Introduction
The substrate for atrial fibrillation (AF) is atrial fibrosis, the hallmark of structural remodeling (SRM) (1, 2). AF begets AF and increasing AF burden leads to structural and functional remodeling, creating a substrate that is favorable for sustaining AF propagation (3) and paroxysmal AF eventually becomes persistent or permanent AF. Catheter ablation of AF results in improvement of symptoms and quality of life, in addition to significantly lowering recurrence rate of AF (4, 5). The goal of ablation is to identify and eliminate the AF trigger(s) and modify the substrate of AF to halt the vicious cycle of arrhythmia and remodeling.
Identification of the arrhythmic substrate and assessment of the structural and functional changes in the atria by non-invasive modalities can be useful in selecting patients for rhythm control therapy, including catheter ablation, early in the disease process. It can also help in monitoring the effects of therapies over time. Delayed enhancement-MRI (DEMRI) to characterize fibrotic or scarred tissue has been used extensively to study ventricular myocardium. The research at our center has shown that DEMRI can accurately quantify the extent of left atrial (LA) SRM and can predict procedural outcome in different stages of SRM (6, 7). However, the degree of reverse remodeling of LA after ablation in various stages of SRM is unknown.
LA function by strain and strain rate is an emerging technology (8, 9). It is feasible and overcomes the limitations of tissue Doppler imaging (TDI) (10, 11) The extent of SRM by DEMRI inversely correlates with LA strain as reported earlier by our group (9).
We conducted this study to compare echocardiographic measures of structural and functional reverse remodeling after catheter ablation of AF in patients with pre-ablation mild vs. moderate-severe LA SRM by DEMRI. In addition, we evaluated the pre-ablation echocardiographic and MRI remodeling parameters and their relationship to procedural outcome.
Methods
We conducted a single center, prospective, cross-sectional study at the University Of Utah Hospital. The study was approved by the investigational review board at University of Utah and was compliant with the Health Insurance Portability and Accountability Act of 1996. No extramural funding was used to support this work. We enrolled 76 patients referred to the AF clinic who were considered for catheter ablation. Prior to ablation, they were evaluated with 3D DEMRI to define the PVs, location of the esophagus, LA anatomy and characterization of the LA wall tissue. Two dimensional (2D) transthoracic echocardiography (TTE) was performed prior to catheter ablation and repeated ≥3 months after ablation. Exclusion criteria were patients with sub-optimal echocardiographic and/or MRI images and mechanical cardiac valves.
Atrial fibrillation ablation procedure
Pulmonary vein antrum isolation (PVAI), in addition to LA posterior and septal debulking was performed under intra-cardiac echocardiographic (ICE) guidance. The procedure has been described elsewhere (6, 7). Briefly, a 10-F, 64-element, phased-array ultrasound catheter (AcuNav, Siemens, Mountain View, California) was used to visualize the interatrial septum and to guide the transseptal puncture. A circular mapping catheter (Lasso, BioSense Webster, Diamond Bar, CA, USA) and an irrigated tip ablation catheter (Thermocool, BioSense Webster, Diamond Bar, CA, USA) were inserted into the LA. Intracardiac echocardiogram was used to define the PV ostia and their antra and to help position the circular mapping catheter and ablation catheter at the desired sites. Temperature and power were set to maximum of 50° and 50 Watts (pump flow rate at 30 ml/min), respectively.
Follow-up after catheter ablation
After the procedure, all patients were monitored overnight in a telemetry unit. Warfarin was continued to maintain an international normalized ratio of 2.0 to 3.0 for 3 months in paroxysmal and 6 months in persistent AF. Event monitors were placed for a minimum of 2 months after their PVAI. Patients were instructed to activate the monitors any time they felt symptomatic. Patients were assessed every 3 months to determine the success of the ablation procedure by clinical examination and Holter monitoring for 8 days at the 3, 6 and 12 months follow-up, in addition to 2D echocardiography. Recurrences were determined from patient reporting, electrocardiogram, event monitoring and Holter monitoring. Success was defined as a lack of any atrial arrhythmias (atrial tachycardia, atrial fibrillation/flutter) lasting for >30 seconds while off antiarrhythmic medications, following a blanking period of 8 weeks as per Heart Rhythm Society (HRS) consensus document guidelines (12). Patients with recurrence during the blanking period were offered electrical cardioversion and/or anti-arrhythmic drugs.
DEMRI
As previously described (6), DEMRI studies were performed on a 1.5 Tesla MR system (Avanto Siemens Healthcare, Erlangen, Germany). High resolution DE images of LA were acquired in 15 minutes after contrast agent injection (0.1 mmol/kg, Multihance, Bracco Diagnostic Inc., Princeton, NJ)) using a three dimensional (3D) respiration-navigated, inversion-recovery gradient echo pulse sequence (TR/TE=2.38/5.4 ms, flip angle of 20°, bandwidth=220 Hz/pixel, FOV=360×360×100 mm, matrix size=288×288×40, voxel size=1.25×1.25×2.5 mm). The inversion pulse was applied every heart beat and fat saturation was applied immediately before data acquisition (23 views per heart beat) during LA diastole. To preserve magnetization preparation in image volume, navigator was acquired immediately after data acquisition block. Typical scan time for DEMRI study was 5-10 minutes depending on patient heart rate and respiration pattern.
For this study, we included only good quality images and 41 (60%) patients were in sinus rhythm and 27 (40%) patients were in AF during pre-ablation MRI and echocardiographic studies. Only 3 (4%) patients were in AF during post-ablation follow-up studies. Also those in AF had good heart rate control with average heart rate of 70 ± 20 beats per minute. To quantify the extent of baseline LA wall SRM, the LA epicardial and endocardial borders on DEMRI images were traced and isolated from the remaining structures. A computerized algorithm generated a histogram of pixel intensities and at 3 standard deviation (SD) from the mean, it was defined as significant SRM. The degree of enhancement or SRM was reported as percent of the total LA wall volume.
Transthoracic echocardiography
Echocardiography was performed using standard views and harmonic imaging (Sequoia, Siemens, Mountain View, CA). Patients with AF had echocardiographic acquisition over 2-seconds duration or for 2 heart beats. In the parasternal long-axis views, LA maximum antero-posterior (AP) diameter was measured. In the apical four chamber view, we measured LV end-diastolic (EDV) and end-systolic volumes (ESV), LV stroke volume (SV) index and LVEF was calculated by Simpson’s method. In the same view, LA maximum and minimum volumes were measured and LA emptying fraction was calculated as maximum volume − minimum volume / maximum volume. LA maximum volume was also measured by biplane area-length method (0.85 × area 1 × area 2 divided by the length) indexed to body surface area (13). LA superior-inferior diameter was measured from the mitral annular plane to the posterior wall of the LA. Pulsed-wave Doppler at the tips of mitral valve leaflets allowed us to measure diastolic parameters which included transmitral early (E) and late (A) diastolic filling velocities, E/A ratio, and E deceleration time (DT). The LV early diastolic tissue velocity (E’) was measured by TDI of the medial mitral annulus. E/E’ was calculated and a value >15 was considered to represent elevated LV filling pressure (14). In AF, E/E’ was averaged with 5 cardiac cycles. Mitral regurgitation was semi-quantitatively assessed by color doppler across mitral valve and graded as none/trace (0), mild (1), moderate (2), moderately severe (3) and severe (4) respectively (15).
Velocity vector imaging (VVI)
Offline analysis of the gray scale images obtained by 2D echocardiography was done by using Velocity Vector Imaging® software (Siemens Medical Solutions USA). The endocardium of LA wall was manually traced starting from the medial to the lateral mitral annulus and was automatically tracked along the border throughout one or more cardiac cycles.
In the apical 4-chamber view, the regional analysis consisted of placing the sample in the mid-septal and mid-lateral LA walls in the same cardiac cycle. In the mid septal wall, sample was placed 1-2 cm proximal to the medial mitral annulus and the fossa of ovalis was avoided for optimal tracking of the endocardium. In the lateral wall, sample was placed 1-2 cm proximal to the lateral mitral annulus and the point of entry of the pulmonary veins was avoided. Thus strain versus time and SR versus time curves were generated from these regions of interest. The strains and strain rates from the two regions were averaged to give average LA strain and strain rate. The VVI software averaged the strain and strain rate for at least 2 cardiac cycles in AF. Speckle tracking has been reported to be a feasible and reproducible method to assess LA longitudinal deformation properties and reference values in healthy individuals have been reported by Cameli et al (11). We assessed the strain and strain rate in 25 normal controls and found the average LA strain of 68 ± 20% and average strain rate of 2.5 ± 0.5 units/second (9).
Statistical analysis
Continuous variables are presented as mean ± SD and dichotomous data are presented as numbers and percentages. The echocardiographic parameters in group 1 and group 2 at baseline, and at 6 ± 3 months after catheter ablation are compared using Student’s paired and unpaired t tests respectively. Pearson’s correlation and Chi-square are reported for comparison between dichotomous variables. A p-value of ≤ 0.05 was considered statistically significant. Univariate and multivariate linear regression analysis were done to assess the correlation between different variables. Univariate and multivariate Cox regression analysis for recurrence of AF were done to evaluate the predictive value of several pre and post ablation clinical and echocardiographic parameters.
Results
After excluding patients with sub-optimal echocardiographic images (n=6/76, 8%) and mechanical valves (n=2, 3%), 68 patients were divided them in to 2 groups based on the degree of delayed enhancement of LA wall by MRI as group 1 with mild LA SRM (≤10%, n=31) and group 2 with moderate-severe LA SRM (>10%, n=37). Figure 1 demonstrates DEMRI examples of 2 patients in different stages of LA SRM. The average degree of SRM was 6.8±2.5% in group 1 and 26.0±13.6% in group 2 (p<0.005). In all, 26 (38%) patients had paroxysmal AF and 42 (62%) patients had persistent AF as defined by the HRS consensus document on AF ablation (12). Group 2 showed a trend towards more patients with persistent AF than group 1, but it was not statistically significant (68% vs. 55%, p=0.2). There were no significant differences in the number of patients with hypertension (42% vs. 43%), coronary artery disease (10% vs. 19%), on statin therapy (26% vs. 43%) or angiotensin converting enzyme inhibitor (ACEI)/ angiotensin receptor blocker (ARB) 29% vs. 43%) between the two groups. There were no major complications from ablation in either group and follow-up was similar in both groups. After the blanking period, AF recurrence was documented in 20 patients. Of these, 16 patients underwent repeat ablation.
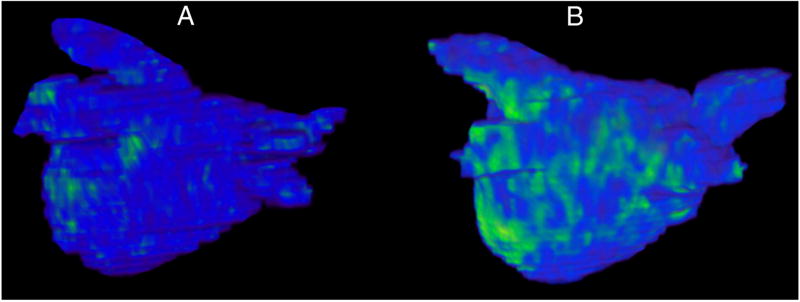
Figure 1.
Pre-ablation delayed enhancement-MRI images of the left atrium in two patients with atrial fibrillation,
(a) Patient in group 1 with mild (7%) structural remodeling (SRM) and (b) Patient in group 2 with moderate-severe SRM (34%) SRM. The blue areas represent normal myocardium and the green areas are hyperenhanced areas which represent pathological myocardium, presumed to be fibrosis.
The patients in group 1 were younger (57±15 vs. 66±13 years, p=0.009) and had a male preponderance (80% vs. 59%, p=0.07) as compared to group 2. Overall, females were older than males (69±13 vs. 59±14 years, p=0.007) and had significantly more LA SRM (25±16 vs. 14±12, p=0.003) and higher E/E’ (15±9 vs. 10±5, p=0.02) than males.
Structural reverse remodeling of LA in mild vs. moderate/severe stages of SRM after catheter ablation
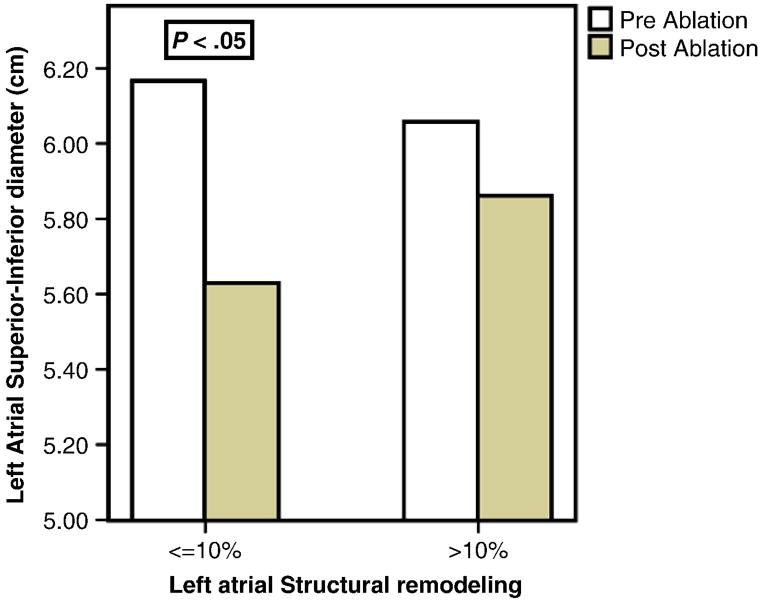
At 6 ± 3 months after ablation, patients in group 1 showed a significant reduction in LA biplane volume index, LA AP diameter and superior-inferior diameter. Patients in group 2 had reduction in LA biplane volume index and in AP diameter but not in superior-inferior diameter (Figure 2, Table 1).
Figure 2.
Improvement in left atrial superior-inferior diameter by transthoracic echocardiography before and 6 ± 3 months after catheter ablation of atrial fibrillation.
Table 1.
Comparison of structural and functional echocardiographic parameters before and 6 ± 3 months after catheter ablation of atrial fibrillation
| Variable | Group 1: Left atrial wall fibrosis <10% (n=31) | Group 2: Left atrial wall fibrosis >10% (n=37) | ||
|---|---|---|---|---|
| Before ablation | After ablation | Before ablation | After ablation | |
| Left Atrial (LA) anterior-posterior diameter (cm) | 4.1±0.7 | 3.7±0.6* | 4.1±0.8 | 3.9±0.7* |
| LA superior-inferior diameter (cm) | 6.1±1.0 | 5.7±1.0* | 6.1±1.0 | 5.8±0.9 |
| LA Emptying fraction (%) | 47±16 | 48±12 | 43±16 | 48±14* |
| LA biplane volume index (ml/m2) | 35±14 | 30±11* | 38±11 | 32±11* |
| LA average strain (%) | 31±16 | 41±11* | 29±15 | 34±19 |
| LA average strain rate (units/sec) | 1.6±1.0 | 2.0±0.6* | 1.2±0.6 | 1.5±0.8 |
| Left Ventricle (LV) Ejection fraction (%) | 52±12 | 59±8* | 52±11 | 54±12 |
| LV stroke volume index (ml/m2) | 27±8 | 31±9 | 26±7 | 28±7 |
| LV end-systolic volume index (ml/m2) | 23±8 | 21±6 | 24±11 | 24±14 |
| LV filling pressure (E/E’) | 9.2±3.3 | 10.8±5.3 | 13.5±8.0 | 13.7±5.0 |
p-value in the same group
Functional reverse remodeling of LA in mild vs. moderate/severe stages of SRM after catheter ablation
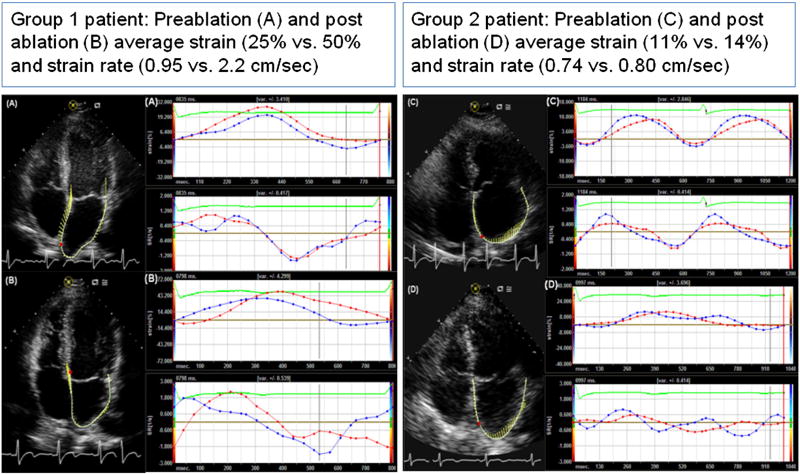
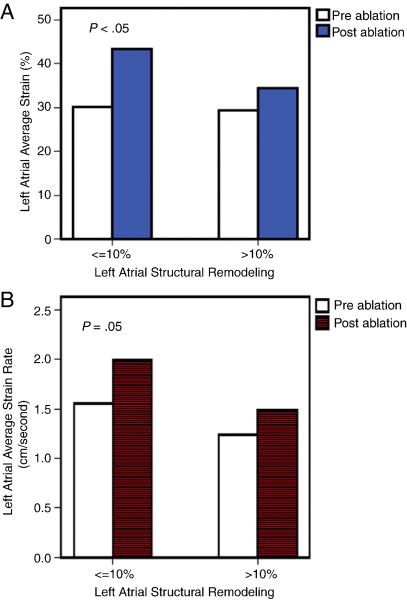
At 6 ± 3 months after ablation, patients in group 1 showed a significant improvement in the average LA strain and strain rate as compared to the strain and strain rate in group 2 (Figure 3 shows pre and post-ablation strain and strain rate imaging; A and B for a patient in group 1, and C and D for a patient in group 2). The average (Δ↑) post-ablation increase in LA strain in group 1 and 2 was 14 % vs. 4% (p<0.05) and increase in strain rate was 0.56 units/second vs. 0.15 units/second (p=0.04) (Figure 4). Overall, there was a significant increase in LA emptying fraction (44±16% to 47±13%, p=0.05) and in LVEF (53±11% to 56±11%, p=0.05) post ablation. (Table 1).
Figure 3.
Vector velocity imaging (VVI) of left atrium:
Strain (%) and strain rate (SR, units/s) vs. time (milliseconds) curves obtained by VVI, before and 6 ± 3 months after catheter ablation of atrial fibrillation. Patient in group 1 with mild (≤10%) LA structural remodeling (SRM) by delayed enhancement MRI had significant improvement in mid-septal and mid-lateral strain and strain rate (A-preablation and B-post-ablation) as compared to the patient in group 2 with moderate-severe LA SRM (>10%) (C-pre-ablation and D-post-ablation). The red and blue curves represent the mid-septal and mid-lateral left atrial samples respectively.
Figure 4.
Functional reverse remodeling of left atrium after ablation:
Strain and strain rate by vector velocity imaging: The average left atrial strain (a) and strain rate (b) significantly improved after catheter ablation of atrial fibrillation in patients with mild (≤10%) left atrial structural remodeling (SRM) as compared to moderate-severe (>10%) SRM assessed by delayed enhancement MRI.
Subgroup analysis of patients with persistent AF and paroxysmal AF showed significant improvement in average LA strain and strain rate in those with mild LA SRM of ≤ 10% as compared to those with moderate-severe SRM of >10%, similar to the entire group.
Predictors of successful ablation of AF
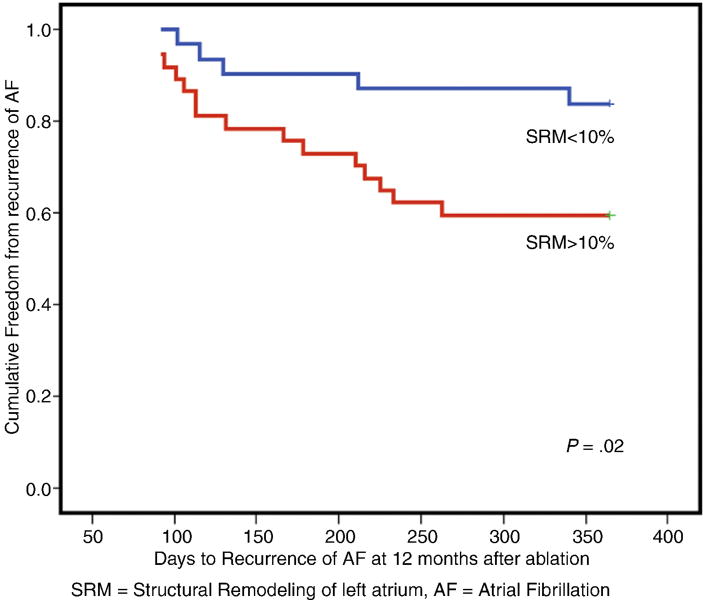
At 12 months follow-up post ablation, 48 (71%) patients remained free of AF with significantly more recurrences in group 2 compared to group 1 (41% vs. 16%, p=0.02). A Kaplan Meier curve shows the difference in long-term recurrence free days after ablation in the 2 groups (Figure 5). Patients with recurrence had more LA SRM at baseline (25±18% vs. 14±11%, p<0.05) as compared to those without recurrence, but had no significant difference in LA and LV parameters prior to ablation. Without recurrence, the average LA strain and strain rate, LA emptying fraction, LA superior-inferior diameter, biplane maximum volume index, and LVEF improved significantly as compared to those who had recurrence of AF (Table 2). In the patients with recurrence, there was no significant difference in the number of patient with paroxysmal (29% vs. 44%) vs. persistent AF (71% vs. 55%) as compared to those without recurrence.
Figure 5.
Long-term recurrence of atrial fibrillation after catheter ablation of atrial fibrillation, Kaplan Meier curves in patients with mild (≤10%) left atrial structural remodeling (SRM) and moderate-severe (>10%) SRM assessed by delayed enhancement MRI.
Table 2.
Differences in the echocardiographic parameters in patients with and without recurrence of atrial fibrillation 12 months after ablation
| Variable | Without recurrence (n=46) | With recurrence (n=18) | ||
|---|---|---|---|---|
| Before ablation | After ablation | Before ablation | After ablation | |
| Left Atrium (LA) wall fibrosis (%) by DEMRI | 14±11 | 25±18* | ||
| LA anterior-posterior diameter (cm) | 4.1±0.7 | 3.8±0.7* | 4.3±0.7 | 4.1±0.6 |
| LA superior-inferior diameter (cm) | 6.0±1.0 | 5.6±0.9* | 6.4±0.8 | 6.1±0.9 |
| LA Emptying fraction (%) | 44±16 | 51±12* | 46±15 | 41±13 |
| LA end-systolic volume index (ml/m2) | 24±11 | 15±9 | 19±11 | 24±14 |
| LA maximum biplane volume index (ml/m2) | 35±12 | 30±11* | 42±12 | 36±14* |
| LA average strain (%) | 31.8±17.1 | 40.5±16.3* | 24.5±15.6 | 30.8±17.9 |
| LA average strain rate (units/sec) | 1.5±0.8 | 1.8±0.7* | 1.2±0.9 | 1.4±0.7 |
| Left Ventricle (LV) Ejection fraction (%) | 53±12 | 56±11* | 52±9 | 57±10 |
| LV stroke volume index (ml/m2) | 26±8 | 30±8* | 29±8 | 29±7 |
| LV end-systolic volume index (ml/m2) | 24±11 | 24±13 | 23±8 | 21±9 |
| LV filling pressure (E/E’) | 12.4±7.6 | 11.8±4.6 | 10.5±4.7 | 14.2±6.3 |
p-value in the same group
Pre-ablation predictors of recurrence of AF: Using Cox multivariate stepwise regression analysis, pre-ablation LA wall fibrosis predicted recurrence after adjusting for, paroxysmal vs. persistent AF, pre-ablation LA AP diameter, superior-inferior diameter, LA maximum biplane volume index, LA emptying fraction, average LA strain and LVEF (p<0.02, hazard ratio (HR): 1.04, 95% confidence interval (CI): 1.01-1.08).
Post-ablation predictors of recurrence of AF: Using Cox multivariate stepwise regression analysis, early post-ablation LA emptying fraction predicted long-term recurrence at 12 months, after adjusting for paroxysmal vs. persistent AF, post-ablation LA AP diameter, superior-inferior diameter, LA maximum biplane volume index, average LA strain and LVEF (p<0.006, HR: 0.97, 95% CI: 0.96-1.0).
Discussion
In this study, we demonstrated significant structural and functional reverse remodeling of the LA by 2D echocardiography and strain and strain rate imaging when catheter ablation of AF was performed in LA with mild SRM by DEMRI. It is also associated with less recurrence of AF. The benefits of ablation of AF seem to be dependent on the stage of LA SRM and not on paroxysmal or persistent nature of AF. Advanced age and female gender had a predisposition for more advanced LA SRM. To our knowledge, this is the first study reporting the degree of reverse remodeling of LA after catheter ablation of AF in various stages of LA SRM quantified by DEMRI.
Structural and functional remodeling of the left atrium in atrial fibrillation
The hallmark of SRM is LA myocardial fibrosis which leads to progressive LA dilation. Longer duration of AF is known to cause progressive remodeling and increased LA size and volume (13, 16, 17) and persistent AF is considered to be an advanced stage of arrhythmia as compared to paroxysmal AF. The patients in our study with persistent AF had larger LA size and volume and reduced strain as compared to patients with paroxysmal AF prior to ablation.
An interesting finding in our study was the lack of difference in the number of patients with paroxysmal and persistent AF when they were classified as mild and moderate-severe LA SRM by DEMRI. This raises an intriguing question about the mechanisms of AF initiation and persistence. Some patients diagnosed as paroxysmal AF may have more progressive LA SRM and resistance to rhythm control therapies while other patients with persistent AF may have mild LA SRM and good response to rhythm control therapies. Identifying the exact pathogenesis of AF in a patient may facilitate individualizing rhythm control therapies. With catheter ablation, areas of LA SRM visualized on DEMRI may be targeted to potentially cure the arrhythmia. Of note, detection of diffuse LA SRM by DEMRI does not serve as an absolute marker. It simply shows the severity of LA pathology. Focal areas of SRM can be targeted as absolute markers for ablation.
The older patients with AF, especially females, had more LA SRM by DEMRI in our study. Females also had elevated LV filling pressure, measured by E/E’. Cardiac fibrosis is a diffuse process occurring in both the ventricles and the atria, and is triggered by higher filling pressure and vice-versa (18). With increasing age, the degree of atrial fibrosis increases as shown by Gramley et al (19). With DEMRI we can demonstrate the AF substrate in high risk patients.
Reverse remodeling of left atrium after catheter ablation of atrial fibrillation
In the structurally remodeled LA, we measured both AP and superior-inferior diameter as LA enlarges in an asymmetric fashion. The extensively remodeled atria can take a longer time to recovery of atrial contractile dysfunction after conversion to sinus rhythm (20, 21). Therkelsen et al evaluated patients with persistent AF using MRI and showed reversal of atrial remodeling beginning immediately after CV (22). They reported a reduction in atrial volumes and increase in atrial EF much earlier than improvement in LVEF and mass after cardioversion. Marsan et al used 3-D echocardiography and they also showed significant structural and functional improvement after catheter ablation (17). In these studies, LA function was assessed by LA emptying/EF.
In our study, the LA with mild SRM had a significant improvement in LA compliance, measured by strain and strain rate as compared to those with moderate-severe SRM at short-term follow up. There was structural reverse remodeling in both groups with reduction in LA size and volume. Assessment at longer duration after ablation is needed to see if there is late functional recovery with advanced SRM as diseased LA may lag behind in functional recovery after successful ablation. It could be speculated that the improvement in strain measurements post ablation could be related to presence of sinus rhythm. However, in our previous study we reported that the rhythm, sinus or AF, during strain imaging did not affect LA strain measurements as it measures only the compliance or reservoir function of LA and not the contractile function (9), suggesting a favorable change in the atrial intrinsic myocardial function. Larger areas of SRM are presumed to be fibrotic and are nonviable areas which do not contribute to improvement in LA strain after restoration of sinus rhythm.
Predictors of long-term success of catheter ablation of AF
Successful ablation was achieved in 71% of our patients at one year follow-up. In other studies, the recurrence of AF after ablation has been variable between 40-86% (4, 23-25). The ability to predict recurrence prior to rhythm control therapies in AF can be helpful in deciding long-term anticoagulation and anti-arrhythmic therapy (5). The type of AF did not predict the recurrence in our study. Mild LA SRM pre-ablation and improvement in LA emptying fraction early post-ablation were found to be the only predictors of long-term successful ablation. Neither pre- or post-ablation LA strain and strain rate nor other echocardiographic parameters were useful in predicting success. This is in contrast to the findings by Di Salvo et al (26) and Wang et al (27) who reported that patients with higher LA strain prior to cardioversion were less likely to have recurrence. Higher post-ablation strain and strain rate predicted success after ablation in the study by Schneider et al (24). By DEMRI, the LA SRM is quantified in 3D view and gives a complete assessment of the LA pathology. Probably that’s the reason why the 3D LA SRM predicted recurrence while 2D LA dimension did not.
Limitations
The main limitation of our study was a short duration of follow up by echocardiography. The success of ablation at long-term follow up needs to be assessed to see how many need repeat ablation for better outcome. It is also important to see if the degree of LA and LV reverse remodeling seen at medium-term follow up is maintained and progressive at longer duration. LA strain and strain rate were assessed in only the septal and lateral walls of the LA since those walls were consistently imaged without significant drop-out. Strain data from ablated regions, mainly the posterior wall could not be obtained due to imaging limitations. The average strain may reflect the overall function of the LA and can be reliably measured in the same location. There are limitations inherent to imaging when patients are in AF which can be fairly overcome by imaging with good heart rate control as done in our patients. Quantification of LA wall fibrosis in thin-walled LA by DEMRI is limited by the inadequate spatial resolution from partial volume effects and cannot give accurate information on the transmurality of fibrosis.
Conclusion
The reverse remodeling of the LA structure and function after catheter ablation of AF is significantly better when performed in the early stage of arrhythmia with mild LA SRM by DEMRI. The benefits of ablation of AF and long term success seem to be dependent on the stage of LA SRM and not on paroxysmal or persistent nature of AF, as traditionally defined. The degree of LA wall SRM pre-ablation and improvement in LA emptying fraction early post-ablation predict success of ablation at one year follow up. Longer duration of follow-up is necessary to evaluate other identifiable characteristics that lead to recurrence after successful ablation. Moreover, this also has important clinical implications such as continuing anticoagulation and anti-arrhythmic medications after ablation.
Acknowledgments
Disclosures: The authors acknowledge the computational support and resources provided by the Scientific Computing and Imaging Institute and the NIH NCRR Center for Integrative Biomedical Computing (www.sci.utah.edu/cibc), NIH NCRR Grant No. 5P41RR012553-02. Nassir Marrouche is partially supported by grants from Siemens Medical and Surgivision.
Footnotes
The authors are solely responsible for the design and conduct of this study, all study analyses and drafting and editing of the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kallergis EM, Manios EG, Kanoupakis EM, et al. Extracellular matrix alterations in patients with paroxysmal and persistent atrial fibrillation: biochemical assessment of collagen type-I turnover. J Am Coll Cardiol. 2008;52:211–5. doi: 10.1016/j.jacc.2008.03.045. [DOI] [PubMed] [Google Scholar]
- 2.Yoshihara F, Nishikimi T, Sasako Y, et al. Plasma atrial natriuretic peptide concentration inversely correlates with left atrial collagen volume fraction in patients with atrial fibrillation: plasma ANP as a possible biochemical marker to predict the outcome of the maze procedure. J Am Coll Cardiol. 2002;39:288–94. doi: 10.1016/s0735-1097(01)01719-3. [DOI] [PubMed] [Google Scholar]
- 3.Allessie MA. Atrial electrophysiologic remodeling: another vicious circle? J Cardiovasc Electrophysiol. 1998;9:1378–93. doi: 10.1111/j.1540-8167.1998.tb00114.x. [DOI] [PubMed] [Google Scholar]
- 4.Oral H, Pappone C, Chugh A, et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006;354:934–41. doi: 10.1056/NEJMoa050955. [DOI] [PubMed] [Google Scholar]
- 5.Stabile G, Bertaglia E, Senatore G, et al. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (Catheter Ablation For The Cure Of Atrial Fibrillation Study) Eur Heart J. 2006;27:216–21. doi: 10.1093/eurheartj/ehi583. [DOI] [PubMed] [Google Scholar]
- 6.Oakes RS, Badger TJ, Kholmovski EG, et al. Detection and Quantification of Left Atrial Structural Remodeling With Delayed-Enhancement Magnetic Resonance Imaging in Patients With Atrial Fibrillation. Circulation. 2009 doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGann CJ, Kholmovski EG, Oakes RS, et al. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J Am Coll Cardiol. 2008;52:1263–71. doi: 10.1016/j.jacc.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 8.D’Andrea A, De Corato G, Scarafile R, et al. Left atrial myocardial function in either physiological or pathological left ventricular hypertrophy: a two-dimensional speckle strain study. Br J Sports Med. 2008;42:696–702. doi: 10.1136/bjsm.2007.041210. [DOI] [PubMed] [Google Scholar]
- 9.Kuppahally SS, Akoum N, Burgon NS, et al. Left Atrial Strain and Strain Rate in Patients with Paroxysmal and Persistent Atrial Fibrillation: Relationship to Left Atrial Structural Remodeling Detected by delayed Enhancement-MRI. Circ Cardiovasc Imaging. doi: 10.1161/CIRCIMAGING.109.865683. [DOI] [PubMed] [Google Scholar]
- 10.D’Andrea A, Caso P, Salerno G, et al. Left ventricular early myocardial dysfunction after chronic misuse of anabolic androgenic steroids: a Doppler myocardial and strain imaging analysis. Br J Sports Med. 2007;41:149–55. doi: 10.1136/bjsm.2006.030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameli M, Caputo M, Mondillo S, et al. Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc Ultrasound. 2009;7:6. doi: 10.1186/1476-7120-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation--excutive summary. Rev Port Cardiol. 2007;26:383–446. [PubMed] [Google Scholar]
- 13.Tsang TS, Barnes ME, Bailey KR, et al. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76:467–75. doi: 10.4065/76.5.467. [DOI] [PubMed] [Google Scholar]
- 14.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–94. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 15.Thomas JD. How leaky is that mitral valve? Simplified Doppler methods to measure regurgitant orifice area. Circulation. 1997;95:548–50. doi: 10.1161/01.cir.95.3.548. [DOI] [PubMed] [Google Scholar]
- 16.Petersen P, Kastrup J, Brinch K, et al. Relation between left atrial dimension and duration of atrial fibrillation. Am J Cardiol. 1987;60:382–4. doi: 10.1016/0002-9149(87)90253-0. [DOI] [PubMed] [Google Scholar]
- 17.Marsan NA, Tops LF, Holman ER, et al. Comparison of left atrial volumes and function by real-time three-dimensional echocardiography in patients having catheter ablation for atrial fibrillation with persistence of sinus rhythm versus recurrent atrial fibrillation three months later. Am J Cardiol. 2008;102:847–53. doi: 10.1016/j.amjcard.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 18.Martos R, Baugh J, Ledwidge M, et al. Diastolic heart failure: evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation. 2007;115:888–95. doi: 10.1161/CIRCULATIONAHA.106.638569. [DOI] [PubMed] [Google Scholar]
- 19.Gramley F, Lorenzen J, Knackstedt C, et al. Age-related atrial fibrosis. Age (Dordr) 2009;31:27–38. doi: 10.1007/s11357-008-9077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapiro EP, Effron MB, Lima S, et al. Transient atrial dysfunction after conversion of chronic atrial fibrillation to sinus rhythm. Am J Cardiol. 1988;62:1202–7. doi: 10.1016/0002-9149(88)90260-3. [DOI] [PubMed] [Google Scholar]
- 21.van den Berg MP, Tjeerdsma G, Jan de Kam P, et al. Longstanding atrial fibrillation causes depletion of atrial natriuretic peptide in patients with advanced congestive heart failure. Eur J Heart Fail. 2002;4:255–62. doi: 10.1016/s1388-9842(01)00232-x. [DOI] [PubMed] [Google Scholar]
- 22.Therkelsen SK, Groenning BA, Svendsen JH, et al. Atrial and ventricular volume and function evaluated by magnetic resonance imaging in patients with persistent atrial fibrillation before and after cardioversion. Am J Cardiol. 2006;97:1213–9. doi: 10.1016/j.amjcard.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 23.Beukema WP, Elvan A, Sie HT, et al. Successful radiofrequency ablation in patients with previous atrial fibrillation results in a significant decrease in left atrial size. Circulation. 2005;112:2089–95. doi: 10.1161/CIRCULATIONAHA.104.484766. [DOI] [PubMed] [Google Scholar]
- 24.Schneider C, Malisius R, Krause K, et al. Strain rate imaging for functional quantification of the left atrium: atrial deformation predicts the maintenance of sinus rhythm after catheter ablation of atrial fibrillation. Eur Heart J. 2008;29:1397–409. doi: 10.1093/eurheartj/ehn168. [DOI] [PubMed] [Google Scholar]
- 25.Shin SH, Park MY, Oh WJ, et al. Left atrial volume is a predictor of atrial fibrillation recurrence after catheter ablation. J Am Soc Echocardiogr. 2008;21:697–702. doi: 10.1016/j.echo.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 26.Di Salvo G, Caso P, Lo Piccolo R, et al. Atrial myocardial deformation properties predict maintenance of sinus rhythm after external cardioversion of recent-onset lone atrial fibrillation: a color Doppler myocardial imaging and transthoracic and transesophageal echocardiographic study. Circulation. 2005;112:387–95. doi: 10.1161/CIRCULATIONAHA.104.463125. [DOI] [PubMed] [Google Scholar]
- 27.Wang T, Wang M, Fung JW, et al. Atrial strain rate echocardiography can predict success or failure of cardioversion for atrial fibrillation: a combined transthoracic tissue Doppler and transoesophageal imaging study. Int J Cardiol. 2007;114:202–9. doi: 10.1016/j.ijcard.2006.01.051. [DOI] [PubMed] [Google Scholar]