the hypothesis that TRAF1 modulates mouse atherogenesis in vivo.
Methods
A detailed description of all methods is accessible in the online supplement.
Results
TRAF1-deficient mice develop smaller atherosclerotic lesions containing fewer macrophages
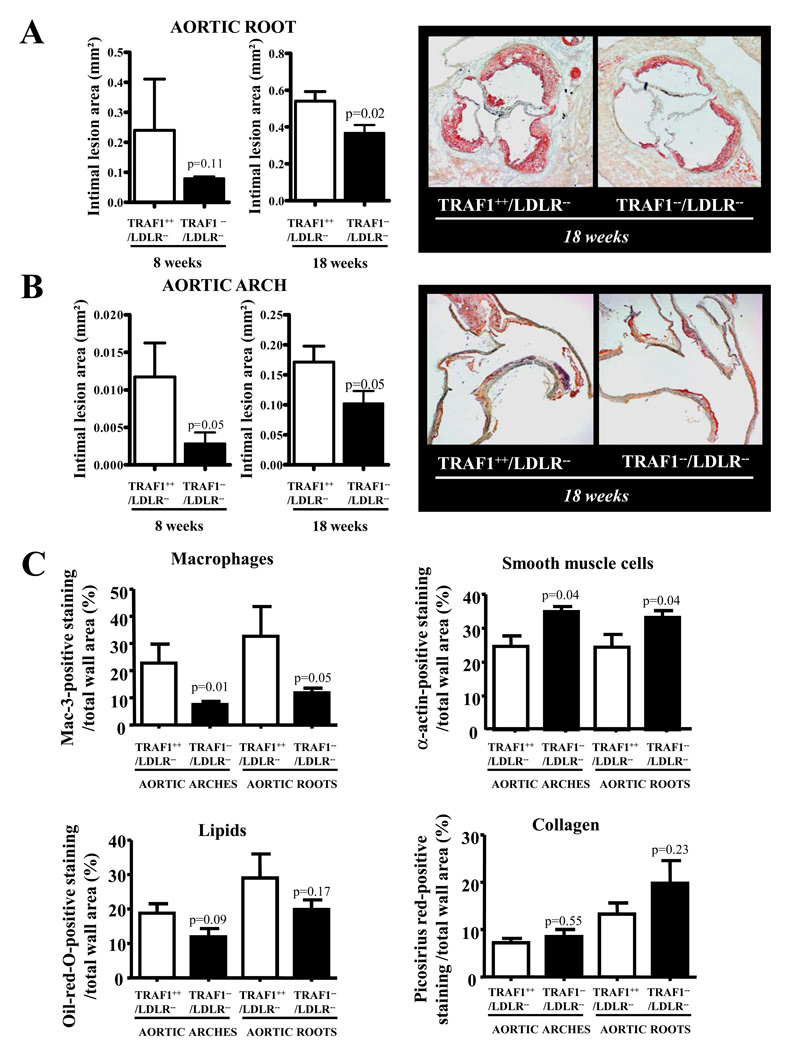
To investigate the influence of TRAF1 on Murine atherogenesis, TRAF1−/−/LDLR−/− and TRAF1+/+/LDLR−/− mice consumed a high-cholesterol diet (HCD) for 8 and 18 weeks. Weights, leukocyte counts, total cholesterol, and triglyceride levels did not differ between the study groups (Data Supplement Table I and II). All mice appeared healthy, active, reproduced normally, and had no obvious abnormalities and a normal life span. TRAF1-deficient animals had smaller intimal lesions of aortic roots (N=8 and 13, p=0.02) and arches (N=8 and 13, p=0.05) compared with controls at both time points, suggesting a pro-atherogenic function of TRAF1 (Fig. 1A and B). Plaques from TRAF1−/−/LDLR−/− mice contained significantly fewer macrophages (p=0.05 and 0.01) and more smooth muscle cells (p=0.04 and 0.04, Fig. 1C). Lipid content tended to decrease while collagen content tended to increase. T cell counts remained unchanged in plaques and adventitia (see Data Supplement Figure I for representative images). Taken together, these features represent characteristics attributed to more stable plaques in humans 24.
Figure 1. TRAF1 deficiency attenuates atherosclerosis in mice.
A and B. TRAF1−/−/LDLR−/− and TRAF1+/+/LDLR−/− mice consumed a high cholesterol diet for 18 weeks and 8 weeks underwent analysis of intimal lesion size in the aortic root (A) and arch (B) (N=8 and 13). Pooled data±SEM are shown on the left; images of representative sections stained for lipid deposition (Oil-red-O) are displayed on the right.
C. Sections of the aortic arches of mice treated as described above were analyzed for macrophage-, smooth muscle cell-, lipid-, and collagen-specific staining. Mac-3-, α-actin-, oil-red-O-, and picosirius red-positive staining in relation to total wall area is displayed as mean±SEM (N=8 and 13).
TRAF1 deficiency on bone marrow derived and resident cells attenuates atherogenesis in mice
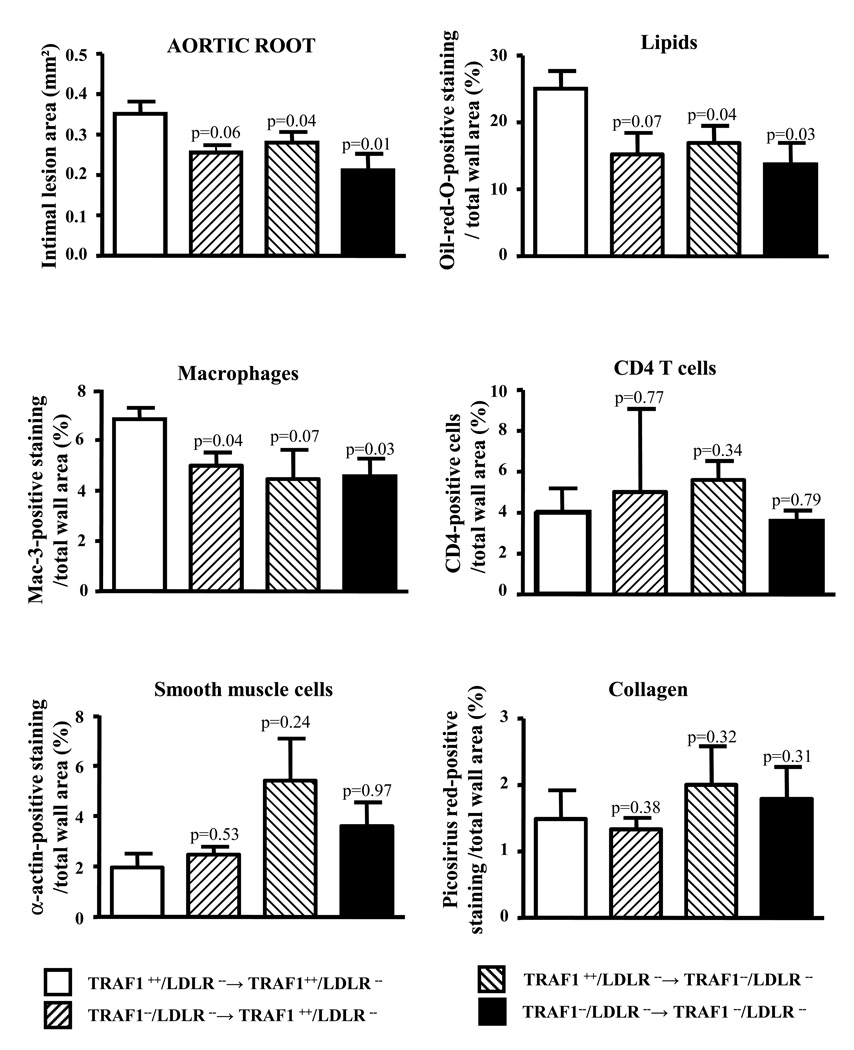
To elucidate the relevance of TRAF1 on bone marrow (BM)-derived and resident cells for atherogenesis, we performed bone marrow transplantations between TRAF1−/−/LDLR−/− and TRAF1+/+/LDLR−/− mice (for study characteristics see Data Supplement Table III). Mice with simultaneous deficiency in TRAF1 on BM-derived and resident cells showed the greatest reduction in intimal lesion size compared with respective wild-type controls (39.9±10.6%, N=8 and 5 per group, p=0.01). TRAF1 deficiency on both, BM-derived and resting cells alone sufficed to attenuate atherogenesis. Analysis of plaque composition revealed significant reduction of macrophage content and lipids while collagen and smooth muscle cell content tended to be increased in the TRAF1-deficient chimers (Fig.2).
Figure 2. TRAF1 deficiency on bone marrow-derived and resident cells attenuates atherogenesis in mice.
Bone marrow transplantations were conducted between TRAF1−/−/LDLR−/− and TRAF1+/+/LDLR−/− mice. Chimers consuming high cholesterol diet for 18 weeks were subjected to analysis of intimal lesion size in the aortic root. Pooled data represent mean±SEM. Mac-3- (macrophage), α-actin- (smooth muscle cell), CD4- (T cell), oil-red-O- (lipid), and picosirius red (collagen)-positive staining in relation to total wall area is displayed as mean ± SEM (N=8 and 5).
TRAF1 deficiency attenuates adhesion of monocytes
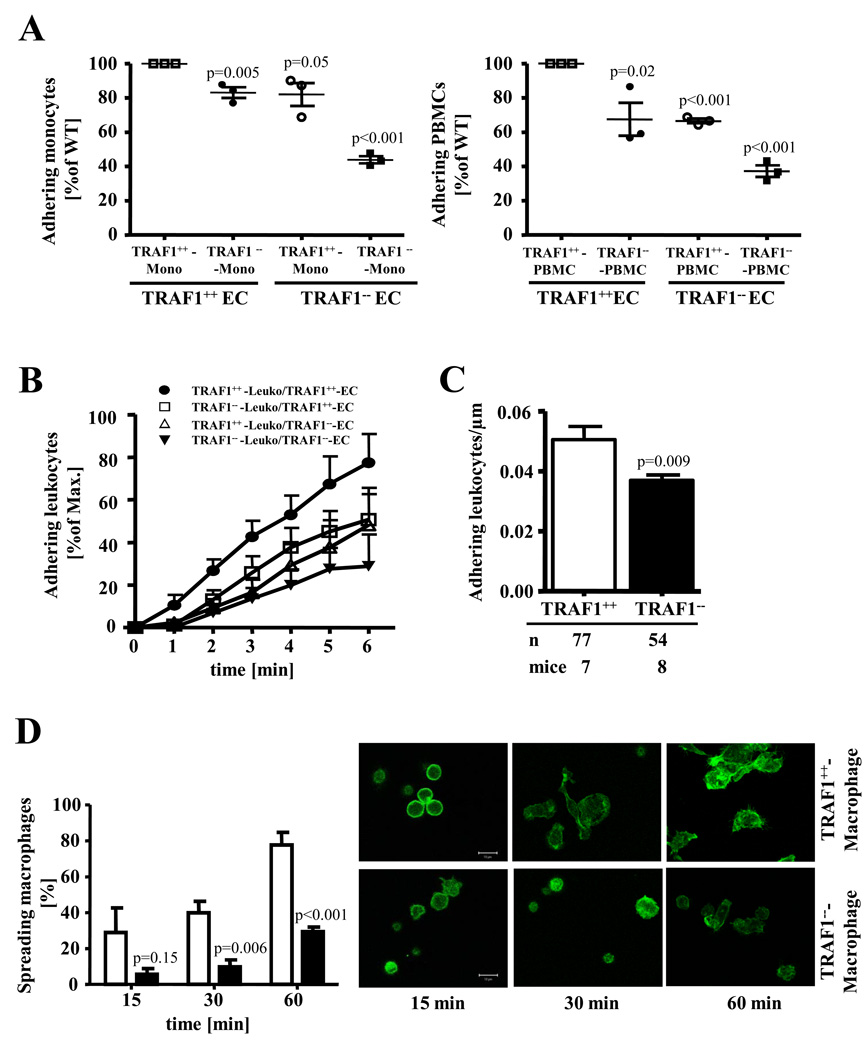
Since we observed markedly decreased macrophage content in plaques from TRAF1−/−/LDLR−/− animals, we tested the hypothesis that TRAF1 modulates adhesion of inflammatory cells, a key step in atherosclerotic plaque formation. TRAF1 deficiency on both monocytes and EC significantly inhibited adhesion compared with respective wild-type controls (N=3, p<0.001, Fig. 3A). Similar findings emerged with PBMCs (N=3, p<0.001, Fig.3A). Under flow conditions relevant to those in human vessels deficiency of TRAF1 on both EC and thioglycollate-elicited peritoneal leukocytes also significantly inhibited adhesion (N=5, p=0.003, Fig.3B). TRAF1 deficiency on either EC or leukocytes alone sufficed to attenuate adhesion of leukocytes (N=5, p=0.04 both, Fig.3B, see also Data Supplement Figure II). Similar results were observed with cells on a LDLR-deficient background (Data Supplement Figure III). In accord, we identified markedly reduced adhesion of leucocytes in the venules of the cremaster muscle in TRAF1-deficient mice compared with wild-type mice in vivo as assessed by intravital microscopy (N=7 and 8, P=0.009; Fig.3C).
Figure 3. TRAF1 deficiency impairs adhesion of monocytes to endothelial cells and attenuates spreading of Murine macrophages.
A. Murine monocytes and PBMCs of TRAF1-deficient and wild-type mice were stained with CFDA and allowed to interact with Murine endothelial cells isolated from TRAF1-deficient and wild-type mice (N=3). Adherent cells were counted under the microscope. Each symbol indicates an individual experiment and donor.
B. Adhesion of PMA-activated thioglycollate-elicited peritoneal leukocytes obtained from TRAF1-deficient and wild-type mice was analyzed on TNFα-activated endothelial cells isolated from TRAF1-deficient and wild-type mice under flow conditions (0.5 dyne/cm2, N=5). Adherent leukocytes were quantified under the microscope. Pooled data represent mean±SEM.
C. Mice were treated intraperitoneally with 200ng TNFα 4h prior to intravital microscopy. Venules (30–50µm) of the cremaster muscle were screened for adhesion of leucocytes. The number of adherent leucocytes was counted manually. Data represent the mean±SEM.
D. Macrophages from wild-type and TRAF1-deficient mice were plated on serum-coated glass cover slips and incubated at 37°C. Cells were stained with Alexa Fluor 594-conjugated phalloidin and confocal microscope performed. Spreading was quantified and expressed as mean±SEM of spreading cells on the left (N=5 each); representative pictures are shown on the right.
TRAF1 deficiency limits actin polymerization and the expression of adhesion molecules in endothelial cells and macrophages
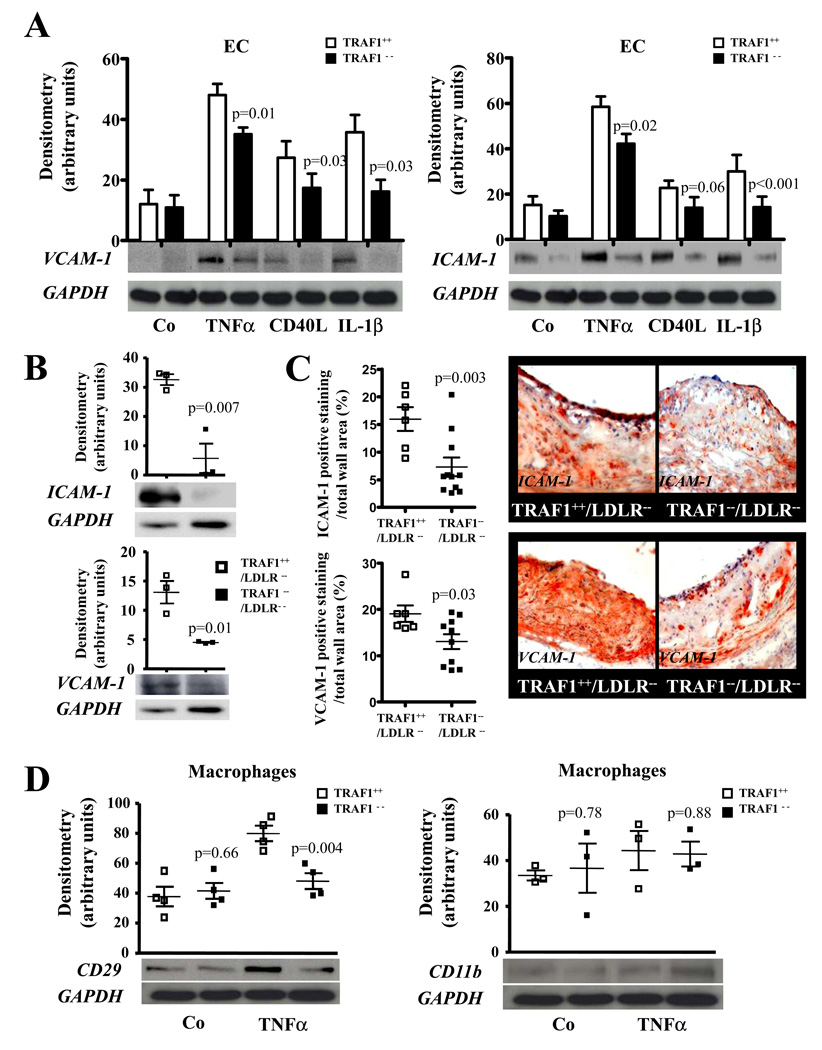
Wild-type peritoneal macrophages quickly spread on glass coverslips whereas the morphology of TRAF1-deficient macrophages remained largely unchanged as assessed by phalloidin staining (N=5 P<0.001, Fig.3D), suggesting that TRAF1 deficiency interferes with actin polymerization. Adhesion molecules regulate protrusion and adhesion. TRAF1 deficiency significantly decreased the expression of ICAM-1 and VCAM-1 on TNFα-stimulated EC by 22±4% (N=7, p=0.02) and 27±2% (N=6, p=0.01, Fig.4A). Respective experiments with TRAF1-deficient or –competent EC from animals also deficient in LDLR generated similar results (Data Supplement Figure III). Furthermore, VCAM-1 and ICAM-1 expression was markedly reduced in both arterial tissue (N=3, p= 0.01 and 0.007) and aortic sections of TRAF1−/−/LDLR−/− mice compared with TRAF1+/+/LDLR−/− mice as assessed by western blotting and immunohistochemistry (Fig.4B and C). TRAF1-deficient BM-derived macrophages showed reduced Integrin beta 1 (CD29) (N=4, p=0.004) expression while no significant difference in CD11b expression was observed (N=3, p=0.88, Fig. 4D). Similar findings were obtained in T cells (Online Supplement Figure IV).
Figure 4. TRAF1 deficiency attenuates expression of adhesion molecules and integrins on Murine endothelial cells and macrophages.
A. Murine endothelial cells isolated from TRAF1-deficient and wild-type mice were stimulated with or without TNFα (20ng/ml), CD40L (10µg/ml), and IL-1β (10ng/ml), and cell lysates were analyzed for VCAM-1 (N=6) and ICAM-1 (N=7) by Western blotting. Pooled densitometric data adjusted for GAPDH given as mean±SEM are shown on top, representative blots below.
B. Arterial tissue of TRAF1−/−/LDLR−/− and TRAF1+/+/LDLR−/− mice was isolated and lysates were examined for VCAM-1 and ICAM-1 protein expression by Western blotting. Data adjusted for GAPDH expression. Data are shown as grouped scatter plot. Representative blots are shown below (N=3).
C. Sections of the aortic roots of TRAF1−/−/LDLR−/− and TRAF1+/+/LDLR−/− mice consuming a high cholesterol diet for 18 weeks underwent immunohistochemical analysis for VCAM-1- and ICAM-1 expression. VCAM-1- (N=6 and 10) and ICAM-1- (N=6 and 11)-positive staining in relation to total wall area is displayed as grouped scatter plot on the left, representative pictures are shown on the right.
D. Murine bone marrow-derived macrophages (BMM) obtained from TRAF1-deficient and wild-type mice were stimulated with or without TNFα (20ng/ml) for 24h, and cell lysates were analyzed for CD29 (N=4) and CD11b (N=3) by Western blotting. Pooled densitometric data adjusted for GAPDH given as grouped scatter plot, representative blots below.
TRAF1 deficiency differentially regulates chemokine and chemokine receptor expression but does not alter inflammatory cell migration
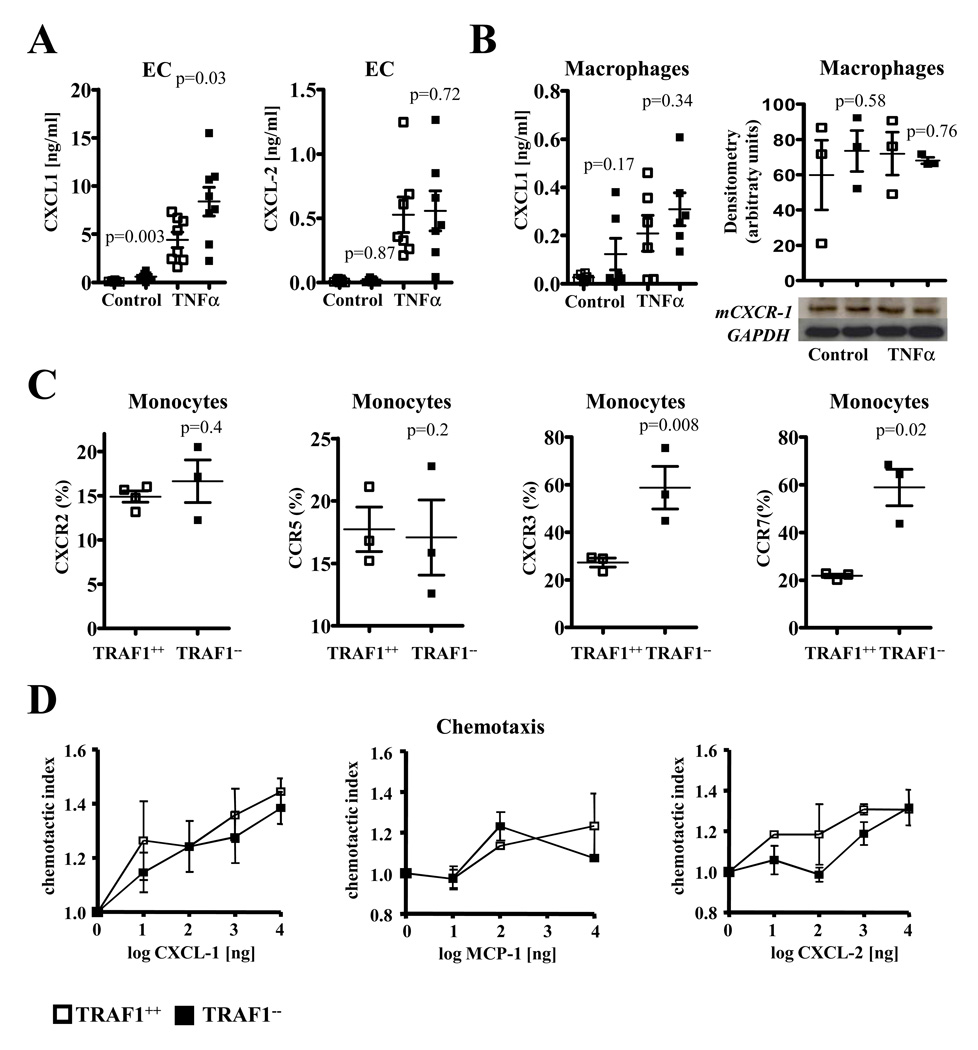
Chemotaxis presents a crucial step in the recruitment of monocytes to the intima 2. We previously reported a slight increase in MCP-1 expression in TRAF1-deficient EC and macrophages 23. Similarly, we observed a significant increase in Kc (CXCL-1) in TRAF1-deficient EC (N=8, p=0.03) but not in macrophages (N=6, p=0.34, 95% CI, 0.02–0.40 and 0.13–0.48). MIP-2 (CXCL-2) expression was not significantly regulated in both cell types (N=7 and 3, p= 0.72 (95% CI, 0.19–0.86 and 0.17–0.94) and 0.81, Fig.5A and B). Western blot analysis confirmed no significant difference between TRAF1-deficient and WT mice in CXCR-1 expression (N=3, p=0.76, Fig.5B). Further evaluation of chemokine receptor expression revealed a significant increase of CCR7 (p=0.02) and CXCR3 (p=0.008) and no significant regulation for CCR5 (p=0.2) and CXCR2 (p=0.4) expression on TRAF1-deficient monocytes compared with respective controls (N=3 each, Fig.5C). Similar results were obtained in TRAF1-deficient CD4 and CD8 T cells before and after αCD3/αCD28 stimulation (Data Supplement Figure V). Migration of inflammatory cells not only depends on chemokines and chemokine receptors but also on the migratory capacity of inflammatory cells. TRAF1 deficiency did not significantly alter the chemotactic index of PBMCs to gradients of various chemokines compared with wild-type controls (N=3 each, Fig.5D).
Figure 5. TRAF1 deficiency differentially regulates chemokine and chemokine receptor expression but does not alter inflammatory cell migration.
A. Murine endothelial cells isolated from TRAF1-deficient and –competent mice were stimulated with or without TNFα (20ng/ml)´ for 24h. Kc (CXCL-1) and MIP-2 (CXCL 2) protein was assayed in the supernatant by ELISA (N=8 and 7). Data are given as mean±SEM and as a grouped scatter blot.
B. Bone marrow-derived macrophages (BMM) of TRAF1-deficient and -competent mice were stimulated with or without TNFα (20ng/ml). CXCL-1 protein was measured in the supernatants by ELISA and CXCR-1 expression in the cell lysates by Western blotting (N=6 and 3). Data are given as mean±SEM and densitometric mean adjusted for GAPDH ±SEM, and as grouped scatter plot, a representative Western blot is shown below where appropriate.
C. Murine monocytes of TRAF1−/−/LDLR−/− and TRAF1+/+/LDLR−/− mice consuming a high cholesterol diet were analyzed for CXCR2, CCR5, CXCR3, and CCR7 expression by FACS (N=3 each). Data represent percentage ±SEM in a grouped scatter blot.
D. PBMCs isolated from TRAF1-deficient and –competent mice were assayed for their migratory capacity towards indicated concentrations of CXCL-1, MCP-1, and CXCL-2 in a modified Boyden chamber (N=3 each). Migrated PBMCs were quantified microscopically and given as percentage of loaded cells.
IL-6 levels decrease in blood and arterial tissue of TRAF1-deficient mice
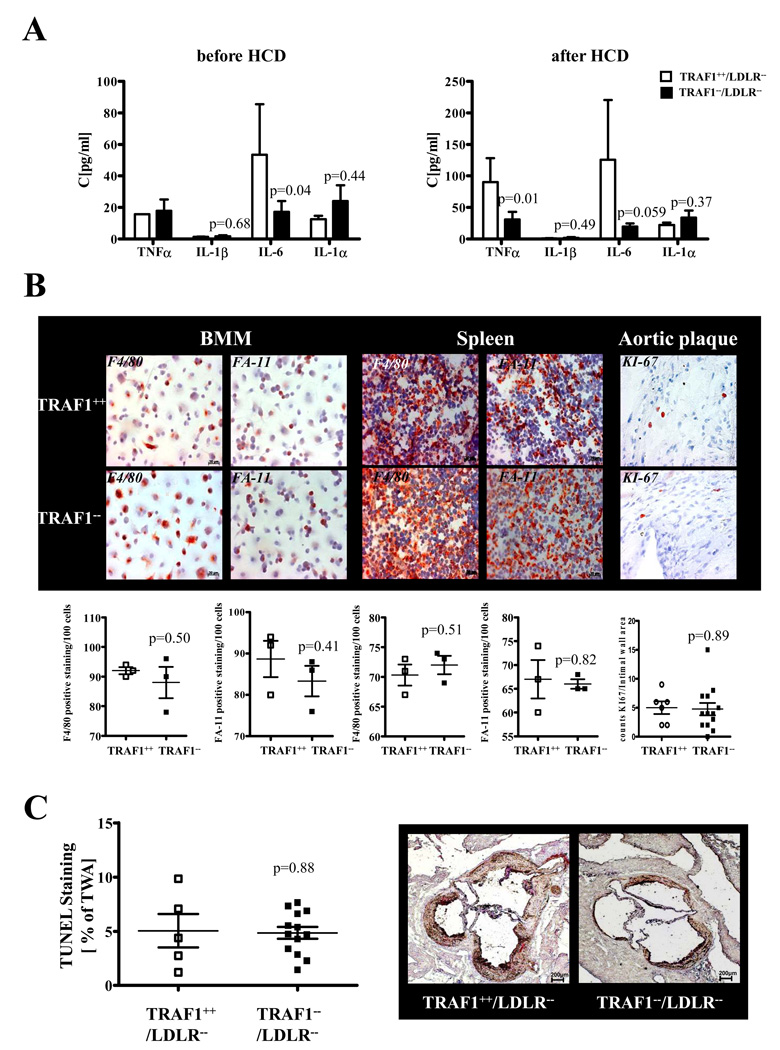
Inflammatory cytokines were assayed in blood and aortic arterial tissue of TRAF1−/−/LDLR−/− and TRAF1+/+/LDLR−/− mice at baseline (N=10 and 16) and after 18 weeks of HCD (N=10 and 18). TRAF1 deficiency provoked a significant reduction of IL-6 (p=0.04 and 0.059, Fig.6A). Furthermore, we identified reduced IL-6 concentrations in arterial tissue (10.56±3.4 vs. 65.63±19, p=0.01) and serum (13.06±5.3 vs. 36.61±7.3, p=0.02) of TRAF1-deficient animals 4h after intraperitoneal injection of 200ng TNFα compared with respective controls. TRAF1-deficient animals expressed decreased levels of TNFα after HCD in serum and arterial tissue (16.53±0.1 vs. 10.63±0.37, P=0.004), while no significant regulation could be observed for IL-1β and IL-1α (95% CI, 9.82–58.52 and 13.60–30.86, Fig.6A).
Figure 6. TRAF1 deficiency downregulates inflammatory cytokines but does not affect macrophage differentiation and cellular apoptosis.
A. Serum from TRAF1−/−/LDLR−/− and TRAF1+/+/LDLR−/− mice was analyzed for TNFα, IL-1β, IL-6, and IL-1α at baseline and after consumption of high cholesterol diet for 18 weeks. Data represent mean±SEM (N=10 and 16 per group)
B. Bone marrow derived macrophages (BMM) fixed on cover slips and sections of spleens of TRAF1−/−/LDLR−/− and TRAF1+/+/LDLR−/− mice were stained with the differentiation markers FA-11 and F4/80 (N=3 each). Similarly, sections of atherosclerotic plaques in the aortic arches of TRAF1−/−/LDLR−/− and TRAF1+/+/LDLR−/− mice fed a high cholesterol diet for 18 weeks were labeled with the proliferation marker Ki-67 (N=6 and 13). Representative images are shown on top. FA-11, F4/80, and Ki-67-positive cells per 100 cells counted were quantified, pooled and are given as grouped scatter plots. C. TRAF1−/−/LDLR−/− and TRAF1+/+/LDLR−/− mice consumed a high cholesterol diet for 18 weeks. Sections of the aortic root were analyzed by TUNEL assay. TUNEL-positive staining in relation to total wall area is displayed as mean±SEM (N=5 and 13).
TRAF1 deficiency does not affect differentiation of monocytes/macrophages
TRAF1-deficient macrophages showed no significant difference in FA-11 and F4/80 expression. The number of Ki-67-positive macrophages did not significantly differ in plaques of TRAF1-deficient and control mice (N=6 and 13, p=0.9; 95% CI, 2.18–7.82 and 2.41–7.13, p=0.9, Fig.6B).
TRAF1 deficiency does not influence apoptosis in atherosclerotic plaques in vivo
TUNEL assays performed on sections of the aortic root and arch of our study animals revealed no significant difference in apoptosis rates in atherosclerotic plaques of TRAF1-deficient and -competent mice (N=5 and 13; p=0.88; 95% CI, 0.76–9.33 and 3.66–6.05, Fig. 6C). These data coincide with previous results of our group, demonstrating similar Caspase-3/7 activation in TRAF1-deficient and -competent EC 23.
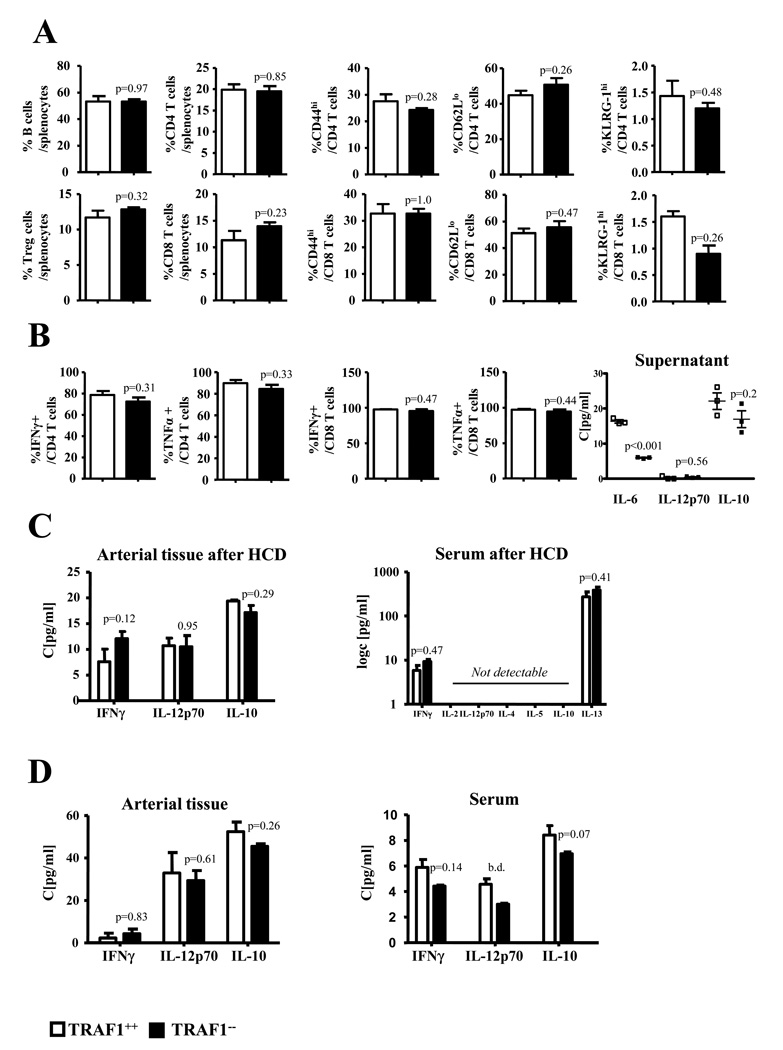
TRAF1 deficiency does not significantly alter general immune responses
CD4+ T lymphocytes comprise several subsets including Th1, Th2, Th17 cells, and Tregs. We tested whether TRAF1 modulates the ratios of these subsets, which may regulate atherogenesis. Splenic CD4, CD8 T cell and Treg numbers of mice consuming HCD did not significantly differ between TRAF1−/−/LDLR−/− and TRAF1+/+/LDLR−/− mice nor did T cell differentiation and activation markers (N=6, Fig.7A). Upon αCD3/αCD28 stimulation activation markers and intracellular IFNγ and TNFα did not significantly differ between splenocytes of both groups. In accord, supernatants showed no significant regulation of IFNγ, IL12p70, and IL-10 (N=3, Fig. 7B). Additionally, we did not observe modulation of Th1 and Th2 cytokines in blood and arterial tissue of TRAF1−/−/LDLR−/− and TRAF1+/+/LDLR−/− mice consuming HCD and respective mice upon intraperitoneal challenge with TNFα (Fig. 7C and D).
Figure 7. TRAF1 deficiency does not alter general immune responses.
(A) Splenocytes were analyzed ex vivo for CD4 and C8 T cells and their activation (CD44hi, CD62lo) and proliferation marker (KLRG-1hi). (B) Splenocytes were activated with αCD3/αCD28 for 3 days and intracellular TNFα and IFNγ determined. Supernatants were assayed for IL-6, IL12p70, and IL-10.
(C) Serum of mice consumed a high cholesterol diet was examined for IFNγ, IL-2, IL12p70, IL-4, IL-5, IL-10 and IL-13. In addition, IFNγ, IL12p70 and IL-10 was assessed in arterial tissue of atherosclerotic and TNFα-stimulated mice as well as in the serum of TNFα -stimulated mice. Data represents mean±SEM.
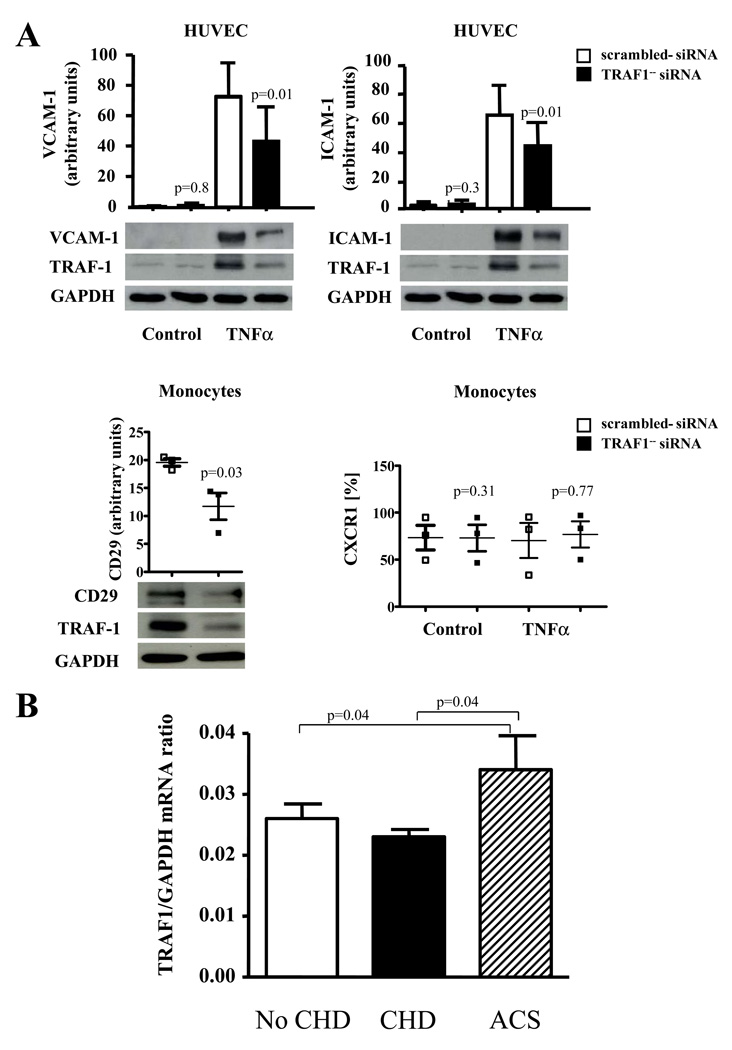
RNA silencing in human cells corroborates the concept that TRAF1 limits the expression of adhesion molecules
TRAF1-silenced EC expressed significantly reduced levels of VCAM-1 and ICAM-1 upon stimulation with TNFα (p= 0.01 and 0.01) and IL-1β (p=0.01 and 0.03) as well as VCAM-1 expression upon CD40L stimulation (p=0.01), confirming our findings in Murine EC (N=4 each, Fig. 8A). TRAF1-silenced human monocytes expressed significantly lower amounts of integrin beta1 compared with respective control-silenced monocytes (N=3, p=0.03), while no significant difference in CXCR1 and CD11b expression could be detected (Fig. 8A).
Figure 8. TRAF1-silencing in EC and monocytes limits the expression of adhesion molecules and integrines and TRAF1 mRNA expression increases in acute coronary syndromes in humans.
A. Human umbilical endothelial cells and human monocytes were transfected with TRAF1-specific- or scrambled siRNA. Cells were stimulated with TNFα (20ng/ml) for 24h and expression levels of VCAM-1 and ICAM-1 (N=4 each) in endothelial cells, and Integrin beta 1(CD29) and CXCR1 (N=3 each) in human monocytes analyzed by Western blotting. Densitometric results adjusted for GAPDH are presented as mean±SEM on top and as grouped scatter blot, representative blots below.
B. 325 patients undergoing coronary angiography were divided into the three groups: no coronary heart disease (No CHD), stable coronary heart disease (CHD), and acute coronary syndromes (ACS). TRAF1 and GAPDH mRNA was analyzed by quantitative real-time PCR in total blood RNA. Spearman correlation coefficients for continuous variables were used to assess univariate correlations of TRAF1 levels with all variables. Results are presented as mean±standard error computed from the average measurements obtained from each group.
TRAF1 mRNA expression is elevated in blood of patients with acute coronary syndrome
We tested the hypothesis that TRAF1 expression in blood correlates with stable and/or acute coronary heart disease in humans 23. Given that TRAF1 constitutes an intracellular protein, we quantified TRAF1 mRNA in total blood RNA of 325 patients undergoing coronary angiography divided into three groups: no coronary heart disease (No CHD), stable coronary heart disease (CHD), and acute coronary syndrome (ACS). Gender and BMI did not significantly differ among the groups, while patients were older in the CHD group and presented with a significantly greater percentage of cardiovascular risk factors such as diabetes and hypertension in the CHD and ACS groups (Table 1). TRAF1/GAPDH mRNA ratios were significantly elevated in patients with ACS compared to CHD and no CHD (0.034±0.0056, 0.023±0.0012, 0.026±0.0024, p=0.04 both, Fig. 8B). After adjusting for age and sex, the association remained significant (beta=0.16, p=0.039). After adjusting for other potential confounders (diabetes, hypertension, BMI, and dyslipidemia) it only approached the limit of statistical significance (beta=0.16, p=0.054).
Table 1.
Demographic and clinical characteristics of clinical study participants
| No CHD (n=77) |
CHD (n=178) |
ACS (n=70) |
|
|---|---|---|---|
| AGE (years) | 62 ±1 | 65±0.6 (p=0.004) | 64±1 |
| BMI (Kg/m2) | 28.2±0.5 | 27.5±0.3 | 27.8±0.5 |
| % men | 71 | 83 | 79 |
| % diabetes | 7.6 | 24.6 (p=0.01) | 25.7 (p=0.03 vs. No CHD) |
| % hypertension | 12.8 | 40.2 (p=0.015) | 15.5 (p=0.04 vs. CHD) |
| % smokers | 9.1 | 27.4 (p=0.039) | 14 |
| SBP | 131±1 | 131±1 | 133±2 |
| DBP | 77±1 | 77±1 | 79±1 |
| CREATININE | 0.94±0.02 | 1.08±0.05 (p=0.02) | 1.03±0.04 (p=0.04 vs. No CHD) |
| GLUCOSE | 110±4 | 114±3 | 120±6 |
| CHOLESTEROL | 202±7 | 182±21 (p=0.016) | 196±9 |
| TRYGLYCERIDES | 150±19 | 151±9 | 171±18 |
| LDL | 116±5 | 96±4 (p=0.019) | 99±7 (p=0.02 vs. No CHD) |
| VLDL | 35±4 | 33±2 | 40±3 (p=0.026 vs. CHD) |
| HDL | 52±3 | 48±1 | 53±5 |
| Creatine kinase | 109±9 | 117±12 | 709±155 (p<0.001 vs. No CHD), (p<0.001 vs. CHD) |
| Pro-BNP | 389±145 | 534±98 | 1330±509 (p=0.02 vs. No CHD), (p=0.03 vs. CHD) |
Discussion
This study reports the novel and surprising finding that TRAF1 deficiency attenuates atherosclerosis in mice. Our data challenge the traditional view of TRAF1 as an inhibitory, primarily anti-inflammatory signaling molecule by identifying TRAF1 as pro-inflammatory mediator of atherosclerosis, a chronic inflammatory disease.
While we previously showed that TRAF1 inhibits certain pro-inflammatory signals in cell types involved in atheromata, the current study demonstrates that TRAF1 acts as pro-inflammatory mediator of atherosclerosis since TRAF1-deficient mice had reduced atherogenesis 23. Our data accord with two recent reports by Oyoshi et al. implicating TRAF1 in the development of allergic lung inflammation in mice, suggesting that TRAF1 may also play an instrumental role in the pathogenesis of inflammatory diseases other than atherosclerosis 30, 31. Notably, overexpression of TRAF1 has also occurred in hematologic disorders such as lymphoma and solid tumors25, 26. Genome-wide studies further showed a correlation between polymorphisms in the TRAF1/C5 locus and the incidence of rheumatoid arthritis and systemic lupus erythematosus27, 28. However, functional analysis of TRAF1 in these diseases is still lacking.
The present study not only showed reduction in overall lesion size in early and delayed TRAF1−/−/LDLR−/− plaques compared with respective controls but that TRAF1-deficient plaques showed histologic features attributed to more stable plaques in humans. Plaques from TRAF1−/−/LDLR−/− animals had a marked reduction in macrophages. Intimal macrophages contribute importantly to atherogenesis, their accumulation progresses during plaque growth, and associates with thrombotic complications 2, 29. Four main processes regulate macrophage content in plaques: adhesion, migration, differentiation, and apoptosis. To gain mechanistic insight into how TRAF1 modulates intraplaque macrophage content, we systematically tested whether TRAF1 affects any of these steps. Interaction of integrins and adhesion molecules such as ICAM-1 and VCAM-1 mediates adhesion of inflammatory cells to the endothelial cell surface. We identified that deficiency of TRAF1 in Murine EC and monocytes reduced adhesion of inflammatory cells to the endothelium in static and dynamic adhesion assays in vitro and in intravital microscopy of the cremaster muscle in vivo. This may be due to decreased expression of VCAM-1 and ICAM-1 which we found both in lysates of EC and aortic arterial tissue of TRAF1-deficient mice. VCAM-1 binds to α4β1 integrin (CD49d/CD29). Interestingly, CD29 was also downregulated in its expression in TRAF1-deficient macrophages, suggesting that TRAF1 on both leukocytes and EC contributes to atherogenesis. This notion is supported by our transplantation study showing that TRAF1 deficiency on both BM-derived and resident cells suffices to reduce lesion formation. In agreement with our findings, Oyoshi et al. demonstrated impaired lymphocyte recruitment to inflamed lungs by reduced expression of VCAM-1 and ICAM-1 in TRAF1-deficient EC 30.
Chemokines pave the way for monocyte extravasation 31. TRAF1 deficiency did not attenuate chemokine expression in EC and macrophages. At the receptor level CCR7, previously associated with regression of atherosclerosis, and CXCR3, known to promote atherogenesis in mice, were both upregulated on monocytes and T cells of TRAF1−/−/LDLR−/− mice 32, 33. CCR5 also known to promote atherosclerosis in mice, however, remained unchanged on monocytes and decreased on T cells34. Nevertheless TRAF1 deficiency did not alter the migratory capacity of PBMCs towards chemokine gradients.
We identified reduced levels of IL-6 and TNFα in serum and arterial tissue of TRAF1-deficient mice consuming HCD. Increasing evidence supports that CD4 T cells play a crucial role in atherogenesis 1. Imbalance of the ratio of the different CD4 T cell subsets Th1, Th2, Th17 and Tregs modulates atherogenesis and plaque destabilization. Bryce et al. demonstrated TRAF1-dependent upregulation of the Th2 cytokines IL-4, IL-5, and IL-13 upon αCD3/αCD28 stimulation in TRAF1-deficient cells compared with wild-type cells 35. In contrast, we observed no major variation in subset ratios and Th1 and Th2 cytokines in our atherosclerotic mice in vitro and in vivo.
Several studies suggested an anti-apoptotic function of TRAF1 36–38. Our data showed no significant difference in apoptosis or proliferation of macrophages in vivo, suggesting that TRAF1 does not influence cell turnover in the atherosclerotic plaque. Since TRAF1 deficiency did not fully prevent atherosclerosis other TRAFs may contribute. TRAF2 and 6 are likely candidates 19, 20.
To investigate the hypothesis that TRAF1 mRNA expression in blood correlates with stable and/or acute coronary heart disease in humans we performed a pilot study. TRAF1 mRNA levels were significantly elevated in patients suffering from ACS, consistent with participation of TRAF1 in plaque instability in vivo. These data match previous findings of our group demonstrating a strong increase of TRAF1 in carotid human atherosclerotic plaques prone to rupture 23. Further studies will have to validate these findings.
In summary, we present the novel finding that TRAF1 deficiency attenuates atherogenesis in mice. We identified impaired monocyte recruitment to the vessel wall as the most likely underlying mechanism. Our data suggest that TRAF1 merits further testing as a therapeutic target in atherosclerosis and other chronic inflammatory diseases.
Supplementary Material
Acknowledgement
We thank Dr. Michael Reth from the Max-Planck-Institute for his great mentorship. Dr. Uwe Schönbeck from Pfizer, USA for the important and fruitful discussions on the topic, Dr. Jens Stein from the University of Bern, Switzerland for excellent training and advice on intravital microscopy, Dr. Marie Follow for her expert advice on qPCR and Dr. Florian Willecke for critical review of the manuscript.
Funding Sources: This work was supported by research grants from the German Research Foundation to AZ (DFG ZI743/3-1 and 3-2) and from the U.S. National Heart Lung Blood Institute to PL (HL34636). Dr. Libby’s laboratory also receives research funding from the DW Reynolds Foundation and the Fondation Leducq. AM was funded by the Excellence Initiative of the German Research Foundation (DFG GSC-4, Spemann Graduate School).
Footnotes
Conflict of Interest Disclosures:
Anna Missiou - Nothing to disclose
Natascha Köstlin - Nothing to disclose
Nerea Varo - Nothing to disclose
Philipp Rudolf - Nothing to disclose
Christian Münkel - Nothing to disclose
Sandra Ernst - Nothing to disclose
Carina Walter - Nothing to disclose
Peter Stachon - Nothing to disclose
Benjamin Sommer - Nothing to disclose
Dietmar Pfeifer - Nothing to disclose
Katja Zirlik - Nothing to disclose
Lindsey MacFarlane - Nothing to disclose
Dennis Wolf - Nothing to disclose
Erdyni Tsitsikov - Nothing to disclose
Christoph Bode - Nothing to disclose
Peter Libby - Nothing to disclose
Andreas Zirlik - Nothing to disclose
Subject codes: [130] Animal models of human disease, [134] Pathophysiology, [138] Cell signalling/signal transduction, [96] Mechanism of atherosclerosis/growth factors
Literature
- 1.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6(7):508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24(11):2137–2142. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 4.Chi H, Messas E, Levine RA, Graves DT, Amar S. Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high-fat diet in a murine apolipoprotein E heterozygote model: pharmacotherapeutic implications. Circulation. 2004;110(12):1678–1685. doi: 10.1161/01.CIR.0000142085.39015.31. [DOI] [PubMed] [Google Scholar]
- 5.Mach F, Schonbeck U, Sukhova GK, Atkinson E, Libby P. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature. 1998;394(6689):200–203. doi: 10.1038/28204. [DOI] [PubMed] [Google Scholar]
- 6.Bradley JR, Pober JS. Tumor necrosis factor receptor-associated factors (TRAFs) Oncogene. 2001;20(44):6482–6491. doi: 10.1038/sj.onc.1204788. [DOI] [PubMed] [Google Scholar]
- 7.Dempsey PW, Doyle SE, He JQ, Cheng G. The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev. 2003;14(3–4):193–209. doi: 10.1016/s1359-6101(03)00021-2. [DOI] [PubMed] [Google Scholar]
- 8.Rothe M, Sarma V, Dixit VM, Goeddel DV. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science (New York, N.Y. 1995;269(5229):1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- 9.Zapata JM, Reed JC. TRAF1: lord without a RING. Sci STKE. 2002;2002(133):PE27. doi: 10.1126/stke.2002.133.pe27. [DOI] [PubMed] [Google Scholar]
- 10.Carpentier I, Beyaert R. TRAF1 is a TNF inducible regulator of NF-kappaB activation. FEBS Lett. 1999;460(2):246–250. doi: 10.1016/s0014-5793(99)01356-3. [DOI] [PubMed] [Google Scholar]
- 11.Dunn IF, Sannikova TY, Geha RS, Tsitsikov EN. Identification and characterization of two CD40-inducible enhancers in the mouse TRAF1 gene locus. Molecular immunology. 2000;37(16):961–973. doi: 10.1016/s0161-5890(01)00015-3. [DOI] [PubMed] [Google Scholar]
- 12.Fotin-Mleczek M, Henkler F, Hausser A, Glauner H, Samel D, Graness A, Scheurich P, Mauri D, Wajant H. Tumor necrosis factor receptor-associated factor (TRAF) 1 regulates CD40-induced TRAF2-mediated NF-kappaB activation. J Biol Chem. 2004;279(1):677–685. doi: 10.1074/jbc.M310969200. [DOI] [PubMed] [Google Scholar]
- 13.Song HY, Rothe M, Goeddel DV. The tumor necrosis factor-inducible zinc finger protein A20 interacts with TRAF1/TRAF2 and inhibits NF-kappaB activation. Proc Natl Acad Sci U S A. 1996;93(13):6721–6725. doi: 10.1073/pnas.93.13.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsitsikov EN, Laouini D, Dunn IF, Sannikova TY, Davidson L, Alt FW, Geha RS. TRAF1 is a negative regulator of TNF signaling. enhanced TNF signaling in TRAF1-deficient mice. Immunity. 2001;15(4):647–657. doi: 10.1016/s1074-7613(01)00207-2. [DOI] [PubMed] [Google Scholar]
- 15.Pryhuber GS, Huyck HL, Roper JM, Cornejo J, O'Reilly MA, Pierce RH, Tsitsikov EN. Acute tumor necrosis factor-alpha-induced liver injury in the absence of tumor necrosis factor receptor-associated factor 1 gene expression. The American journal of pathology. 2005;166(6):1637–1645. doi: 10.1016/s0002-9440(10)62474-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie P, Hostager BS, Munroe ME, Moore CR, Bishop GA. Cooperation between TNF receptor-associated factors 1 and 2 in CD40 signaling. J Immunol. 2006;176(9):5388–5400. doi: 10.4049/jimmunol.176.9.5388. [DOI] [PubMed] [Google Scholar]
- 17.Duckett CS, Gedrich RW, Gilfillan MC, Thompson CB. Induction of nuclear factor kappaB by the CD30 receptor is mediated by TRAF1 and TRAF2. Molecular and cellular biology. 1997;17(3):1535–1542. doi: 10.1128/mcb.17.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukundan L, Bishop GA, Head KZ, Zhang L, Wahl LM, Suttles J. TNF receptor-associated factor 6 is an essential mediator of CD40-activated proinflammatory pathways in monocytes and macrophages. J Immunol. 2005;174(2):1081–1090. doi: 10.4049/jimmunol.174.2.1081. [DOI] [PubMed] [Google Scholar]
- 19.Donners MM, Beckers L, Lievens D, Munnix I, Heemskerk J, Janssen BJ, Wijnands E, Cleutjens J, Zernecke A, Weber C, Ahonen CL, Benbow U, Newby AC, Noelle RJ, Daemen MJ, Lutgens E. The CD40-TRAF6 axis is the key regulator of the CD40/CD40L system in neointima formation and arterial remodeling. Blood. 2008;111(9):4596–4604. doi: 10.1182/blood-2007-05-088906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sotoudeh M, Li YS, Yajima N, Chang CC, Tsou TC, Wang Y, Usami S, Ratcliffe A, Chien S, Shyy JY. Induction of apoptosis in vascular smooth muscle cells by mechanical stretch. Am J Physiol Heart Circ Physiol. 2002;282(5):H1709–H1716. doi: 10.1152/ajpheart.00744.2001. [DOI] [PubMed] [Google Scholar]
- 21.Urbich C, Mallat Z, Tedgui A, Clauss M, Zeiher AM, Dimmeler S. Upregulation of TRAF-3 by shear stress blocks CD40-mediated endothelial activation. J Clin Invest. 2001;108(10):1451–1458. doi: 10.1172/JCI13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo D, Luo Y, He Y, Zhang H, Zhang R, Li X, Dobrucki WL, Sinusas AJ, Sessa WC, Min W. Differential functions of tumor necrosis factor receptor 1 and 2 signaling in ischemia-mediated arteriogenesis and angiogenesis. The American journal of pathology. 2006;169(5):1886–1898. doi: 10.2353/ajpath.2006.060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zirlik A, Bavendiek U, Libby P, MacFarlane L, Gerdes N, Jagielska J, Ernst S, Aikawa M, Nakano H, Tsitsikov E, Schonbeck U. TRAF-1, -2, -3, -5, and -6 are induced in atherosclerotic plaques and differentially mediate proinflammatory functions of CD40L in endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27(5):1101–1107. doi: 10.1161/ATVBAHA.107.140566. [DOI] [PubMed] [Google Scholar]
- 24.Sukhova GK, Schonbeck U, Rabkin E, Schoen FJ, Poole AR, Billinghurst RC, Libby P. Evidence for increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques. Circulation. 1999;99(19):2503–2509. doi: 10.1161/01.cir.99.19.2503. [DOI] [PubMed] [Google Scholar]
- 25.Zhang B, Wang Z, Li T, Tsitsikov EN, Ding HF. NF-kappaB2 mutation targets TRAF1 to induce lymphomagenesis. Blood. 2007;110(2):743–751. doi: 10.1182/blood-2006-11-058446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zapata JM, Krajewska M, Morse HC, 3rd, Choi Y, Reed JC. TNF receptor-associated factor (TRAF) domain and Bcl-2 cooperate to induce small B cell lymphoma/chronic lymphocytic leukemia in transgenic mice. Proc Natl Acad Sci U S A. 2004;101(47):16600–16605. doi: 10.1073/pnas.0407541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plenge RM, Seielstad M, Padyukov L, Lee AT, Remmers EF, Ding B, Liew A, Khalili H, Chandrasekaran A, Davies LR, Li W, Tan AK, Bonnard C, Ong RT, Thalamuthu A, Pettersson S, Liu C, Tian C, Chen WV, Carulli JP, Beckman EM, Altshuler D, Alfredsson L, Criswell LA, Amos CI, Seldin MF, Kastner DL, Klareskog L, Gregersen PK. TRAF1-C5 as a risk locus for rheumatoid arthritis--a genomewide study. N Engl J Med. 2007;357(12):1199–1209. doi: 10.1056/NEJMoa073491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurreeman FA, Goulielmos GN, Alizadeh BZ, Rueda B, Houwing-Duistermaat J, Sanchez E, Bevova M, Radstake TR, Vonk MC, Galanakis E, Ortego N, Verduyn W, Zervou MI, Consortium S, Roep BO, Dema B, Espino L, Urcelay E, Boumpas DT, van den Berg LH, Wijmenga C, Koeleman BP, Huizinga TW, Toes RE, Martin J. The TRAF1-C5 region on chromosome 9q33 is associated with multiple autoimmune diseases. Ann Rheum Dis. 2009 doi: 10.1136/ard.2008.106567. [DOI] [PubMed] [Google Scholar]
- 29.Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, Weissleder R. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci U S A. 2006;103(27):10340–10345. doi: 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oyoshi MK, Bryce P, Goya S, Pichavant M, Umetsu DT, Oettgen HC, Tsitsikov EN. TNF receptor-associated factor 1 expressed in resident lung cells is required for the development of allergic lung inflammation. J Immunol. 2008;180(3):1878–1885. doi: 10.4049/jimmunol.180.3.1878. [DOI] [PubMed] [Google Scholar]
- 31.Smith DF, Galkina E, Ley K, Huo Y. GRO family chemokines are specialized for monocyte arrest from flow. Am J Physiol Heart Circ Physiol. 2005;289(5):H1976–H1984. doi: 10.1152/ajpheart.00153.2005. [DOI] [PubMed] [Google Scholar]
- 32.Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc Natl Acad Sci U S A. 2006;103(10):3781–3786. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veillard NR, Steffens S, Pelli G, Lu B, Kwak BR, Gerard C, Charo IF, Mach F. Differential influence of chemokine receptors CCR2 and CXCR3 in development of atherosclerosis in vivo. Circulation. 2005;112(6):870–878. doi: 10.1161/CIRCULATIONAHA.104.520718. [DOI] [PubMed] [Google Scholar]
- 34.Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117(13):1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 35.Bryce PJ, Oyoshi MK, Kawamoto S, Oettgen HC, Tsitsikov EN. TRAF1 regulates Th2 differentiation, allergic inflammation and nuclear localization of the Th2 transcription factor, NIP45. Int Immunol. 2006;18(1):101–111. doi: 10.1093/intimm/dxh354. [DOI] [PubMed] [Google Scholar]
- 36.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281(5383):1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 37.Sabbagh L, Pulle G, Liu Y, Tsitsikov EN, Watts TH. ERK-dependent Bim modulation downstream of the 4-1BB-TRAF1 signaling axis is a critical mediator of CD8 T cell survival in vivo. J Immunol. 2008;180(12):8093–8101. doi: 10.4049/jimmunol.180.12.8093. [DOI] [PubMed] [Google Scholar]
- 38.Speiser DE, Lee SY, Wong B, Arron J, Santana A, Kong YY, Ohashi PS, Choi Y. A regulatory role for TRAF1 in antigen-induced apoptosis of T cells. The Journal of experimental medicine. 1997;185(10):1777–1783. doi: 10.1084/jem.185.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.