Abstract
Liver fibrosis is mediated by the transformation of hepatic stellate cells (HSC) from a quiescent to an activated state. To understand the role of HSC in liver immunity, we investigated the effect of this transition on T cell stimulation in vitro. Unlike quiescent HSC, activated HSC did not induce proliferation of antigen-specific T cells. Phenotypic analysis of quiescent and activated HSC revealed that activated HSC expressed the coinhibitory molecule B7-H4. Silencing B7-H4 by siRNA in activated HSC restored the ability of T cells to proliferate, differentiate and regain effector recall responses. Furthermore, expression of B7-H4 on HSC inhibits early T cell activation and addition of exogenous IL-2 reversed the T cell anergy induced by activated HSC.
Conclusions
These studies reveal a novel role for activated HSC in the attenuation of intrahepatic T cell responses via expression of the coinhibitory molecule B7-H4, and may provide fundamental insight into intrahepatic immunity during liver fibrogenesis.
Keywords: Stellate cell, B7-family members, B7-H4, T cell proliferation, T cell anergy, liver fibrosis
Introduction
Chronic liver disease is the tenth leading cause of mortality in the United States (1). Whether a viral infection such as HCV, or a non-infectious insult such as alcohol or a genetic disease is the inciting agent, each shares a common route to eventual liver failure characterized by inflammation, fibrosis, and cirrhosis (2). Hepatic stellate cells (HSC) are the major cell types in the liver responsible for liver fibrosis (3). These cells are non-parenchymal cells that comprise 5-8% of the normal liver, located in the space of Disse between the endothelial layer and parenchymal hepatocytes (4), and serve as a depot of vitamin A (5).
In the healthy liver, HSC are present in a quiescent form and perform multiple physiologic functions including directing hepatic development and regeneration as well as producing lipoproteins, growth factors and cytokines (5). However, during liver injury, HSC become activated (6), leading to a phenotypic and functionally consequent transformation. Activated HSC (AHSC) are the major mediators of liver fibrosis through extracellular matrix deposition (type I and type III collagen), secretion of the vasoconstrictor endothelin-1 which contributes to portal hypertension, and reduced production of matrix metalloprotease-1 necessary for the degradation of collagen type 1 (5).
In addition to their role in liver fibrosis, recent evidence has also placed HSC at the center of the intrahepatic immune response (7). HSC secrete chemokines, express various Toll-like receptors and are phagocytic (7). HSC have been shown to prevent graft rejection in a transplantation model by inhibiting T cell responses (8) and have also been shown to expand regulatory T cells in an IL-2 dependent manner (9). AHSC interact with, and in fact, also engulf lymphocytes through phagocytosis during liver fibrosis (10). Thus, the role that the AHSC may play in instructing antigen-specific T cell responses during fibrogenesis is of great interest.
HSC share many features with professional antigen presenting cells (APCs) (11), and have been shown to process and present antigen to T cells (12). T cell activation requires interaction of the T cell receptor (TCR) with cognate peptide antigen presented in the context of MHC on APC, as well as the interaction of ligand-receptor pairs providing costimulatory signals. Once T cells are activated, coinhibitory molecules play a role in dampening the T cell response serving as an “off-switch.” APC regulate T cell responses through the balance of signals delivered through costimulatory and coinhibitory molecules, with B7 family ligands expressed on APC playing a major role in T cell mediated immunity (13). Accumulated evidence demonstrates that there are at least seven members present in the B7 family namely, B7-1 (CD80), B7-2 (CD86), B7-H1 (PD-L1), B7-DC (PD-L2), B7-H2, B7-H3 and B7-H4 (14). B7-1 and B7-2 interact with the receptor CD28 on T cells to provide costimulatory signals as well as interacting with CTLA4 to provide coinhibitory signals. B7-H1 and B7-DC interact with PD-1 to induce T cell apoptosis. B7-H2 interacts with ICOS on T cells and provides a costimulatory signal. B7-H3 and B7-H4 are newly identified ligands that inhibit T cell responses by interacting with as yet unidentified receptors (15).
In the present study, we have investigated the impact of AHSC upon adaptive immune responses in the context of B7 family members, using an in vitro mouse model. AHSC express the coinhibitory molecule B7-H4, which provides a signal to dampen antigen-specific T cell responses. This work bears important implications for the dysfunctional immune responses that are often described in liver fibrosis, which is the natural environment of AHSC, and suggests a major role of these cells in modulating T cell immunity.
Materials and Methods
Mice
C57BL/6 mice (Jackson laboratory) were used for HSC isolation. CD8+ T cells specific for LCMV glycoprotein gp33-41 were isolated from spleens of P14 TCR transgenic mice.
HSC isolation and activation
HSC were isolated from mouse livers as previously described (16). Briefly, liver of C57BL/6 mice was perfused through the portal vein with HBSS (Ca++Mg++ free), followed by 1mg protease from Streptomyces griseus (Sigma-Aldrich) / gram body weight in DMEM/F-12, and finally with 5mg collagenase type IV (Sigma-Aldrich) in DMEM/F-12. Liver tissue was mechanically disrupted, and further digested for 20 min. Highly buoyant HSC were isolated by gradient centrifugation with Optiprep® (Axis-Shield PoC AS, Oslo, Norway) and washed with HBSS. HSC were cultured in non-tissue culture-treated plates in DMEM supplemented with 10% fetal calf serum and 1% penicillin/streptomycin. HSC that were freshly isolated ex vivo or cultured on untreated plastic plates for 1 day were considered to be QHSC. AHSC were obtained from the plate by scrapping after continuous culture for seven days.
HSC and T cell co-culture assays
Quiescent, activated, or siRNA-transfected HSC (more detail in the supplementary methods section) were pulsed with various concentrations of gp33 peptide (KAVYNFATM) or infected with vaccinia virus expressing LCMV gp33 epitope (kind gift from Dr. Rafi Ahmed) in DMEM containing 10% FCS. After washing, either CFSE labeled, unlabelled or effector CD8+ T cells were added. Proliferation and cytokine production of the CD8+ T cells were analyzed. Detail methodologies are included in the supplementary section.
Results
Activated HSC inhibits CD8+ T cell proliferation
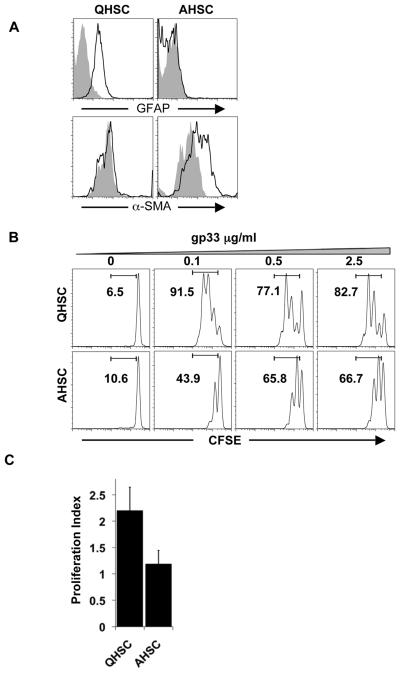
Recent work has demonstrated that HSC can act as APC to induce CD8+T cell proliferation in vitro (12); however, the impact of the transition of HSC from quiescence to activation on antigen specific T cell proliferation is unknown. HSC isolated from the liver are quiescent for 1-2 days and will attain an activated phenotype after 6 days of culture on non-treated tissue culture plates (5). QHSC express the marker Glial Fibrillary Acidic protein (GFAP) which is subsequently downregulated in AHSC, while alpha-smooth muscle actin (α-SMA) is upregulated upon activation of HSC (Fig. 1A) (17). We compared the ability of QHSC and AHSC to induce T cell proliferation in a 3 day culture of CFSE labeled-P14 TCR transgenic CD8+ T cells with HSC pulsed with cognate peptide gp33 derived from LCMV (18). Whereas peptide-pulsed QHSC are able to stimulate division of antigen-specific T cells, AHSC are unable to achieve the same amount of cell proliferation, as reflected both in the percentage and index of T cell division (Fig. 1B, C). Next we investigated whether the reduction in T cell proliferation after stimulation with AHSC is contact dependent or mediated by soluble factors. AHSCs secrete cytokines known to induce T cell proliferation such as IL-6 and RANTES (19) (Fig. S1A). Indeed, coculturing of CFSE labeled, anti-CD3 stimulated T cells with conditioned medium from AHSC improves T cell proliferation rather than abrogating it (Fig. S1B). Therefore, although AHSC secrete T cell stimulatory cytokines, they provide a more dominant, non-secreted inhibitory signal that prevents T cell proliferation.
Figure 1. AHSC inhibit CD8+ T cell proliferation.
(A) Murine QHSC and AHSC were stained with GFAP and α-SMA. F4/80+ cells were excluded from the FACS analysis. Gray histograms represent staining with an isotype control antibody. (B) QHSC and AHSC were pulsed with gp33 peptide and cultured with CFSE labeled P14 TCR transgenic CD8+ T cells. After 3 days, proliferation of CD8+ T cells was determined by dilution of CFSE. Representative histograms are shown gated on CD3+CD8+ cells. (C) Proliferation index analysis was performed with Flow Jo software, and calculated as the average number of cell divisions. Average and standard deviation is shown from three independent experiments.
B7-H4 is upregulated on AHSC
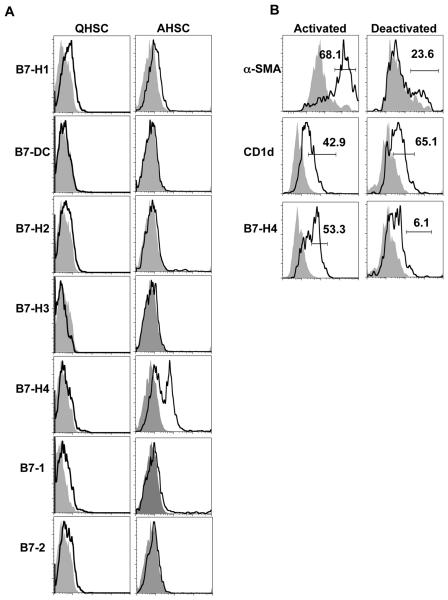
We investigated the expression of seven costimulatory and coinhibitory molecules from the B7 family in QHSC and AHSC. In concurrence with previous reports, both QHSC and ASHC show low levels of expression of the costimulatory molecules B7-1 and B7-2 (Fig. 2A). However, only AHSC express the coinhibitory molecule B7-H4 (Fig. 2A). No other differential expression patterns of the costimulatory or coinhibitory molecules are detected. We did not detect appreciable levels of B7-H4 in other liver APC (CD11c+ dendritic cells or Kupffer cells), or in splenic dendritic cells directly ex vivo (Fig. S2). To assess whether B7-H4 expression was tied to the activation status of HSC, we reversed the activation state of AHSC in vitro. Reversal of HSC activation is considered to be an important process during reversal fibrosis (20). Deactivation of AHSC by culturing HSC on a basement membrane matrix in vitro (21) reduces the expression of the activation marker α-SMA as well as B7-H4 expression, while no change in the constitutive HSC marker CD1d were observed (Fig. 2B). Thus, AHSC express the coinhibitory molecule B7-H4, and this expression is specifically associated with the activated state.
Figure 2. AHSC expresses B7-H4.
(A) Flow cytometric analysis of the B7 family of costimulatory and coinhibitory molecules in QHSC and AHSC. Representative histograms are gated on F4/80− and either GFAP or α-SMA+ cells. (B) AHSC were cultured on a basement membrane matrix and were stained for expression of molecules as indicated. Representative data is shown from two independent experiments.
B7-H4 on AHSC inhibits T cell proliferation
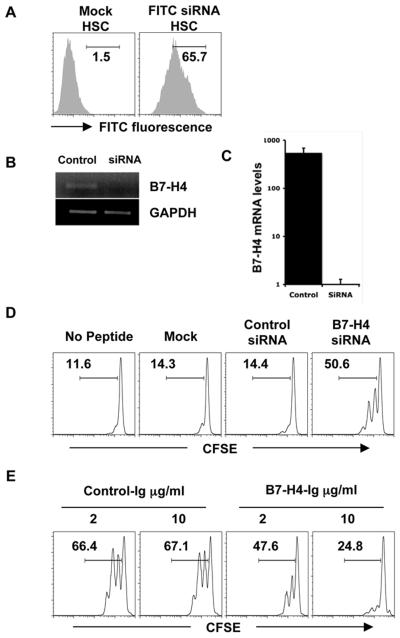
To evaluate the function of B7-H4 in AHSC-T cell interactions, we silenced the expression of B7-H4 in AHSC using siRNA. FITC labeled siRNA is efficiently internalized by HSC (Fig. 3A), and qualitative and quantitative RT-PCR using primers specific for B7-H4 and GAPDH demonstrate that the expression of B7-H4 is efficiently silenced in B7-H4 siRNA treated HSC (Fig. 3B, C). AHSCs treated with B7-H4 siRNA, a non-targeting control siRNA or mock transfection were pulsed with 0.02 ug/ml gp33 peptide, and cultured together with CFSE labeled P14 TCR transgenic CD8+ T cells for 3 days. B7-H4-silenced AHSC induces efficient T cell proliferation in comparison to those treated with control siRNA or mock transfected, as measured by CFSE dilution (Fig. 3D). In concordance with a previous report, anti-CD3/CD28 induced T cell proliferation is also inhibited by the addition of B7-H4-Ig but not by control-Ig (Fig. 3E) (22). Thus, our results demonstrate that B7-H4 on AHSC inhibits the proliferation of CD8+ T cells.
Figure 3. B7-H4 on AHSC inhibits T cell proliferation.
(A) AHSC were transfected with FITC-labeled siRNA or mock, and uptake of siRNA was evaluated by flow cytometry. (B) AHSC transfected with either B7-H4 siRNA or control siRNA were analyzed for the B7-H4 RNA expression using qualitative and (C) quantitative RT-PCR. Results are shown as fold difference of B7-H4 amplification over GAPDH control. (D) Mock or control siRNA or B7-H4 siRNA transfected HSC were pulsed with gp33 peptide and cocultured with CFSE labeled P14 CD8+ T cells for 3 days and T cell proliferation assayed by flow cytometry. Numbers indicate percent divided cells. (E) CFSE-labeled CD8+ T cells were added to plate-bound anti-CD3/CD28 along with the indicated concentrations of B7-H4-Ig or control-Ig and cultured for three days. Proliferation was assessed by CFSE dilution. Representative data is shown from six independent experiments.
B7-H4 on AHSC inhibits cytokine production by T cells
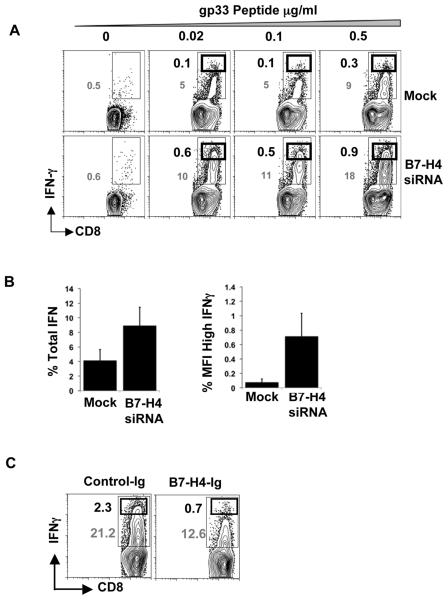
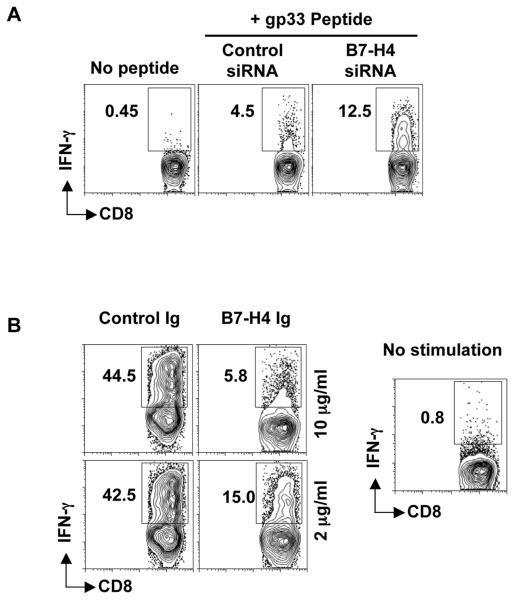
We assessed the generation of cytokine secreting T cells stimulated by AHSC with or without B7-H4. CD8+ T cells from P14 transgenic mice were cultured with AHSC treated with B7-H4 siRNA or mock transfection and pulsed with various concentrations of cognate peptide. B7-H4-knockdown AHSC generates higher levels of IFNγ secreting antigen-specific T cells, suggesting an effect of B7-H4 both on T cell division and functional capacity (Fig. 4A). IL-2 production by T cells was also restored by B7-H4 knockdown, though at a relatively lesser magnitude (data not shown). We also observed a higher mean fluorescence intensity (MFI) of IFNγ staining in the CD8+ T cells, as well as a larger frequency of high IFNγ producing CD8+ T cells after stimulation with B7-H4 silenced AHSC compared to control AHSC (Fig. 4B). Confirming the central role of B7-H4 in these results, stimulation of CD8+ T cells with anti-CD3/CD28 antibodies in the presence of B7-H4-Ig induced the generation of less IFNγ compared to stimulation with control-Ig (Fig. 4C). Altogether, our results demonstrate that B7-H4 on activated HSC inhibits the generation of cytokine producing T cells.
Figure 4. B7-H4 on AHSC inhibits cytokine producing CD8+T cells.
(A) Mock or B7-H4 siRNA transfected HSC were pulsed with gp33 peptide and cocultured with P14 CD8+ T cells for three days. The CD8+ T cells were then restimulated with plate bound anti-CD3/CD28 antibodies (1ug/ml) for 5 hours and intracellular IFNγ levels were analyzed. Thin black gate and grey numbers represent the percentage frequency of total IFNγ producing CD8+ T cells. Thick black gate and black numbers represent the frequency of high IFNγ producing CD8+ T cells. Plots are gated on CD3+CD8+ cells. Representative data is shown from two independent experiments (B) Fold difference in the frequency of total (left panel) or high MFI (right panel) IFNγ producing CD8+ T cells between B7-H4 knockdown and control HSC. Average and standard deviation is shown from three independent experiments. (C) CD8 + T cells cultured with anti-CD3/CD28 in the presence of B7-H4-Ig or control-Ig for three days were restimulated with plate coated anti-CD3/CD28 antibodies (1ug/ml) for 5 hours and intracellular IFNγ was measured. Representative data is shown from three independent experiments.
B7-H4 on AHSC reduces the T cell recall response
We investigated the influence of B7-H4-expressing HSC on recall responses of previously primed effector CD8+ T cells. Effector T cells upon re-encountering cognate antigen, are capable of producing IFNγ. We generated CD8+ T cell blasts or effector CD8+ T cells by pulsing P14 splenocytes with 1ug/ml gp33 peptide and subsequently purifying them after six days of expansion. They were then cocultured with gp33 peptide-pulsed B7-H4 knockdown and control AHSC for six hours. As shown IFNγ production is reduced after stimulation with B7-H4 expressing AHSC compared to B7-H4 knockdown AHSC (Fig. 5A). CD8+ T cell blasts or effector CD8+ T cells were also generated using anti-CD3/CD28, and restimulated with anti-CD3/CD28 in the presence of B7-H4-Ig or control-Ig. The addition of B7-H4-Ig reduces IFNγ production in a dose-dependent manner, while high levels of IFNγ +CD8+ T cells are seen with treatment with control-Ig (Fig. 5B). These results indicate that B7-H4 on AHSC attenuates the recall effector T cell response.
Figure 5. B7-H4 on AHSC reduces the recall response.
(a) B7-H4 knockdown and control siRNA transfected AHSC were peptide pulsed and cocultured with effector CD8+ T cells for five hours. IFNγ expression on CD8+ T cells was analyzed using intracellular cytokine staining. (b) Effector CD8+ T cells were cultured with anti-CD3/CD28 antibodies and B7-H4-Ig or control-Ig and IFNγ was measured as above. Representative data is shown from two independent experiments.
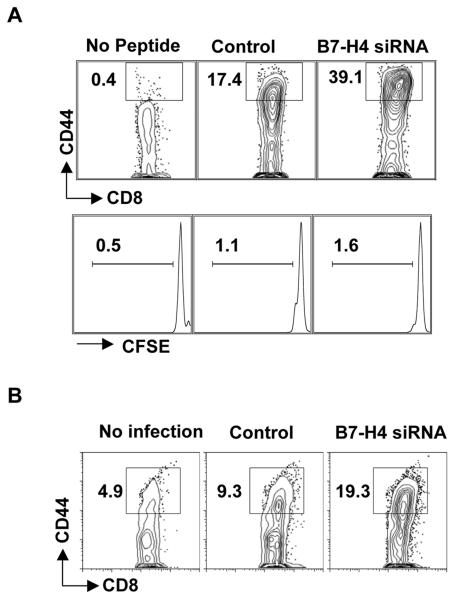
B7-H4 on AHSC inhibits T cell activation
CD8+ T cells that are stimulated by B7-H4 silenced HSC exhibit a highly activated phenotype with a high frequency of CD44hi cells, even before cell division has ensued (Fig. 6A). Thus, in concordance with previous reports (22, 23) B7-H4 inhibits or delays T cell activation. To show whether HSC were capable of processing and presenting antigen and whether inhibition of T cells by B7-H4 on infected HSC still occurred, AHSC were infected with 5 pfu/ml with vaccinia virus expressing gp33 epitope of LCMV for 24 hours. Subsequent to vaccinia infection expressing gp33, AHSC were then transfected with B7-H4 siRNA or control siRNA. Two days post transfection, P14 transgenic CD8+ T cells were added and the level of activation based on CD44 expression was evaluated. Our results demonstrate that in the absence of B7-H4, the vaccinia-gp33 infected HSC induces T cell activation efficiently as compared to the control siRNA treatment (Fig. 6B). However, it is important to note that similar to stimulation with peptide pulsed HSC, the effect of B7-H4 mediated inhibition of T cell activation and proliferation, is dose dependent.
Figure 6. B7-H4 on AHSC inhibits the T cell activation.
(A) CD8+T cells stimulated with B7-H4 knockdown or control AHSC were analyzed for the surface expression of CD44 after 24 hrs by flow cytometry. Percentage of CD44hi + cells of total CD8+ T cells are shown (Top). CFSE dilution after 24 hrs is also shown (Bottom). (B) P14 CD8+ T cells were stimulated with vaccinia-gp33 infected followed by B7-H4 siRNA or control siRNA transfected HSC were analyzed for the surface expression of CD44 after 24 hrs by flow cytometry. Representative data is shown from four independent experiments.
B7-H4 mediated inhibition of T cells by AHSC can be reversed by exogenous IL-2
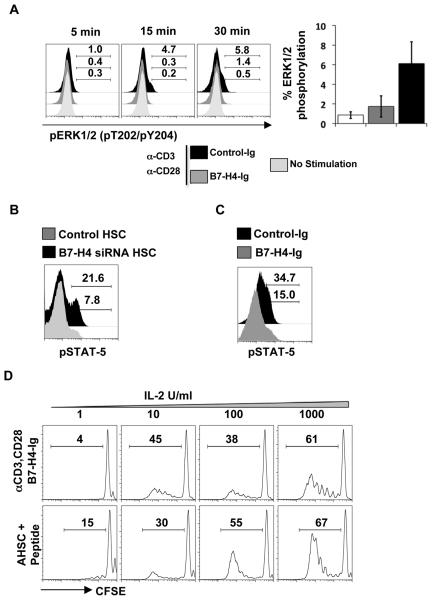
To elucidate the mechanism of B7-H4 inhibition of T cells, we assessed the early signaling pathway and phosphorylation state of various signal transduction molecules on T cells. T cell activation is associated with MAPK signaling through ERK1/2. We evaluated the initiation of MAPK signaling via the presence of phosphorylated ERK molecules in T cells with and without B7-H4-Ig. Phosphorylated ERK1/2 could be detected in T cells with control-Ig as early as 5-15 minutes after stimulation. In contrast, the addition of B7-H4-Ig inhibits the phosphorylation of ERK-1/2 in CD8+T cells (Fig. 7A). Together, our results demonstrate that B7-H4 on AHSC inhibits early steps of CD8+ T cell activation. Additionally, CD8+ T cells that are stimulated with B7-H4 knockdown AHSC expressed higher levels of phosphorylated STAT-5 as compared to control HSC (Fig. 7B). Similarly CD8+ T cells stimulated in the presence of B7-H4-Ig demonstrate lower levels of phosphorylated STAT5 molecules as compared to T cells stimulated in the presence of control-Ig (Fig. 7C). Previous reports have shown that STAT-5 phosphorylation is induced via the IL-2 signaling pathway (24). In addition, lower levels of IL-2 receptor CD25 were observed on T cells stimulated with B7-H4 expressing AHSC compared to the B7-H4 silenced AHSC (data not shown). This demonstrates that the T cells are potentially anergized by AHSC. It has been well established that IL-2 signaling prevents T cell anergy (25) and in fact, addition of exogenous IL-2 overcomes the inhibition of CD8+ T cell proliferation initiated by B7-H4-Ig and by AHSC in a dose-dependent manner (Fig. 7D). Importantly, these results demonstrate that HSC B7-H4 mediated T cell anergy can be overcome through provision of exogenous IL-2.
Figure 7. Exogenous IL-2 can overcome the B7-H4 mediated inhibition of T cells by AHSC.
(A) CD8+ T cells stimulated with anti-CD3/CD28 and either B7-H4-Ig or control-Ig proteins at indicated timepoints were analyzed for pERK1/2 by flow cytometry (left). Average of ERK1/2 phosphorylation at 30 min time point is shown from of three independent experiments. (B) P14 CD8+T cells stimulated with peptide pulsed B7-H4 knockdown or control AHSC and (C) anti-CD3/CD28 in the presence of B7H4-Ig or control-Ig for two days were analyzed for the presence of phosphorylated STAT-5 molecules. Grey histograms represent expression levels in CD8+ T cells stimulated with control AHSC or B7-H4-Ig or control-Ig and black histograms represent expression levels in T cells stimulated with B7-knockdown AHSC or control-Ig. (D) B6 or P14 CD8+ T cells were labeled with CFSE and cultured with either anti-CD3/CD28 and B7-H4-Ig or AHSC pulsed with cognate peptide. Various concentrations of IL-2 was added on to the culture and proliferation was assessed as described before. Representative data is shown from two independent experiments for each panel.
Discussion
These studies reveal a novel potential mechanism for liver T cell anergy, which is mediated by the coinhibitory molecule B7-H4 on AHSC. To our knowledge, this is the first report of the functional role for B7-H4 in the liver. B7-H4 is a GPI anchored coinhibitory molecule identified through database sequence analysis of B7 family like molecules and has been shown to inhibit T cell proliferation by interacting with an unknown receptor on T cells (22, 23, 26). Expression of B7-H4 has been shown in several human cancers such as ovarian carcinoma (27), breast cancer (28), brain tumors (29), prostate cancer (30), and renal cell carcinoma (31). B7-H4 expressing tumor macrophages from human ovarian carcinoma were immunosuppressive contributing to tumor escape from the immune response (32). Some studies have demonstrated an inverse correlation between B7-H4 expression and tumor T cell infiltration (33). Our results show that AHSC, through the expression of this coinhibitory molecule B7-H4, may occupy a more important niche in modulating intrahepatic immune responses than previously recognized. Other B7 family ligands, present on professional APC, have been widely implicated in intrahepatic immunity: B7-H1 mediated inhibition of T cells in the liver has been shown by several groups (34-36) and B7-1, B7-DC have also been reported for their role in immune modulation in the liver (37). In the present study we have demonstrated for the first time the role of B7-H4 on the activated HSC suggesting a link between fibrogenesis and immune modulation. We have shown that compared to QHSC, in vitro AHSC inhibit peptide antigen-induced T cell proliferation, and may contribute to the fibrotic liver's tolerogenic environment. While QHSC do stimulate T cell proliferation, they are present at very low frequency so that their effect on T cell responses in the normal liver is largely unkown. In contrast, in the injured or fibrotic liver, HSC exist in a predominantly activated state and acquire proliferative capacity themselves (5). We hypothesize that HSC activated in vivo also upregulate B7-H4 expression, and then dominate the liver environment with T cell inhibitory signals leading to attenuation of the immune response.
T cell responses can be divided into two major stages; (i) primary and (ii) recall responses. Antigen specific primary T cell responses have been shown in the liver (38). Similarly, T cells activated in the peripheral lymphoid tissues that traffic through the liver have poor survival, earning the liver the reputation as a graveyard for activated T cells (39). It has been shown that the coinhibitory molecule B7-H1 expressed on hepatocytes promotes priming but inhibits recall T cell responses (40). In contrast, we report here that B7-H4 on AHSC inhibits both priming and recall CD8+ T cell responses. Inhibiting T cell responses at different stages also renders HSC's key role in modulating intrahepatic immunity in fibrosis.
Here we provide evidence that B7-H4 expression on AHSC induced T cell inactivation or anergy that could be reversed by exogenous IL-2. The rescue mechanism from B7-H4 is similar to B7-H1 mediated T cell inhibition since the B7-H1 (PD-L1)-PD-1 inhibitory pathway can also be overcome by provision of exogenous IL-2 (41). This may have interesting implications in chronic viral diseases such as hepatitis C virus infection as the inhibitory effects of B7-H4 on T cells may be perpetuated or amplified by a relative deficiency of IL-2 (42, 43).
Still unknown is the cellular regulation of B7-H4 in AHSC, although our studies are starting to offer some intriguing clues. In tumor macrophages the upregulation of B7-H4 is dependent on IL-6 and IL-10 (32). Interestingly, AHSC also secrete IL-6; however, while QHSC can be isolated from IL-6 knockout mice, these cells do not seem to proliferate or transition to an activated state (data not shown). Altogether, it will be of further interest to investigate whether B7-H4 expression on HSC results in, is coincidental with, or is a consequence of HSC proliferation and activation.
In summary, our results demonstrate that AHSC inhibit T cell responses in a B7-H4 dependent manner. In the tumor microenvironment, B7-H4 attenuates T cell responses and the tumors use this mechanism to evade the T cell immunity. In the present study, our results suggest that AHSC proliferate, perpetuate fibrosis and inhibit intrahepatic T cell immunity. AHSC expressed B7-H4 provides a novel link between liver fibrosis and impaired intrahepatic immunity and highlights the potential importance of targeting interventions toward the AHSC in hepatotropic infections such as HCV.
Supplementary Material
Acknowledgements
The authors would like to thank Hannah Scarborough for excellent technical assistance, Benton Lawson for assistance with rRT-PCR experiments and Chris Ibegbu with flow cytometry. We would like to thank our colleagues Holly L. Hanson and Huiming Hon for their critical discussions.
This study was supported in part from the American Liver Foundation Thomas F. Nealon, III Postdoctoral Research Fellowship Honoring Zachery Rue (RC), the Bill and Melinda Gates Foundation Grand Challenges in Global Health (AG, GC#12 to Rafi Ahmed), EVC/CFAR Immunology Core P30 AI050409 (AG), Cancer Research Institute Investigator Award (AG), the Yerkes Research Center Base Grant RR-00165 and the Public Health Service DK083356 and AI070101 (AG).
Abbreviations used in this paper
- HSC
hepatic stellate cells
- QHSC
quiescent hepatic stellate cells
- AHSC
activated hepatic stellate cells
- APCs
antigen presenting cells
- TCR
T cell receptor
- MHC
major histocompatibility complex
- LCMV
lymphocytic choreomeningitis virus
- CFSE
Carboxyfluorescein diacetate succinimidyl ester
- GFAP
Glial Fibrillary Acidic protein
- α-SMA
alpha smooth muscle actin
- MFI
mean fluorescence intensity
- MAPK
mitogen activated protein kinase
- IFNγ
interferon gamma
- IL-2
interlukin 2
- HCV
hepatitis C virus
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- FACS
fluorescence activated cell sort
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Lim YS, Kim WR. The global impact of hepatic fibrosis and end-stage liver disease. Clin Liver Dis. 2008;12:733–746, vii. doi: 10.1016/j.cld.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Lefton HB, Rosa A, Cohen M. Diagnosis and epidemiology of cirrhosis. Med Clin North Am. 2009;93:787–799, vii. doi: 10.1016/j.mcna.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Friedman SL. Hepatic fibrosis -- overview. Toxicology. 2008;254:120–129. doi: 10.1016/j.tox.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 5.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winau F, Quack C, Darmoise A, Kaufmann SH. Starring stellate cells in liver immunology. Curr Opin Immunol. 2008;20:68–74. doi: 10.1016/j.coi.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Chen CH, Kuo LM, Chang Y, Wu W, Goldbach C, Ross MA, et al. In vivo immune modulatory activity of hepatic stellate cells in mice. Hepatology. 2006;44:1171–1181. doi: 10.1002/hep.21379. [DOI] [PubMed] [Google Scholar]
- 9.Jiang G, Yang HR, Wang L, Wildey GM, Fung J, Qian S, et al. Hepatic stellate cells preferentially expand allogeneic CD4+ CD25+ FoxP3+ regulatory T cells in an IL-2-dependent manner. Transplantation. 2008;86:1492–1502. doi: 10.1097/TP.0b013e31818bfd13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muhanna N, Horani A, Doron S, Safadi R. Lymphocyte-hepatic stellate cell proximity suggests a direct interaction. Clin Exp Immunol. 2007;148:338–347. doi: 10.1111/j.1365-2249.2007.03353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinas O, Bataller R, Sancho-Bru P, Gines P, Berenguer C, Enrich C, et al. Human hepatic stellate cells show features of antigen-presenting cells and stimulate lymphocyte proliferation. Hepatology. 2003;38:919–929. doi: 10.1053/jhep.2003.50392. [DOI] [PubMed] [Google Scholar]
- 12.Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 14.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 15.Yi KH, Chen L. Fine tuning the immune response through B7-H3 and B7-H4. Immunol Rev. 2009;229:145–151. doi: 10.1111/j.1600-065X.2009.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Passino MA, Adams RA, Sikorski SL, Akassoglou K. Regulation of hepatic stellate cell differentiation by the neurotrophin receptor p75NTR. Science. 2007;315:1853–1856. doi: 10.1126/science.1137603. [DOI] [PubMed] [Google Scholar]
- 17.Yang L, Jung Y, Omenetti A, Witek RP, Choi S, Vandongen HM, et al. Fate-mapping evidence that hepatic stellate cells are epithelial progenitors in adult mouse livers. Stem Cells. 2008;26:2104–2113. doi: 10.1634/stemcells.2008-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pircher H, Baenziger J, Schilham M, Sado T, Kamisaku H, Hengartner H, et al. Characterization of virus-specific cytotoxic T cell clones from allogeneic bone marrow chimeras. Eur J Immunol. 1987;17:159–166. doi: 10.1002/eji.1830170202. [DOI] [PubMed] [Google Scholar]
- 19.Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol. 1998;161:4652–4660. [PubMed] [Google Scholar]
- 20.Kisseleva T, Brenner DA. Role of hepatic stellate cells in fibrogenesis and the reversal of fibrosis. J Gastroenterol Hepatol. 2007;22(Suppl 1):S73–78. doi: 10.1111/j.1440-1746.2006.04658.x. [DOI] [PubMed] [Google Scholar]
- 21.Gaca MD, Zhou X, Issa R, Kiriella K, Iredale JP, Benyon RC. Basement membrane-like matrix inhibits proliferation and collagen synthesis by activated rat hepatic stellate cells: evidence for matrix-dependent deactivation of stellate cells. Matrix Biol. 2003;22:229–239. doi: 10.1016/s0945-053x(03)00017-9. [DOI] [PubMed] [Google Scholar]
- 22.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, et al. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–861. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 23.Prasad DV, Richards S, Mai XM, Dong C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 2003;18:863–873. doi: 10.1016/s1074-7613(03)00147-x. [DOI] [PubMed] [Google Scholar]
- 24.Imada K, Leonard WJ. The Jak-STAT pathway. Mol Immunol. 2000;37:1–11. doi: 10.1016/s0161-5890(00)00018-3. [DOI] [PubMed] [Google Scholar]
- 25.Dure M, Macian F. IL-2 signaling prevents T cell anergy by inhibiting the expression of anergy-inducing genes. Mol Immunol. 2009;46:999–1006. doi: 10.1016/j.molimm.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci U S A. 2003;100:10388–10392. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi IH, Zhu G, Sica GL, Strome SE, Cheville JC, Lau JS, et al. Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family. J Immunol. 2003;171:4650–4654. doi: 10.4049/jimmunol.171.9.4650. [DOI] [PubMed] [Google Scholar]
- 28.Tringler B, Zhuo S, Pilkington G, Torkko KC, Singh M, Lucia MS, et al. B7-h4 is highly expressed in ductal and lobular breast cancer. Clin Cancer Res. 2005;11:1842–1848. doi: 10.1158/1078-0432.CCR-04-1658. [DOI] [PubMed] [Google Scholar]
- 29.Yao Y, Wang X, Jin K, Zhu J, Wang Y, Xiong S, et al. B7-H4 is preferentially expressed in non-dividing brain tumor cells and in a subset of brain tumor stem-like cells. J Neurooncol. 2008;89:121–129. doi: 10.1007/s11060-008-9601-x. [DOI] [PubMed] [Google Scholar]
- 30.Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, et al. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci U S A. 2007;104:19458–19463. doi: 10.1073/pnas.0709802104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krambeck AE, Thompson RH, Dong H, Lohse CM, Park ES, Kuntz SM, et al. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci U S A. 2006;103:10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyatake T, Tringler B, Liu W, Liu SH, Papkoff J, Enomoto T, et al. B7-H4 (DD-O110) is overexpressed in high risk uterine endometrioid adenocarcinomas and inversely correlated with tumor T-cell infiltration. Gynecol Oncol. 2007;106:119–127. doi: 10.1016/j.ygyno.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 34.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 35.Yu MC, Chen CH, Liang X, Wang L, Gandhi CR, Fung JJ, et al. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40:1312–1321. doi: 10.1002/hep.20488. [DOI] [PubMed] [Google Scholar]
- 36.Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med. 2003;198:39–50. doi: 10.1084/jem.20022235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oikawa T, Takahashi H, Ishikawa T, Hokari A, Otsuki N, Azuma M, et al. Intrahepatic expression of the co-stimulatory molecules programmed death-1, and its ligands in autoimmune liver disease. Pathol Int. 2007;57:485–492. doi: 10.1111/j.1440-1827.2007.02129.x. [DOI] [PubMed] [Google Scholar]
- 38.Bertolino P, Bowen DG, McCaughan GW, Fazekas de St Groth B. Antigen-specific primary activation of CD8+ T cells within the liver. J Immunol. 2001;166:5430–5438. doi: 10.4049/jimmunol.166.9.5430. [DOI] [PubMed] [Google Scholar]
- 39.Crispe IN, Dao T, Klugewitz K, Mehal WZ, Metz DP. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev. 2000;174:47–62. doi: 10.1034/j.1600-0528.2002.017412.x. [DOI] [PubMed] [Google Scholar]
- 40.Wahl C, Bochtler P, Chen L, Schirmbeck R, Reimann J. B7-H1 on hepatocytes facilitates priming of specific CD8 T cells but limits the specific recall of primed responses. Gastroenterology. 2008;135:980–988. doi: 10.1053/j.gastro.2008.05.076. [DOI] [PubMed] [Google Scholar]
- 41.Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, et al. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32:634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 42.Semmo N, Day CL, Ward SM, Lucas M, Harcourt G, Loughry A, et al. Preferential loss of IL-2-secreting CD4+ T helper cells in chronic HCV infection. Hepatology. 2005;41:1019–1028. doi: 10.1002/hep.20669. [DOI] [PubMed] [Google Scholar]
- 43.Radziewicz H, Ibegbu CC, Hon H, Osborn MK, Obideen K, Wehbi M, et al. Impaired hepatitis C virus (HCV)-specific effector CD8+ T cells undergo massive apoptosis in the peripheral blood during acute HCV infection and in the liver during the chronic phase of infection. J Virol. 2008;82:9808–9822. doi: 10.1128/JVI.01075-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.