Abstract
Dietary interventions have been consistently proposed as a part of a comprehensive strategy to lower the incidence and severity of coronary heart disease (CHD), in the process providing long-term cardioprotection. Replacement of dietary saturated fatty acids (SFA) with higher intakes of monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA) has been reported to be inversely associated with risk of CHD. The observed lower incidence of CHD among populations consuming a Mediterranean-type diet, mainly enriched in MUFA from olive oil, has long supported the belief that MUFA are an optimal substitution for SFA. However, both epidemiologic and interventional studies suggest that although substituting MUFA-rich foods for SFA-rich foods in the diet can potentially lower total plasma cholesterol concentrations, this substitution does not lower the extent of coronary artery atherosclerosis. In addition, although recent evidence suggests that the source of MUFA (animal fat vs vegetable oils) may differentially influence the correlation between MUFA intake and CHD mortality, animal studies suggest that neither source is cardioprotective.
Keywords: Fatty acids, Dietary fat, Lipoprotein metabolism, Atherosclerosis, Cholesterol, Coronary heart disease, Low-density lipoproteins, High-density lipoproteins
Introduction
Lifestyle and dietary modifications are strongly recommended as an efficient, early interventional approach to modifying risk factors for coronary heart disease (CHD). For decades, numerous studies have examined the role of individual dietary fatty acids and how they affect serum cholesterol concentrations and CHD [1]. Two main findings stemmed from the seminal studies of Keys [2], de Lorgeril and Salen [3], Keys et al. [4], and Hegsted et al. [5]: 1) intake of saturated fatty acids (SFA) was the dietary factor most often correlated with both higher total plasma cholesterol (TPC) levels and higher rates of CHD; and 2) lower rates of CHD were found in populations consuming diets where olive oil is the key fat source (ie, Mediterranean diet). Accordingly, subsequent investigations sought to identify the best substitute for SFA among several candidates, including monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), carbohydrates, and protein. By using predictive equations, Keys et al. [4] and Hegsted et al. [5] first postulated that oleic acid, the major MUFA in olive oil, was neutral in its effect on serum cholesterol concentrations, being more similar to an equivalent amount of carbohydrate.
Over 20 years later, Mattson and Grundy [6, 7] re-examined the potential use of MUFA as a replacement for SFA, using genetically modified safflower oil, also termed oleinate, as the source of MUFA. Metabolic ward experiments suggested that a high level of MUFA provided a similar degree of low-density lipoprotein cholesterol (LDL-C) lowering as did PUFA [6]. Although it is still relatively popular to recommend MUFA-enriched diets in place of SFA-rich diets in cardiovascular disease prevention [8], recent evidence from both human and animal studies calls for a careful, controlled appraisal of the benefits of MUFA [9••, 10••].
Olive Oil Versus Other MUFA-Enriched Oils: Do They Have Different Effects on Plasma Cholesterol?
The mean daily intake of MUFA worldwide, as percentage of total energy, is estimated to range from 3.5% (in China) to 22.3% (in Greece). This wide variance indicates that dietary recommendations should account for region-specific food patterns and dietary habits [11]. Data obtained from the United States Department of Agriculture indicate that oleic acid (cis C18:1 n-9) comprises more than 92% of the MUFA consumed in MUFA-containing foods, whereas about 60% to 80% of oleic acid intake comes from olive oil. Vegetable oils (including olive oil, high-oleic safflower oil, high-oleic sunflower oil, and canola oil) are the richest sources of MUFA, followed by nuts (macadamia, hazelnuts, and pecans), fruit (avocado), and animal products (ground beef, pork, eggs, and bacon) [12]. Some of the available data are shown in Fig. 1. Fats from both animal and plant sources typically have percentages of MUFA≥20%, whereas very few fats are much lower in their percentage of MUFA. MUFAs coexist with SFA in many foods, especially in natural and processed animal products. Accordingly, in some cases, high MUFA and SFA intakes may be correlated, in which case MUFA might be positively associated with the progression of CHD [13].
Fig. 1.
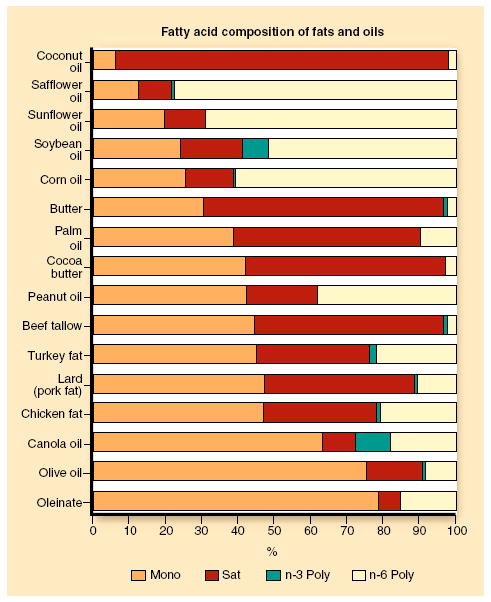
Average fatty acid percentage compositions of selected representative samples of fats and oils. Mono monounsaturated; n-3 omega 3; n-6 omega-6; Poly polyunsaturated; Sat saturated. (Data from Reeves and Weihrauch [52], and Rudel LL [unpublished data].)
In earlier studies, MUFA intake included both cis- and trans-isomers of oleic acid such as elaidic acid (C18:1 trans n-9, the most abundant trans MUFA in the Western diet) and vaccenic acid (C18:1 trans n-7), the former being the primary isomer found in partially hydrogenated vegetable oils whereas the latter is the most common trans isomer found in beef and dairy products. Mounting evidence has indicated that all fatty acids with a double bond in the trans configuration will promote higher LDL-C and lower high-density lipoprotein cholesterol (HDL-C) concentrations, so their intake is highly correlated with the progression of CHD [14, 15]. Thus, it is important to consider cis-MUFA and trans-MUFA separately.
Neutral effects of MUFA on plasma cholesterol levels in humans were first reported in the late 1950s and early 1960s by Keys et al. [2, 4], who used olive oil as the source of MUFA. Other MUFA-rich oils, such as canola or high-oleic safflower oil (oleinate), were not widely available at that time. Later, Mattson and Grundy [6] used oleinate as the source of MUFA, and suggested that MUFA may be a preferred candidate for SFA replacement in the diet because lower LDL-C was found with their MUFA-enriched diet without any lowering of HDL-C concentrations, as was found with their n-6 PUFA comparator diet.
Olive oil differs from other MUFA-rich oils (Fig. 1) in that it is 1) lower in PUFA (compared with canola and oleinate), 2) higher in SFA (compared with canola, oleinate, and high-oleic sunflower oils), 3) lower in plant sterols than other MUFA-rich oils (110 mg/100 g vs 250–440 mg/100 g), and 4) higher in squalene content (500 mg/100 g vs trace amounts in canola and oleinate) [16]. Although direct comparisons of consumption of olive oil and other MUFA-rich oils have been infrequent in humans, the fatty acid percentage composition differences may, in part, confound results regarding the ability of MUFA from different sources to modulate plasma cholesterol levels. All food fats are complex mixtures of fatty acids (Fig. 1), and interactions may not always be obvious.
Differences in the proportion of dietary energy as MUFA may contribute to the variance among previous reports. Further, whereas Keys et al. [2, 4] focused on the effects of MUFA on TPC, Mattson and Grundy [6] evaluated MUFA effects on plasma LDL-C and HDL-C levels. This may have some relevance when the effects of MUFA are compared with carbohydrate. Mensink et al. [17] reported that when olive oil was isocalorically replaced with complex carbohydrate, TPC and LDL-C concentrations were only slightly lower, whereas HDL-C decreased and fasting triglyceride concentrations increased. In addition, the type of olive oil could affect the degree of atheroprotection. For example, virgin olive oil retains most of its lipophilic components (including alpha-tocopherol, beta-carotene, and phenolic flavonoid compounds with strong antioxidant properties), whereas refined olive oil loses most of its antioxidants during the refining process [18]. As a result, compared with virgin olive oil, refined olive oil was less effective at increasing the resistance of LDL to oxidative stress [19] and at reducing TPC and the TPC/ HDL-C ratio in adults with mild abdominal obesity [20].
Although most human and animal studies have examined the atheroprotective effects of dietary sources of MUFA, one cannot exclude the relative contribution of MUFA synthesized de novo from SFA or from glucose. Fatty acid elongases (Elovl-5 and Elovl-6) and desaturases (stearoyl-CoA-desaturase 1 [SCD1]) mediate the conversion of SFA into MUFA such as palmitoleate (C16:1 n-7) or oleate (C18:1 n-9) and are rate-limiting enzymes for MUFA biosynthesis. Recently, Green et al. [21] comprehensively analyzed enzymes involved in MUFA synthesis. Using the rat insulinoma-1 cell line, they showed that Elovl-5 preferentially converts palmitoleic acid (C16:1 n-7) to C18:1 n-7 whereas Elovl-6 elongates mostly palmitic acid (C16:0) to stearic acid (C18:0), which can then be further desaturated by SCD1 to oleic acid. These findings, along with the observation that Elovl enzymes can elongate exogenous fatty acids, provide suggestions on how de novo synthesis of specific n-7 versus n-9 MUFA species may occur in vivo. Nevertheless, whether modulating MUFA synthesis via Elovl-5 and Elovl-6 would have different physiologic outcomes is yet to be determined.
The possibility that selected dietary MUFA species may have different effects on cholesterol metabolism is supported by some human and animal studies. Palmitoleic acid (enriched in macadamia nuts) has been reported to increase TPC and LDL-C compared with oleic acid in hypercholesterolemic individuals [22] but appears to provide a more favorable lipid and lipoprotein profile compared with a typical Western diet [23, 24]. Recently, increased proportions of palmitoleic acid, along with oleic and palmitic acid, in serum cholesteryl esters were associated with a higher incidence of death from cardiovascular disease, whereas higher levels of cholesteryl linoleate were associated with a lower incidence of CVD death [10••]. Although the interest of nutritionists and researchers has focused on oleic acid and olive oil, growing experimental evidence casts doubt on the notion that increasing MUFA intake per se is an effective strategy to provide cardioprotection. Nevertheless, apparently based on the higher life expectancy associated with a Mediterranean dietary pattern with olive oil as the key fat source, the United States Food and Drug Administration in 2004 authorized the use of health claims for olive oil. A recent summary of the data linking MUFA and CHD recommends reconsideration of any recommendation of MUFA as a proper substitute for SFA [25].
Epidemiologic Evidence for Cardioprotection Based on the Mediterranean Diet
A large body of epidemiologic evidence based on the “Mediterranean dietary pattern” (MDP) has been used to support the notion that oleic acid–rich diets confer cardioprotection [26-29]. However, no primary prevention trial or prospective cohort study has assessed the association between adherence to an MDP and the incidence of a first coronary event. In the mid 1990s, Trichopoulou et al. [30] first described the MetDiet Score System as a tool to assess conformity with the MDP; however this tool does not differentiate oleic acid (or MUFA) from the other components of the MDP and does not account for other factors in Mediterranean diets, such as moderate consumption of red wine and a significant consumption of omega-3 fatty acid-rich foods. The early definition of MDP (a high intake of olive oil–derived MUFA, reduced intake of meat and dairy products, and increased consumption of green leafy vegetables and fruit) is not the current dietary pattern in Mediterranean regions [3]. Contrary to popular thought, Mediterranean populations do eat dairy products (although mainly derived from goat’s milk instead of cow’s milk), pork, and are not vegetarian.
Most dietary intervention studies have used surrogate markers for CHD risk (such as LDL-C and HDL-C levels) and not CHD mortality rate. As reviewed earlier by Brown et al. [25], when the extent of atherosclerosis (as the underlying cause of CHD) was directly assessed following dietary intervention, both human and animal studies consistently documented that MUFA were not atheroprotective compared with SFA. Studies in non-human primates and transgenic mouse models helped to identify the hepatic enzyme acyl-CoA: cholesterol-O-acyltransferase 2 (ACAT2) as a critical player in mediating MUFA-induced alterations in atherosclerosis and provided experimental evidence for the use of plasma LDL cholesteryl oleate as a predictor (or biomarker) for atherosclerosis [31-34, 35••, 36].
There is no consensus on the best way to measure fatty acid intake and its relationship to incidence of CHD. Some studies support the use of serum cholesteryl ester (CE) fatty acid composition to evaluate dietary fatty acids [37, 38], whereas others have related serum fatty acid composition with mortality or CHD end points [39, 40]. As a result, studies that measure MUFA intake using plasma fatty acid compositions or MUFA enrichment in either plasma CE or phospholipid (PL) may not always accurately reflect MUFA intake. Of note, PL-MUFA levels do not necessarily reflect the dietary intake because MUFA can be synthesized de novo. On the other hand, PL-PUFA may be more reflective of fatty acid intake because humans cannot synthesize these FA [41, 42]. However, the proportion of MUFA and PUFA within different plasma lipid classes varies considerably.
There is an increasing interest in elucidating the atheroprotective potential of minor, though highly bioactive, non-MUFA components in olive oil. Results from the EUROLIVE Study suggest that more work is needed to pinpoint which dietary components (possibly not from olive oil) are responsible for the anti-inflammatory, antioxidant, and vasodilatory properties observed in people consuming a Mediterranean-type diet [43, 44].
The relative benefits of MUFA-rich diets compared with carbohydrate-enriched or protein-enriched diets in the management of CHD risk factors (eg, elevated blood pressure, high plasma triglycerides, and postprandial dyslipidemia) is still a matter of debate, especially in individuals at high risk of metabolic abnormalities for whom the preferred replacement for SFA is unresolved. Recent data from the Dietary Effects on Lipoproteins and Thrombogenic Activity (DELTA) trial [45] and OmniHeart study [46] provide suggestions of relevance, although their findings are based only on surrogate markers of CHD. DELTA trial investigators found that when 7% of energy from SFA was replaced with MUFA, a significant reduction in TPC, LDL-C, and HDL-C could be measured in metabolic syndrome patients [45]. Similarly, the OmniHeart Study in healthy individuals showed that a partial substitution of carbohydrate with either protein or monounsaturated fat can lower both blood pressure and triglyceride levels [46].
Can Plant-Derived and Animal-Derived MUFA have Different Influences on Cardiovascular Risk?
The Atherosclerosis Risk in Communities (ARIC) study, a cohort study of healthy middle-aged adults, provided the first evidence of the association between plasma PL and CE fatty acid composition and the surrogate marker for CHD, carotid artery wall intima-medial thickness [47, 48]. After adjustment for age and energy intake, SFA and MUFA intake—along with animal fat and cholesterol—were positively correlated with carotid artery wall thickness, as was plasma cholesteryl oleate, whereas vegetable fat and PUFA intakes, and plasma cholesteryl linoleate, were negatively correlated. Although the ARIC study paved the way for new investigations aimed at identifying correlations between CHD risk and plasma fatty acid composition, the caveats mentioned previously discussing the difficulty of associating plasma fatty acid composition with dietary fatty acid intake still apply.
With the exception of the work by Laaksonen et al. [39], the relationship between serum MUFA and CHD mortality has been poorly documented. The Uppsala Longitudinal Study of Adult Men (ULSAM) [10••] studied approximately 2000 men with a mean age at baseline of 50 years who were re-examined at ages 60, 70, 77, and 82 years to assess relationships between dietary fat biomarkers, desaturase indexes, and mortality. Dietary oleic acid was mainly derived from meat and dairy products in this Swedish population. Serum cholesteryl palmitate, palmitoleate, and oleate percentages were positively and significantly related to both total and cardiovascular mortality whereas that for cholesteryl linoleate was inversely associated. The most robust evidence to date relating MUFA intake to CHD risk comes from a pooled analysis of 11 cohort studies that comprised 344,696 individuals, 5249 CHD events, and 2155 coronary deaths [9••]. Using substitution models, Jakobsen et al. [9••] tested the hypothesis that energy intake from unsaturated fatty acids or carbohydrate should replace energy intake from SFA to prevent CHD. They found a direct association between substitution of MUFA for SFA and risk of CHD events, but no association with coronary deaths. On the other hand, for each 5% of energy from SFA replaced by PUFA, a significant inverse association between PUFA and risk of coronary events and deaths was demonstrated.
As with the ULSAM Study, a major source of MUFA in these 11 studies was animal fat (meat and dairy products), so confounding from other dietary components in these sources cannot be excluded. However, when the data were adjusted to account for different effects of cis-MUFA versus trans-MUFA, no changes in the hazard ratio were found. These data support previous studies [10••, 25, 32-34, 36] in suggesting that for CHD prevention, isocaloric replacement of SFA with PUFA is preferable to replacement with MUFA or carbohydrate.
Interestingly, this work has intensified the debate about the source of MUFA (animal-derived vs plant-derived) and how this might account for different degrees of cardioprotection [49]. In northern European and US populations, MUFA and SFA intakes often were linked, which may have obscured potential benefits of MUFA intake. On the other hand, the observation that earlier studies consistently used plant-derived MUFA (e.g, olive and vegetable oils) may support the notion that other components of these oils can contribute to a cardioprotective profile. However, in studies in non-human primates and mice [32-34, 35••, 36], dietary MUFA from vegetable sources did not reduce the extent of atherosclerosis compared with SFA, whereas PUFA offered protection. On balance then, fatty acid composition, per se, regardless of the fat source, appears to be a significant part of the answer.
Based on the current MDP, studies promoting adherence to MDP may not provide clear-cut answers about cardio-protective effects. Additionally, nutrigenomics has shown us that the individual responses to dietary fat may vary; accordingly, a personalized dietary regimen may be desirable for each individual to achieve protection from cardiovascular disease [50, 51]. In this context, it is possible that SFA replacement with MUFA might be beneficial for selected individuals. Nevertheless, many types of evidence have shown that PUFA replacement for SFA has effectively reduced CHD risk in both healthy and dyslipidemic individuals and in populations where the source of MUFA is either animal or vegetable [47], suggesting that this substitution is overall more beneficial for protection from cardiovascular disease.
Conclusions
Cumulative evidence suggests that dietary and lifestyle modifications may help prevent cardiovascular disease and should be considered as a primary treatment before cholesterol-lowering or blood pressure–lowering drugs are used. A major area of uncertainty for the past 50 years has been the relationship among the type of dietary fat, CHD, and associations with well-known CHD risk factors (e.g, serum cholesterol, hypertension, obesity, and inflammation). Based on the higher life expectancy associated with a MDP (where olive oil is the key fat source) and subsequent epidemiologic evidence, in 2004 the United States Food and Drug Administration authorized the use of health claims for olive oil; however, a recent appraisal based on a large body of experimental evidence suggests that MUFA might not be the proper substitute for SFA [25]. The debate continues on the optimal dietary fatty acid composition, but the evidence supporting MUFA as the healthy dietary fatty acid is weak. Even when considering the food source of MUFA (plant vs animal), there is little evidence to support recommendations to increase dietary MUFA for CHD prevention. On the other hand, increasing dietary PUFA consistently appears to provide benefit.
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
Contributor Information
Chiara Degirolamo, Department of Translational Pharmacology, Consorzio Mario Negri Sud, via Nazionale 8/A, 66030, S. Maria Imbaro, CH, Italy, degirolamo@negrisud.it.
Lawrence L. Rudel, Department of Pathology, Section on Lipid Sciences, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157-1040, USA, lrudel@wfubmc.edu
References
Papers of particular interest, published recently, have been highlighted as:
-
••
Of major importance
- 1.Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659–669. doi: 10.1001/archinternmed.2009.38. [DOI] [PubMed] [Google Scholar]
- 2.Keys A. Coronary heart disease in seven countries. Circulation. 1970;41(Suppl1):1–211. [PubMed] [Google Scholar]
- 3.de Lorgeril M, Salen P. The Mediterranean diet: rationale and evidence for its benefits. Curr Atheroscl Rep. 2008;10:518–522. doi: 10.1007/s11883-008-0080-5. [DOI] [PubMed] [Google Scholar]
- 4.Keys A, Anderson JT, Grande F. Prediction of serum-cholesterol responses of man to changes in fats in the diets. Lancet. 1957;2:960–966. doi: 10.1016/s0140-6736(57)91998-0. [DOI] [PubMed] [Google Scholar]
- 5.Hegsted DM, McGandy RB, Myers ML, Stare FJ. Quantitative effects of dietary fat on serum cholesterol in man. Am J Clin Nutr. 1965;17:281–295. doi: 10.1093/ajcn/17.5.281. [DOI] [PubMed] [Google Scholar]
- 6.Mattson FH, Grundy SM. Comparison of effects of dietary saturated, monounsaturated and polyunsaturated fatty acids on plasma lipids and lipoproteins in man. J Lipid Res. 1985;26:194–202. [PubMed] [Google Scholar]
- 7.Grundy SM. Comparison of monounsaturated fatty acids and carbohydrates for lowering plasma cholesterol. N Engl J Med. 1986;314:745–748. doi: 10.1056/NEJM198603203141204. [DOI] [PubMed] [Google Scholar]
- 8.Lloyd-Jones D, Adams RJ, Todd M, et al. Heart Disease and Stroke Statistics 2010 Update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 9••.Jakobsen MU, O’Reilly E, Heitmann BL, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr. 2009;89:1425–1432. doi: 10.3945/ajcn.2008.27124.. This large follow-up study provides powerful evidence that over a wide range of intakes, MUFA does not provide CHD prevention.
- 10••.Warensjo E, Sundstrom J, Vessby B, et al. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a population-based prospective study. Am J Clin Nutr. 2008;88:203–209. doi: 10.1093/ajcn/88.1.203.. This study showed for the first time that when coronary mortality is assessed over a period of 30 years, serum MUFA levels are positively associated with coronary deaths.
- 11.Elmadfa I, Kornsteiner M. Dietary fat intake-a global perspective. Ann Nutr Metab. 2009;54(Suppl 1):8–14. doi: 10.1159/000220822. [DOI] [PubMed] [Google Scholar]
- 12.USDA National Nutrient database for standard reference. [May 17, 2009]; Available at www.ars.usda.gov/fnic/nutrientdata.
- 13.Ros E. Dietary cis-monounsaturated fatty acids and metabolic control in type 2 diabetes. Am J Clin Nutr. 2003;78(Suppl):617S–625S. doi: 10.1093/ajcn/78.3.617S. [DOI] [PubMed] [Google Scholar]
- 14.Watts GF, Jackson P, Burke V, Lewis B. Dietary fatty acids and progression of coronary artery disease in men. Am J Clin Nutr. 1996;64:202–209. doi: 10.1093/ajcn/64.2.202. [DOI] [PubMed] [Google Scholar]
- 15.Brouwer IA, Wanders AJ, Katan MB. Effect of animal and industrial trans fatty acids on HDL and LDL cholesterol levels in humans—a quantitative review. PLoS ONE. 2010;5:e9434. doi: 10.1371/journal.pone.0009434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Truswell AS, Choudhury N. Monounsaturated oils do not all have the same effect on plasma cholesterol. Eur J Clin Nutr. 1998;52:312–315. doi: 10.1038/sj.ejcn.1600566. [DOI] [PubMed] [Google Scholar]
- 17.Mensink RP, de Groot MJM, van den Broeke LT, et al. Effects of monounsaturated fatty acids vs. complex carbohydrates on serum lipoproteins and apoproteins in healthy men and women. Metabolism. 1989;38:172–178. doi: 10.1016/0026-0495(89)90258-8. [DOI] [PubMed] [Google Scholar]
- 18.Visioli F, Galli C. Antiatherogenic components of olive oil. Curr Atheroscl Rep. 2001;3:64–67. doi: 10.1007/s11883-001-0012-0. [DOI] [PubMed] [Google Scholar]
- 19.Ramirez-Tortosa MC, Urbano G, Lopez-Jurado M, et al. Extra-virgin olive oil increases the resistance of LDL to oxidation more than refined olive oil in free-living men with peripheral vascular disease. J Nutr. 1999;129:2177–2183. doi: 10.1093/jn/129.12.2177. [DOI] [PubMed] [Google Scholar]
- 20.Bos MB, de Vries JH, Feskens EJ, et al. Effect of a high monounsaturated fatty acid diet and a Mediterranean diet on serum lipids and insulin sensitivity in adults with mild abdominal obesity. Nutr Metab Cardiovasc Dis. 2009 Aug 17; doi: 10.1016/j.numecd.2009.05.008. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 21.Green CD, Ozguden-Akkoc CG, Wang Y, et al. Role of fatty acid elongases in determination of de novo synthesized monounsaturated fatty acid species. J Lipid Res. 2010;51:1871–1877. doi: 10.1194/jlr.M004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nestel P, Clifton P, Noakes M. Effects of increasing dietary palmitoleic acid compared with palmitic and oleic acids on plasma lipids of hypercholesterolemic men. J Lipid Res. 1994;35:656–662. [PubMed] [Google Scholar]
- 23.Griel AE, Cao Y, Bagshaw DD, et al. A macadamia nut-rich diet reduces total and LDL-cholesterol in mildly hypercholesterolemic men and women. J Nutr. 2008;138:761–767. doi: 10.1093/jn/138.4.761. [DOI] [PubMed] [Google Scholar]
- 24.Garg ML, Blake RJ, Wills RB. Macadamia nut consumption lowers plasma total and LDL cholesterol levels in hypercholesterolemic men. J Nutr. 2003;133:1060–1063. doi: 10.1093/jn/133.4.1060. [DOI] [PubMed] [Google Scholar]
- 25.Brown JM, Shelness GS, Rudel LL. Monounsaturated fatty acids and atherosclerosis: opposing views from epidemiology and experimental animal models. Curr Atheroscler Rep. 2007;9:494–500. doi: 10.1007/s11883-007-0066-8. [DOI] [PubMed] [Google Scholar]
- 26.Mensink RP, Katan MB. Effects of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb. 1992;12:911–919. doi: 10.1161/01.atv.12.8.911. [DOI] [PubMed] [Google Scholar]
- 27.Gardner CD, Kraemer HC. Monounsaturated versus polyunsaturated dietary fat and serum lipids. A meta-analysis. Arterioscler Thromb Vasc Biol. 1995;15:1917–1927. doi: 10.1161/01.atv.15.11.1917. [DOI] [PubMed] [Google Scholar]
- 28.Hu FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337:1491–1499. doi: 10.1056/NEJM199711203372102. [DOI] [PubMed] [Google Scholar]
- 29.Lòpez-Miranda J, Pèrez-Jimènez F, Ros E, et al. Olive oil and health: Summary of the II international conference on olive oil and health consensus report, Jaèn and Còrdoba (Spain) 2008. Nutr Metab Cardiovasc Dis. 2010;20:284–294. doi: 10.1016/j.numecd.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Trichopoulou A, Kouris-Blazos A, Wahlqvist ML, et al. Diet and overall survival in elderly people. BMJ. 1995;311:1457–1460. doi: 10.1136/bmj.311.7018.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carr TP, Parks JS, Rudel LL. Hepatic ACAT activity in African green monkeys is highly correlated to plasma LDL cholesteryl ester enrichment and coronary artery atherosclerosis. Arterioscler Thromb. 1992;12:1274–1283. doi: 10.1161/01.atv.12.11.1274. [DOI] [PubMed] [Google Scholar]
- 32.Rudel LL, Haines J, Sawyer JK, et al. Hepatic origin of cholesteryl oleate in coronary artery atherosclerosis in African green monkeys. Enrichment by dietary monounsaturated fat. J Clin Invest. 1997;100:74–83. doi: 10.1172/JCI119524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bell AT, III, Wilson MD, Kelley KL, et al. Monounsaturated fatty acyl-coenzyme A is predictive of atherosclerosis in human apoB-100 transgenic, LDLr -/- mice. J Lipid Res. 2007;48:1122–1131. doi: 10.1194/jlr.M600526-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Bell AT, III, Kelley KL, Wilson MD, et al. Dietary fat-induced alterations in atherosclerosis are abolished by ACAT2-deficiency in apoB100 only, LDLr-/- mice. Arterioscler Thromb Vasc Biol. 2007;27:1396–1402. doi: 10.1161/ATVBAHA.107.142802. [DOI] [PubMed] [Google Scholar]
- 35••.Degirolamo C, Shelness GS, Rudel LL. LDL cholesteryl oleate as a predictor for atherosclerosis: evidence from human and animal studies on dietary fat. J Lipid Res. 2009;50(Suppl):S434–S439. doi: 10.1194/jlr.R800076-JLR200.. This review article provides a comprehensive analysis of the current evidence supporting the notion that plasma lipid enrichment with MUFA is predictive for atherosclerosis.
- 36.Rudel LL, Parks JS, Sawyer JK. Compared to dietary monounsaturated and saturated fat, polyunsaturated fat protects African Green monkeys from coronary artery atherosclerosis. Arterioscler Thromb Vasc Biol. 1995;15:2101–2110. doi: 10.1161/01.atv.15.12.2101. [DOI] [PubMed] [Google Scholar]
- 37.Baylin A, Campos H. The use of fatty acid biomarkers to reflect dietary intake. Curr Opin Lipidol. 2006;17:22–27. doi: 10.1097/01.mol.0000199814.46720.83. [DOI] [PubMed] [Google Scholar]
- 38.Arab L. Biomarkers of fat and fatty acid intake. J Nutr. 2003;133(Suppl 3):925S–932S. doi: 10.1093/jn/133.3.925S. [DOI] [PubMed] [Google Scholar]
- 39.Laaksonen DE, Nyyssonen K, Niskanen L, et al. Prediction of cardiovascular mortality in middle-aged men by dietary and serum linoleic and polyunsaturated fatty acids. Arch Intern Med. 2005;165:193–199. doi: 10.1001/archinte.165.2.193. [DOI] [PubMed] [Google Scholar]
- 40.Harris WS, Poston WC, Haddock CK. Tissue n-3 and n-6 fatty acids and risk for coronary heart disease events. Atherosclerosis. 2007;193:1–10. doi: 10.1016/j.atherosclerosis.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 41.Harris WS. The Omega-3 Index: from biomarker to risk marker to risk factor. Curr Atheroscler Rep. 2009;11:411–417. doi: 10.1007/s11883-009-0062-2. [DOI] [PubMed] [Google Scholar]
- 42.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as biomarker of dietary intake. Prog Lipid Res. 2008;47:348–380. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Mi Covas, V R-G, de la Torre R, Kafatos A, et al. Minor components of olive oil: evidence to date of health benefits in humans. Nutr Rev. 2006;64(Suppl 1):20–30. [Google Scholar]
- 44.Cicero AF, Nascetti S, Lòpez-Sabater MC, et al. Changes in LDL fatty acid composition as a response to olive oil treatment are inversely related to lipid oxidative damage: the EUROLIVE Study. J Am Coll Nutr. 2008;27:314–320. doi: 10.1080/07315724.2008.10719705. [DOI] [PubMed] [Google Scholar]
- 45.Berglund L, Lefevre M, Ginsberg HN, et al. Comparison of monounsaturated fat with carbohydrates as a replacement for saturated fat in subjects with a high metabolic risk profile: studies in the fasting and postprandial states. Am J Clin Nutr. 2007;86:1611–1620. doi: 10.1093/ajcn/86.5.1611. [DOI] [PubMed] [Google Scholar]
- 46.Furtado JD, Campos H, Appel LJ, et al. Effect of protein, unsaturated fat, and carbohydrate intakes on plasma apolipoprotein B and VLDL and LDL containing apolipoprotein C-III: results from the OmniHeart Trial. Am J Clin Nutr. 2008;87:1623–1630. doi: 10.1093/ajcn/87.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tell GS, Evans GW, Folsom AR, et al. Dietary fat intake and carotid artery wall thickness: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol. 1994;139:979–989. doi: 10.1093/oxfordjournals.aje.a116947. [DOI] [PubMed] [Google Scholar]
- 48.Ma J, Folsum AR, Lewis L, Eckfeldt JH. Relation of plasma phospholipids and cholesterol ester fatty acid composition to carotid artery intima-media thickness in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 1997;65:551–559. doi: 10.1093/ajcn/65.2.551. [DOI] [PubMed] [Google Scholar]
- 49.Rohem E. The evidence-based Mediterranean diet reduces coronary heart disease risk, and plant-derived monounsaturated fats may reduce coronary heart disease risk. Am J Clin Nutr. 2009;90:697–698. doi: 10.3945/ajcn.2009.28148. [DOI] [PubMed] [Google Scholar]
- 50.Corella D, Ordovas JM. Nutrigenomics in cardiovascular medicine. Circ Cardiovasc Genet. 2009;2:637–651. doi: 10.1161/CIRCGENETICS.109.891366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lefevre M, Champagne CM, Tulley RT, et al. Individual variability in cardiovascular disease risk factor responses to low-fat and low-saturated-fat diets in men: body mass index, adiposity, and insulin resistance predict changes in LDL cholesterol. Am J Clin Nutr. 2005;82:957–963. doi: 10.1093/ajcn/82.5.957. [DOI] [PubMed] [Google Scholar]
- 52.Reeves JB, III, Weihrauch JL. Agriculture Handbook No 8–4 USDA Science and Education Administration. Washington, DC: US Government Printing Office; 1979. Composition of Foods: Fats and Oils Raw, Processed, Prepared. Consumer and Food Economics Institute. Revised June 1979. [Google Scholar]


