Abstract
Humoral immune responses to mismatched donor human leukocyte antigen (HLA) and major histocompatibility (MHC) class I related chain A (MICA) have been reported to contribute to immunopathogenesis of antibody mediated rejection (AMR) in early period (EP) and cardiac allograft vasculopathy (CAV) in late period (LP) following cardiac transplantation (HTx). The goal of this study is to define the roles of donor specific antibodies (DSA) and anti-MICA in AMR and CAV. 95 post-HTx recipients were enrolled; 43pts in EP (≤12months post-HTx) and 52pts in LP (>12months post-HTx). Development of DSA and anti-MICA were serially monitored using Luminex. Development of DSA (AMR+:n=6/8,75%, AMR−:n=4/35,11%, p=0.009) and anti-MICA (AMR+:n=5/8,63%, AMR−:n=4/35,11%, p=0.002) was significantly associated with AMR. AMR+DSA+ pts demonstrated increased anti-MICA levels compared to AMR+DSA− pts (p=0.01). Serial monitoring revealed DSA (2.7±1.4months) preceded development of anti-MICA (6.5±2.1months) in recipients diagnosed with AMR at 8.3±2.5months post-HTx. Development of DSA (CAV+:n=8/12,67%, CAV−: n=5/40,13%, p=0.004) and anti-MICA (CAV+:n=9/12,75%, CAV−:n=5/40,13%, p=0.001) was significantly associated with CAV. CAV+DSA+ pts demonstrated increased anti-MICA levels compared to CAV+DSA− pts (p=0.01). Abs to HLA are associated with and precede development of anti-MICA in AMR and CAV. Therefore, DSA and anti-MICA can be used as non-invasive markers for monitoring AMR and CAV.
Keywords: MICA, HLA, cardiac transplantation, antibody mediated rejection, cardiac allograft vasculopathy
1. Introduction
The immediate and long term success of human cardiac transplantation (HTx) is impeded by the development of antibody mediated rejection (AMR) and cardiac allograft vasculopathy (CAV) respectively. During the early post-operative period, it is estimated that 20–40% of HTx recipients develop AMR [1–5]. During the late post-HTX period, cardiac allograft vasculopathy (CAV) is a pathognomic feature of allograft dysfunction and contributes to increased mortality [6–9]. Alloantibodies directed against mismatched donor major histocompatibility complex (MHC) molecules have been linked to increased allograft failure during both early and late post-operative period [10–12]. However, a significant proportion of solid organ recipients demonstrate allograft failure although no antibodies (Abs) against major histocompatibility antigens are detected [13–15]. Increasingly, studies have demonstrated that Abs against non-classical MHC molecules, such as MHC class I polypeptide-related sequence A (MICA) can induce complement-dependent cytotoxicity and have been implicated in acute and chronic solid organ allograft rejection [13, 16, 17]. This extends to post-HTx recipients where a positive correlation between the development of circulating non-MHC Abs with allograft dysfunction has been demonstrated [18].
MICA is a highly polymorphic cell surface glycoprotein expressed on endothelial cells as well as fibroblasts and activated monocytes [19]. MICA is a ligand for NKG2D, which is an activating immunoreceptor found on natural killer (NK) cells and on CD8+ T cells [20, 21]. The NKG2D receptor acts as a co-stimulatory signal for CD8+ T cells which complements T-cell receptor mediated antigen recognition of target cells [22, 23]. The increased surface expression of MICA on graft endothelial cells during episodes of rejection can induce allorecognition leading to an amplified humoral and cellular mediated immune response, as substantiated by enhanced detection of anti-MICA in serum of allograft recipients with acute and chronic rejection [24, 25].
The incidence of anti-MICA varies considerably, between 3% in healthy individuals to more than 30% after kidney and heart transplantation [26]. In spite of confounding factors such as lack of sensitivity and/or specificity in detection systems and lack of test standardization which may contribute to the varying incidence of anti-MICA in different patient populations, many studies have suggested that MICA plays an important role in the alloimmune response following solid organ transplantation [17, 27]. In the case of HTx recipients, studies have demonstrated that nearly 40% of patients develop Abs against MICA during the first year post-transplant and are at an increased risk for the development of severe acute rejection [24]. Similarly, a recent retrospective study found that more than 20% of patients with cardiac rejection episodes had Abs against MICA, with the resultant multivariate analysis identifying anti-MICA positivity as an independent risk factor for the development of CAV [28]. Further, studies have demonstrated increased titers of anti-MICA in serum accompanied by an enhanced MICA expression on allograft endomyocardial biopsies in patients with increased episodes of acute cardiac allograft rejection [29].
It is important to elucidate whether there is a causative role for Abs to MICA in inducing adverse early or late cardiac allograft events. There is some evidence for the role of MICA in AMR in renal transplant recipients [30, 31]. However, there is a dearth of studies that have examined the role of MICA in AMR in HTx, which is a frequent cause of early adverse graft function. Given the evidence for the role of DSA in recipients who were subsequently diagnosed with AMR and CAV in early and late post-HTx period respectively, the objective of our present study was to evaluate the association between the development of DSA to mismatched HLA and serum levels of Abs against 10 commonly found MICA antigens in post-HTx patients. Our results demonstrate that DSA is significantly associated with the detection of anti-MICA in patients with AMR and CAV. Importantly, serial monitoring of postoperative sera in the early period indicated that detection of DSA precedes the detection of Abs to MICA in patients that developed AMR, thereby suggesting a temporal relationship between DSA and development of Abs to MICA following HTx.
2. Material and Methods
2.1 Study Population
In a protocol approved by the Institutional Review Board, 95 patients who underwent adult HTx at Barnes-Jewish Hospital/Washington University were enrolled in the study. Patient sera was collected and stored at −70°C. For the 43 patients in the early period (EP, ≤ 12 months post-HTx), serum samples were obtained every month following enrollment in the study. This coincided with the performance of surveillance or clinically indicated endomyocardial biopsy. In many cases, we were able to obtain a serum sample prior to HTx and immediately following HTx as well. For the 52 patients in the late period (LP, >12 months post-HTx), serum samples were obtained annually following enrollment in the study and it coincided with the performance of surveillance or clinically indicated angiogram. Utilizing guidelines recommended by the International Society of Heart and Lung Transplantation (ISHLT), a diagnosis of AMR was reached by examining clinical, histological and serological criteria [7]. Clinical criteria included patient symptoms (fatigue, palpitations), objective evidence of cardiac dysfunction (decreased ejection fraction and/or restrictive physiology on echocardiography, intravenous inotrope administration). Histological and immunopathologic criteria included capillary endothelial swelling as a marker of acute capillary injury, macrophages or neutrophils in capillaries and lack of features consistent with cellular rejection, positive C4d capillary staining and CD68 positivity for capillary macrophages. Serological criteria included detection of DSA to mismatched donor HLA. Once the attending transplant cardiologist assessed each of the above criteria and reached a diagnosis of AMR, initial treatment was initiated which included methylprednisone, plasmapheresis and intravenous immunoglobulins (IVIG). A diagnosis of CAV was reached based on angiographic evidence of coronary artery stenosis. Angiographic evidence of coronary artery stenosis (>50% luminal diameter) of 1 vessel or less was designated as none/minimal CAV while coronary artery stenosis (>50% luminal diameter) of 2 vessels or more was designated as moderate/severe CAV.
2.2 Detection of DSA by Luminex
Luminex technology (Biosource International Inc, CA) which utilizes a solid-phase assay, was used to identify DSA in patient sera. Primary Ab coated beads and incubation buffer were initially placed into 96-well filter plates. On an oribital shaker at room temperature, samples and standards were incubated with the primary Ab beads. Subsequently, the wells were washed and biotinylated secondary Abs were added prior to a 30 minute incubation period. Strepatividin-R-phyocoerythrin solution was added (after the wells were washed) and incubated for 15 minutes. The wells were washed for a final time and binding was determined with a dual-laser flow analyzer, the Luminex-100 system version 1.7. Data analysis was performed using the MasterPlex QT 1.0 system (MiraiBio Group, CA) and detection was compared to standard curves using a five-parameter regression formula.
2.3 Detection of Abs against MICA by Luminex
As described in a previous study from our laboratory, Abs to MICA were determined using LABScreen® assay (Luminex Technology, Biosource International Inc, CA) in 96-well filter plates according to the manufacturer’s specifications (One Lambda Inc, CA) [27]. The assay filter plate was pre-wet with 300µl of wash buffer (cat. # LSPWABUF) and on a platform plate shaker at low speed, it was incubated for 10 minutes. After aspirating the buffer with a Millipore vacuum manifold, 5µl of LABScreen beads along with 20µl of serum was dispensed into test wells. A negative control serum sample (OLI Cat. #LS-NC) was used in conjunction with a healthy volunteer’s serum to establish a cutoff for each test. With gentle shaking, the mixture was incubated for 30 minutes and washed with 275µl of wash buffer. 100µl of 1X PE conjugated anti-human IgG was added to each well and incubated for 30 minutes. Lastly, 80µl of 1X PBS was added and the samples were read using LABScan™ 100™ machine.
Serum samples were tested at 1:3 dilution for Abs against a panel of 10 commonly found MICA antigens (MICA *001, *002, *004, *007, *009, *012, *017, *018, *019, and *027). The fluorescent signal was measured using LABScan™ and analyzed by HLA-Visual™ software (One Lambda Inc, CA). The raw mean fluorescence intensity (MFI) values were normalized with negative control serum (OLI Cat no. LS-NC) and utilizing the formula: ([sample # N beads − sample negative control beads] − [negative control # N beads − negative control beads]). A reading was considered positive if the fluorescent signal of each bead was above the MFI of volunteer control sera and normal control sera provided by the company. Serum samples containing Abs against all 10 tested MICA antigens were used as positive controls and these samples were a kind gift from Miyuki Ozawa (One lambda Inc, CA).
2.4 Statistical Analysis
Data are represented as mean value ± standard deviation. Statistical analysis was performed using GraphPad Prism version 4.03 (GraphPad Software Inc, CA). Two-tailed student t test was used to compare levels of Abs to MICA in cohorts being compared. Correlation analysis was performed using Spearman rank test. Uni- and multivariate analysis was performed using SPSS software (SPSS Inc, IL) and statistical significance was defined at p < 0.05.
3. Results
3.1 Patient Demographics
Ninety five adult HTx recipients were enrolled. Forty three HTx recipients were in the EP cohort while the remaining 52 were in the LP cohort. Forty three patients were followed for development of acute AMR of whom 8 recipients met diagnostic criteria for acute AMR (AMR+). Fifty two patients were followed for development of CAV of whom 12 recipients had angiographic evidence of moderate or severe CAV (CAV+). As presented in Table 1, there is no significant difference in the demographic profile between AMR+ and AMR− recipients or CAV+ and CAV− recipients.
Table 1.
Patient Demographics
| Variable n (%) | Early HTx (n=43) | Late HTx (n=52) | ||||
|---|---|---|---|---|---|---|
| Total patients n = 95 | AMR (n=8) | No AMR (n=35) | p value | CAV (n=12) | No CAV (n=40) | p value |
| Age at HTx (yrs) | 43.6 ± 15.6 | 48.3 ± 17.5 | 0.25 | 52.2 ± 12.8 | 51.2 ± 9.3 | 0.32 |
| Male Gender(M:F) | 6:2 | 26:9 | 0.18 | 10:2 | 32: 8 | 0.29 |
| Ethnicity | ||||||
| Caucasian | 6 (75%) | 27 (77%) | 0.12 | 9 (75%) | 30 (75%) | 0.57 |
| African American | 2 (25%) | 6 (17%) | 0.17 | 2 (16%) | 8 (20%) | 0.35 |
| Other | 0 (0%) | 2 (5.7%) | 1.0 | 1 (8.3%) | 2 (5%) | 0.43 |
| LVAD prior to HTx | 2 (25%) | 8 (23%) | 0.71 | 3 (25%) | 11 (28%) | 0.12 |
| Mean f/u time (yrs) | 1.8 ± 0.8 | 1.6 ± 0.7 | 0.55 | 8.9 ± 4.9 | 10.1 ± 6.4 | 0.31 |
3.2 Antibodies to mismatched HLA (DSA) and MICA develop in AMR+ HTx recipients
Table 2 depicts the diagnostic profile of all EP cohort recipients in our study who were diagnosed with AMR. Of 8 recipients who were AMR+, 6 patients (75%) were DSA+ while 2 patients (25%) were DSA− (Table 3A). DSA+MICA+ patients (n=5, 63%) were more likely to develop AMR compared to DSA+MICA− patients (n=1, 13%, p=0.01). Both patients who were DSA− were MICA−. Conversely, 35 recipients were AMR−, of whom 31 patients (89%) were DSA− and 4 patients (11%) were DSA+. Though the number of patients who MICA alone positive were small, our data demonstrates that DSA− MICA− patients (n=29, 83%) were less likely to develop AMR than DSA−MICA+ patients (n=2, 6%, p=0.006). An equal number of DSA+ patients were MICA+ (n=2) and MICA− (n=2). The development of DSA alone as well as MICA and DSA together correlated significantly with the development of AMR in AMR+DSA+ patients (p=0.02). A significantly high proportion of AMR− patients (83%) were negative for Abs to either of these antigens (p=0.003). This suggests the possibility that alloimmunity, as evidenced by the development of DSA, may play a role in inducing Abs to MICA in HTx recipients that develop AMR.
Table 2.
Diagnosis Criteria in AMR+ Patients
| Patient | Symptoms | Abnormal Echo | Inotropes | Histology | Immunopathology | DSA | |
|---|---|---|---|---|---|---|---|
| CD4 | CD68 | ||||||
| 1 | + | − | + | + | + | + | − |
| 2 | + | + | − | + | + | + | + |
| 3 | + | + | + | − | + | + | + |
| 4 | + | + | + | + | + | − | + |
| 5 | + | + | + | + | − | + | − |
| 6 | − | + | + | − | − | + | + |
| 7 | + | + | + | + | + | − | + |
| 8 | − | + | + | + | + | + | + |
Table 3.
Comparison of DSA and MICA Abs in post HTx sera of AMR (+/−) patients and CAV (+/−) patients
| A | ||||
|---|---|---|---|---|
| AMR+ (n=8) | AMR− (n=35) | |||
| DSA+ (n=6) | DSA− (n=2) | DSA+ (n=4) | DSA− (n=31) | |
| MICA+ | 5 (63%) | 0 | 2 (6%) | 2 (6%) |
| MICA− | 1 (13%) | 2 (25%) | 2 (6%) | 29 (83%) |
| P value | 0.01 | 0.08 | 1.0 | 0.006 |
| B | ||||
|---|---|---|---|---|
| CAV+ (n=12) | CAV− (n=40) | |||
| DSA+ (n=8) | DSA− (n=4) | DSA+ (n=5) | DSA− (n=35) | |
| MICA+ | 6 (50%) | 3 (25%) | 2 (5%) | 3 (8%) |
| MICA− | 2 (17%) | 1 (8%) | 3 (8%) | 32 (80%) |
| P value | 0.03 | 0.04 | 0.45 | 0.003 |
3.3 Antibodies to mismatched HLA (DSA) and MICA develop in CAV+ HTx recipients
Of 12 recipients who were CAV+, 8 patients (67%) were DSA+ while 4 patients (33%) were DSA− (Table 3B). DSA+MICA+ patients (n=6, 50%) were more likely to develop CAV compared to DSA+MICA− patients (n=2, 17%, p=0.03). Of 4 recipients who were DSA−, 3 patients were MICA+ while 1 patient was MICA− (25% vs 8%, p=0.04). Conversely, 40 recipients were CAV−, of whom 35 patients (88%) were DSA− and 5 patients (12%) were DSA+. DSA−MICA− patients (n=32, 80%) were less likely to develop CAV than DSA−MICA+ patients (n=3, 8%, p=0.003). The development of DSA alone, MICA alone as well as MICA and DSA together correlated significantly with the development of CAV in CAV+DSA+ patients (p=0.02). A significantly high proportion of CAV−patients (80%) were negative for Abs to either of these antigens (p=0.002). Taken together, these results strongly suggest that DSA and anti-MICA independently and in conjunction play a significant role in the pathogenesis of CAV in HTx recipients.
3.4 Antibodies to DSA precede the development of Abs to MICA in AMR+ recipients
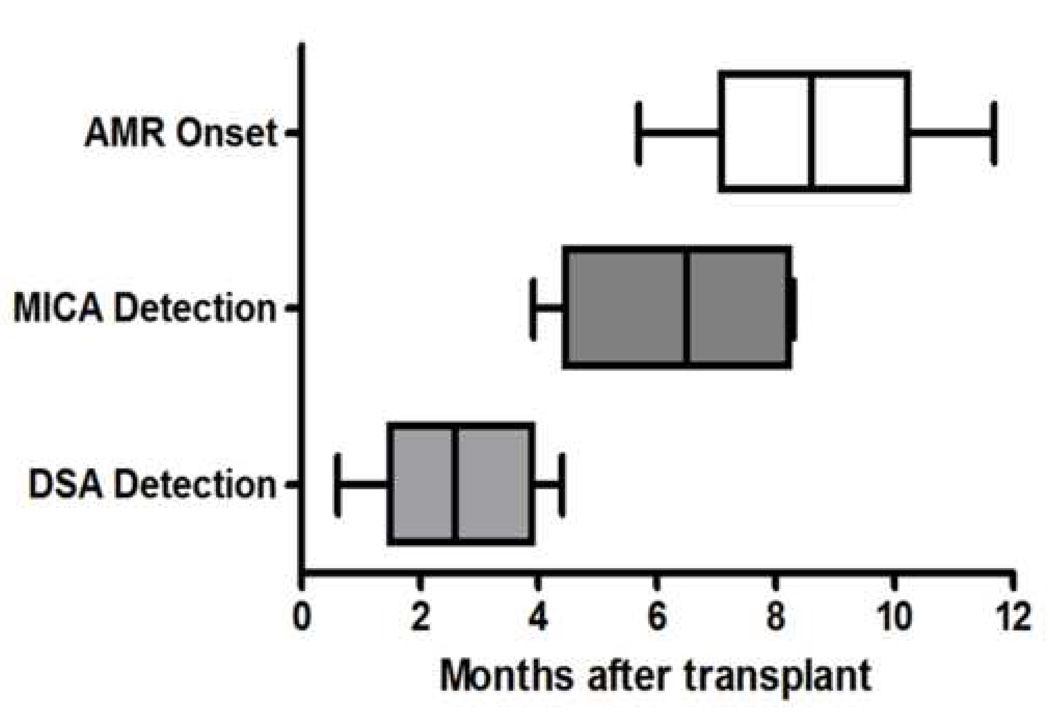
There were 5 AMR+ recipients who were DSA+ and MICA+. To assess the temporal sequence of Abs development, sera from various time points over a 12 month period were serially analyzed for the development of DSA and anti-MICA (Figure 1). Donor specific antibodies to mismatched donor HLA were present at 2.7±1.4 months followed by Abs to MICA at 6.5±2.1 months. This cohort of patients developed AMR at 8.3±2.5 months post-HTx. These results support our contention that alloimmunity, as noted by the presence of DSA, can up-regulate MICA expression which leads to induction of Abs to MICA. Furthermore, the development of Abs to these two antigen targets (mismatched donor HLA and MICA) precedes the clinical diagnosis of AMR.
Figure 1.
Development of DSA, anti-MICA and development of AMR after HTx. DSA and anti-MICA were detected by Labscreen™ Luminex systems. DSA and MICA bars represent the time interval (mean±SD) at which Abs were detected. AMR bars represent mean time when patients with DSA and anti-MICA were clinically diagnosed with AMR. Serial monitoring of DSA and Abs to MICA in the early post-HTx period in 5 DSA+ patients indicated that DSA was detectable at 2.7±1.4 months, followed by anti-MICA at 6.5±2.1 months in patients diagnosed with AMR at 8.3±2.5 months.
3.5 Antibodies titers to MICA peak before clinical diagnosis of AMR in HTx patients
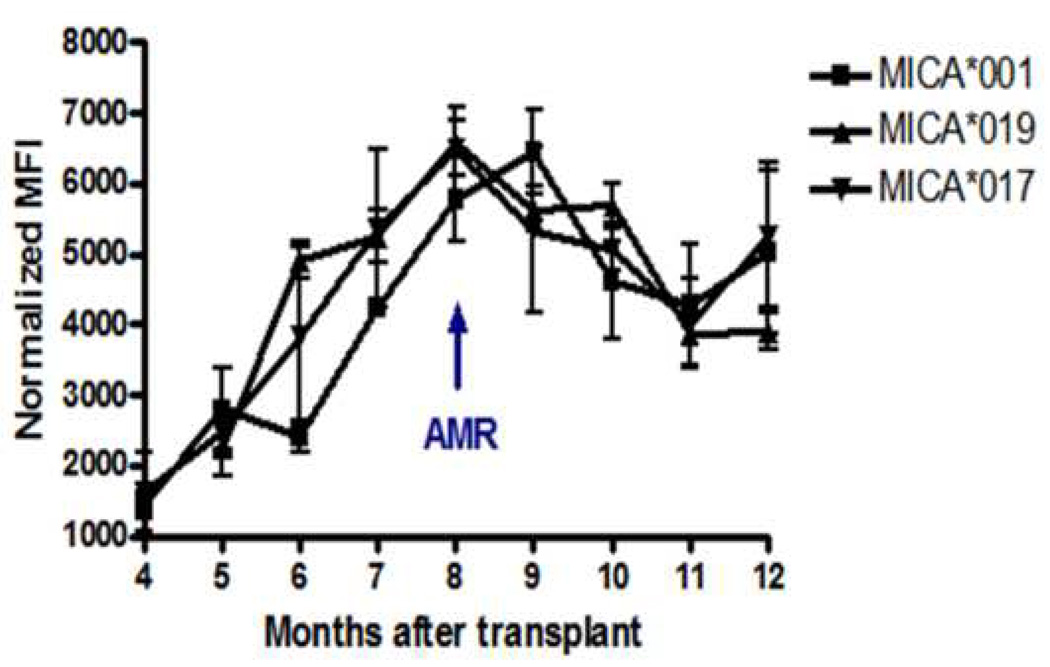
Antibodies to MICA *001,*017 and *019 were frequently noted in AMR+ patients and there were 5 patients who developed Abs to at least one of these antigens. Serial samples from these patients were assessed for MFI of MICA Abs in order to correlate the kinetics of Abs development to the clinical diagnosis of AMR. In this cohort of 5 patients, the mean time for development of AMR was 8.0 ±2.7 months post-HTx. Abs to MICA*001,*019 and *017 initially appeared 5 months post-HTx and peaked 2.8±1.2 months prior to diagnosis of AMR. After clinical diagnosis of AMR, Abs titers diminish, which may be a result of the treatment regimen that the patients received following diagnosis of allograft rejection.
4. Discussion
The development of immune responses to mismatched donor HLA and MICA antigens during post-operative period has been demonstrated following solid organ transplantation [32]. The primary objective of our study was to evaluate the association between donor specific Abs to HLA and anti-MICA in HTx recipients with AMR and CAV during their post-operative period. We serially monitored the levels of DSA to HLA and anti-MICA in serum of 43 HTx recipients during the EP (for development of AMR) and 52 HTx recipients during the LP (for development of CAV). In the EP, 8 patients demonstrated clinical and histological evidence of AMR as outlined by the ISHLT guidelines. Interestingly, 75% (n=6) of AMR+ patients demonstrated DSA and 63% (n=5) demonstrated anti-MICA compared to 11% (n=4) and 6% (n=2) of the 35 AMR− patients respectively (Table 3A). AMR+ patients with DSA (AMR+DSA+) demonstrated a significant association with positive titers of anti-MICA compared to AMR+DSA−, AMR−DSA+, and AMR−DSA− patients (Table 3A). Similarly, monitoring serum levels of DSA and anti-MICA in patients with CAV during the LP indicated that CAV+DSA+ were significantly positive for anti-MICA compared to CAV+DSA−, CAV−DSA+ and CAV−DSA+ patients (Table 3B). The results from our study suggest that increased DSA in AMR+ and CAV+ patients may be up-regulating MICA expression which then facilitates the development of Abs to MICA which is detectable in circulation.
A number of studies have shed light on the association of anti-MICA with adverse cardiac allograft function. In one such report, post-transplant sera from 150 patients were analyzed to examine if MICA Abs are a marker for decreased patient survival, acute cellular rejection and CAV [28]. Anti-MICA was strongly associated with acute rejection (63 vs 29%) and CAV (79 vs 33%). Reports from other investigators have demonstrated a significant correlation between the presence of anti-MICA in pre-and post-HTx sera and severe acute rejection [24, 29]. In one report, 190 pre-and post-transplant serum samples from 44 patients were collected during the first year after HTx [29]. In addition to sera, 10 endomyocardial biopsy samples were also assessed using RT-PCR and immunohistochemistry techniques. Nearly 60% of patients with severe acute rejection had evidence of anti-MICA and typically, the development of Abs to MICA preceded diagnosis of acute cellular rejection. In contrast to above discussed reports supporting a role of MICA in the development of adverse cardiac allograft outcomes, a recent study with a large number of HTx recipients (pre-transplant - 491 pts; post-transplant - 196 pts) noted no effect on pre-transplant and post-transplant production of MICA Abs on number of acute cellular rejection episodes in year 1 or CAV which was assessed at years 3 and 5 [33]. Based on these results, they concluded that MICA Abs do not adversely affect the outcome of HTx. It is of interest that in this study, the investigators did not analyze for the development of DSA which points out the importance of DSA in the development of both AMR as well as CAV following HTx.
In our study, we demonstrated that development of DSA preceded the detection of anti-MICA by nearly 4 months in sera of AMR+ patients. The binding of anti-HLA to the allograft can induce inflammatory cascades resulting in up-regulation of MHC class II, MICA and complement deposition. The resultant inflammatory milieu and increased induction of MHC class II on allograft epithelium facilitates the processing of donor derived peptides including MICA [16, 17, 34]. In addition, MICA can also mediate an allogenic response resulting in enhanced T cell proliferation, cytokine production and cytotoxicity [23]. Therefore we postulate that DSA to mismatched HLA may be involved in the induction of antibody mediated immune responses to MICA which are detectable in HTx recipients with AMR and CAV. Anti-MICA peaked during the clinical diagnosis of AMR and titers reduced following treatment in these patients (Figure 2). These findings are particularly noteworthy when viewed in conjunction with the damage that can be caused due to concurrent presence of Abs against donor mismatched HLA. However, a limitation of the study is that the follow-up for patients in the AMR cohort is less than 2 years.
Figure 2.
Sequential measurements of Luminex detectable anti-MICA in AMR+ patients. Anti-MICA was detected by Labscreen™ Luminex systems. Each sample was run in duplicate at 1:3 dilutions. Data represented is from serial sera from five AMR+ patients. Serial monitoring of Abs to MICA antigens *001, *019 and *017 indicated that titers of MICA Abs increase 2.8 ± 1.2 months before the clinical diagnosis of AMR.
MICA, located in the HLA class I region of chromosome 6, encodes a 383-amino acid polypeptide and is characterized as having three extracellular domains (α1, α2, α3), a transmembrane region and a cytoplasmic tail [35]. MICA is co-dominantly expressed and gene polymorphisms in the α3 domains are associated with specific graft-versus-host disease (GVHD), cancer, allograft rejection and viral infection [26]. MICA is a ligand for NKG2D, which is a well characterized activating receptor on NK and CD8+ T cells which plays a unique role as a link between innate and adaptive immunity in transplantation. MICA, which is present on the cell surface of endothelial cells, fibroblasts, monocytes and keratinocytes can induce the production of Abs that can be detected in the serum. In the setting of organ transplantation, data presented in this communication as well as reports from other groups strongly support the conclusion that development of Abs to MICA are associated with both AMR and CAV resulting in adverse allograft function [21, 35, 36].
To summarize, our study demonstrates a significant relationship between DSA and anti-MICA in cardiac allograft recipients with AMR and CAV during the early and late post-HTX periods respectively. At present, we do not monitor MICA antibodies in our clinical HTx program at Washington University. While DSA is not routinely monitored in our clinical HTx program for all recipients, we do test for the presence of anti-HLA and DSA in selected patients with a presumptive diagnosis of AMR. DSA is not generally tested in patients suspected or diagnosed with CAV. Based on our results, we propose that DSA is involved in the induction of antibody mediated immune responses to MICA in patients with AMR and CAV. DSA and anti-MICA precede the clinical diagnosis of AMR and can be utilized as non-invasive markers for post-transplant monitoring of AMR and CAV.
Acknowledgements
The authors thank Miyuki Ozawa and Kevin Harral (One lambda Inc, CA) for providing positive control sera. There is no financial or professional conflict of interest by any of the authors of this manuscript. This work is supported in part by funding by the Barnes-Jewish-Christian (BJC) Foundation to TM. DSN is supported by NIH Training Grant T32 HL07776.
Abbreviations
- Abs
Antibodies
- AMR
Antibody Mediated Rejection
- CAV
Cardiac Allograft Vasculopathy
- HLA
Human Leukocyte Antigens
- HTx
Heart Transplantation
- MHC
Major Histocompatibility Complex
- MICA
MHC class I chain-related molecule A
- NK cells
Natural Killer cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kfoury AG, Hammond ME. Controversies in defining cardiac antibody-mediated rejection: Need for updated criteria. J Heart Lung Transplant. doi: 10.1016/j.healun.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Michaels PJ, Fishbein MC, Colvin RB. Humoral rejection of human organ transplants. Springer Semin Immunopathol. 2003;25(2):119. doi: 10.1007/s00281-003-0139-x. [DOI] [PubMed] [Google Scholar]
- 3.Taylor DO, Edwards LB, Boucek MM, Trulock EP, Keck BM, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: twenty-first official adult heart transplant report--2004. J Heart Lung Transplant. 2004;23(7):796. doi: 10.1016/j.healun.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Reed EF, Demetris AJ, Hammond E, Itescu S, Kobashigawa JA, Reinsmoen NL, Rodriguez ER, Rose M, Stewart S, Suciu-Foca N, Zeevi A, Fishbein MC. Acute antibody-mediated rejection of cardiac transplants. J Heart Lung Transplant. 2006;25(2):153. doi: 10.1016/j.healun.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Uber WE, Self SE, Van Bakel AB, Pereira NL. Acute antibody-mediated rejection following heart transplantation. Am J Transplant. 2007;7(9):2064. doi: 10.1111/j.1600-6143.2007.01900.x. [DOI] [PubMed] [Google Scholar]
- 6.Kelishadi SS, Azimzadeh AM, Zhang T, Stoddard T, Welty E, Avon C, Higuchi M, Laaris A, Cheng XF, McMahon C, Pierson RN., 3rd Preemptive CD20+ B cell depletion attenuates cardiac allograft vasculopathy in cyclosporine-treated monkeys. J Clin Invest. 120(4):1275. doi: 10.1172/JCI41861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A, Berry GJ, Burke MM, Demetris AJ, Hammond E, Itescu S, Marboe CC, McManus B, Reed EF, Reinsmoen NL, Rodriguez ER, Rose AG, Rose M, Suciu-Focia N, Zeevi A, Billingham ME. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24(11):1710. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Dhaliwal A, Thohan V. Cardiac allograft vasculopathy: the Achilles' heel of long-term survival after cardiac transplantation. Curr Atheroscler Rep. 2006;8(2):119. doi: 10.1007/s11883-006-0049-1. [DOI] [PubMed] [Google Scholar]
- 9.Ramzy D, Rao V, Brahm J, Miriuka S, Delgado D, Ross HJ. Cardiac allograft vasculopathy: a review. Can J Surg. 2005;48(4):319. [PMC free article] [PubMed] [Google Scholar]
- 10.Kaczmarek I, Deutsch MA, Kauke T, Beiras-Fernandez A, Schmoeckel M, Vicol C, Sodian R, Reichart B, Spannagl M, Ueberfuhr P. Donor-specific HLA alloantibodies: long-term impact on cardiac allograft vasculopathy and mortality after heart transplant. Exp Clin Transplant. 2008;6(3):229. [PubMed] [Google Scholar]
- 11.Jaramillo A, Smith MA, Phelan D, Sundaresan S, Trulock EP, Lynch JP, Cooper JD, Patterson GA, Mohanakumar T. Development of ELISA-detected anti-HLA antibodies precedes the development of bronchiolitis obliterans syndrome and correlates with progressive decline in pulmonary function after lung transplantation. Transplantation. 1999;67(8):1155. doi: 10.1097/00007890-199904270-00012. [DOI] [PubMed] [Google Scholar]
- 12.Jindra PT, Hsueh A, Hong L, Gjertson D, Shen XD, Gao F, Dang J, Mischel PS, Baldwin WM, 3rd, Fishbein MC, Kupiec-Weglinski JW, Reed EF. Anti-MHC class I antibody activation of proliferation and survival signaling in murine cardiac allografts. J Immunol. 2008;180(4):2214. doi: 10.4049/jimmunol.180.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizutani K, Terasaki PI, Shih RN, Pei R, Ozawa M, Lee J. Frequency of MIC antibody in rejected renal transplant patients without HLA antibody. Hum Immunol. 2006;67(3):223. doi: 10.1016/j.humimm.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Atz ME, Reed EF. Role of anti-MHC class I antibody in facilitating transplant accommodation. Crit Rev Immunol. 2008;28(6):485. doi: 10.1615/critrevimmunol.v28.i6.20. [DOI] [PubMed] [Google Scholar]
- 15.Grossman EJ, Shilling RA. Bronchiolitis obliterans in lung transplantation: the good, the bad, and the future. Transl Res. 2009;153(4):153. doi: 10.1016/j.trsl.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Dierselhuis M, Goulmy E. The relevance of minor histocompatibility antigens in solid organ transplantation. Curr Opin Organ Transplant. 2009;14(4):419. doi: 10.1097/MOT.0b013e32832d399c. [DOI] [PubMed] [Google Scholar]
- 17.Sumitran-Holgersson S. Relevance of MICA and other non-HLA antibodies in clinical transplantation. Curr Opin Immunol. 2008;20(5):607. doi: 10.1016/j.coi.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Rose ML. De novo production of antibodies after heart or lung transplantation should be regarded as an early warning system. J Heart Lung Transplant. 2004;23(4):385. doi: 10.1016/j.healun.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 19.Bahram S, Bresnahan M, Geraghty DE, Spies T. A second lineage of mammalian major histocompatibility complex class I genes. Proc Natl Acad Sci U S A. 1994;91(14):6259. doi: 10.1073/pnas.91.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, Phillips JH. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285(5428):730. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Stastny P. MICA antigens stimulate T cell proliferation and cell-mediated cytotoxicity. Hum Immunol. 2006;67(3):215. doi: 10.1016/j.humimm.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285(5428):727. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 23.Suarez-Alvarez B, Lopez-Vazquez A, Baltar JM, Ortega F, Lopez-Larrea C. Potential role of NKG2D and its ligands in organ transplantation: new target for immunointervention. Am J Transplant. 2009;9(2):251. doi: 10.1111/j.1600-6143.2008.02526.x. [DOI] [PubMed] [Google Scholar]
- 24.Suarez-Alvarez B, Lopez-Vazquez A, Diaz-Pena R, Diaz-Molina B, Blanco-Garcia RM, Alvarez-Lopez MR, Lopez-Larrea C. Post-transplant soluble MICA and MICA antibodies predict subsequent heart graft outcome. Transpl Immunol. 2006;17(1):43. doi: 10.1016/j.trim.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Suarez-Alvarez B, Lopez-Vazquez A, Diaz-Molina B, Bernardo-Rodriguez MJ, Alvarez-Lopez R, Pascual D, Astudillo A, Martinez-Borra J, Lambert JL, Gonzalez S, Lopez-Larrea C. The predictive value of soluble major histocompatibility complex class I chain-related molecule A (MICA) levels on heart allograft rejection. Transplantation. 2006;82(3):354. doi: 10.1097/01.tp.0000228911.22944.23. [DOI] [PubMed] [Google Scholar]
- 26.Choy MK, Phipps ME. MICA polymorphism: biology and importance in immunity and disease. Trends Mol Med. 16(3):97. doi: 10.1016/j.molmed.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Angaswamy N, Saini D, Ramachandran S, Nath DS, Phelan D, Hachem R, Trulock E, Patterson GA, Mohanakumar T. Development of antibodies to human leukocyte antigen precedes development of antibodies to major histocompatibility class I-related chain A and are significantly associated with development of chronic rejection after human lung transplantation. Hum Immunol. 71(6):560. doi: 10.1016/j.humimm.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kauke T, Kaczmarek I, Dick A, Schmoeckel M, Deutsch MA, Beiras-Fernandez A, Reichart B, Spannagl M. Anti-MICA antibodies are related to adverse outcome in heart transplant recipients. J Heart Lung Transplant. 2009;28(4):305. doi: 10.1016/j.healun.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Suarez-Alvarez B, Lopez-Vazquez A, Gonzalez MZ, Fdez-Morera JL, Diaz-Molina B, Blanco-Gelaz MA, Pascual D, Martinez-Borra J, Muro M, Alvarez-Lopez MR, Lopez-Larrea C. The relationship of anti-MICA antibodies and MICA expression with heart allograft rejection. Am J Transplant. 2007;7(7):1842. doi: 10.1111/j.1600-6143.2007.01838.x. [DOI] [PubMed] [Google Scholar]
- 30.Suarez-Alvarez B, Alonso-Arias R, Bravo-Mendoza C, Lopez-Vazquez A, Ortega T, Baltar JM, Coto E, Ortega F, Lopez-Larrea C. Identification of epitopes and immunodominant regions on the MICA protein defined by alloantibodies from kidney transplant patients. Transplantation. 2009;88(3 Suppl):S68. doi: 10.1097/TP.0b013e3181afeb7a. [DOI] [PubMed] [Google Scholar]
- 31.Amico P, Honger G, Bielmann D, Lutz D, Garzoni D, Steiger J, Mihatsch MJ, Dragun D, Schaub S. Incidence and prediction of early antibody-mediated rejection due to non-human leukocyte antigen-antibodies. Transplantation. 2008;85(11):1557. doi: 10.1097/TP.0b013e31816f612a. [DOI] [PubMed] [Google Scholar]
- 32.Cai J, Terasaki PI. Post-transplantation antibody monitoring and HLA antibody epitope identification. Curr Opin Immunol. 2008;20(5):602. doi: 10.1016/j.coi.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Smith JD, Brunner VM, Jigjidsuren S, Hamour IM, McCormack AM, Banner NR, Rose ML. Lack of effect of MICA antibodies on graft survival following heart transplantation. Am J Transplant. 2009;9(8):1912. doi: 10.1111/j.1600-6143.2009.02722.x. [DOI] [PubMed] [Google Scholar]
- 34.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 35.Molinero LL, Marcos CY, Mirbaha F, Fainboim L, Stastny P, Zwirner NW. Codominant expression of the polymorphic MICA alloantigens encoded by genes in the HLA region. Eur J Immunogenet. 2002;29(4):315. doi: 10.1046/j.1365-2370.2002.00274.x. [DOI] [PubMed] [Google Scholar]
- 36.Zou Y, Stastny P. The role of major histocompatibility complex class I chain-related gene A antibodies in organ transplantation. Curr Opin Organ Transplant. 2009;14(4):414. doi: 10.1097/mot.0b013e32832d835e. [DOI] [PubMed] [Google Scholar]