Abstract
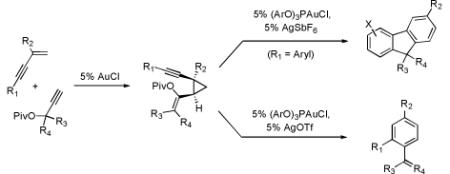 Intermolecular annulation of enynes and propargyl esters to selectively produce styrenes or fluorenes is reported. The divergent arene syntheses involve a Au-catalyzed, two-pot, multi-step process proceeding by cis-diastereoselective cyclopropanation, cycloisomerization, and, finally, annulation or elimination.
Intermolecular annulation of enynes and propargyl esters to selectively produce styrenes or fluorenes is reported. The divergent arene syntheses involve a Au-catalyzed, two-pot, multi-step process proceeding by cis-diastereoselective cyclopropanation, cycloisomerization, and, finally, annulation or elimination.
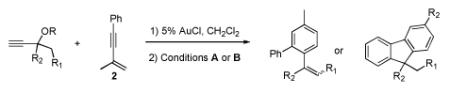
Metal-catalyzed cycloaddition reactions provide attractive and efficient methods for the synthesis of arenes, although regioselectivity presents a major challenge for intermolecular reactions.1 The synthesis of polysubstituted benzenes by the [2+2+2] cyclotrimerization of alkynes2 has been extensively studied, as has the Pd-catalyzed [4+2] cycloaddition of enynes and activated alkynes.3 We report herein a mechanistically distinct intermolecular annulation of enynes and alkynes to produce multiply substituted arenes (eq 1); styrene or fluorene products can be selectively accessed by judicious choice of reaction conditions.
 |
(1) |
Given the synthetic utility of vinyl cyclopropanes,4 we anticipated that alkynyl cyclopropanes derived from the cyclopropanation of 1,3-enynes would provide similar opportunities for organic synthesis.5 In light of our previous work employing propargyl esters as carbene precursors in Au(I)-catalyzed cyclopropanation reactions of olefins,6,7 the reaction of 1 with enyne 2 was investigated.8
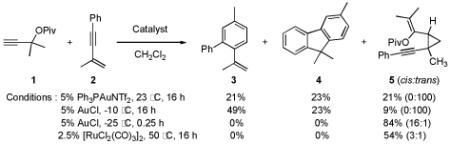
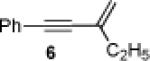
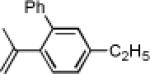
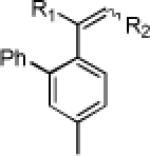
Initially, treatment of 1 and 2 with a cationic phosphinegold(I) complex resulted in a mixture of products, including styrene 3, fluorene 4, and cyclopropane trans-5 (eq 2). The unexpected products 3 and 4 were intriguing; 3 formally results from a completely regioselective [4+2] cross-dimerization of two different enynes, while compounds such as 4 are of interest due to the blue-light emitting properties of polyfluorenes.9,10
 |
(2) |
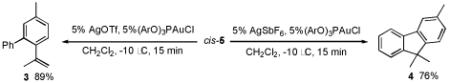
Since cyclopropane 5 was obtained exclusively as the trans-diastereomer, we hypothesized that 3 and 4 arose from the cis-diastereomer. Therefore, we were pleased to find that the less reactive AuCl cleanly catalyzed the synthesis of cyclopropane 5 with high cis-diastereoselectivity and complete regioselectivity. With ready access to cis-5, we next investigated its transformation to 3 and 4.
 |
(3) |
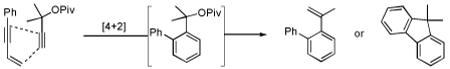
Gratifyingly, either compound could be selectively prepared simply by changing the silver salt co-catalyst in conjunction with triarylphosphitegold(I) chloride (Ar = 2,4-di-tert-butylphenyl).11,12 Thus, reaction of cis-5 with the Au complex and AgOTf provided 3 in 89% yield, while the reaction with AgSbF6 under otherwise identical conditions provided 4 in 76% yield (eq 3).13
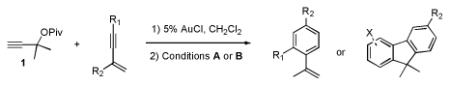
The substrate scope of the two-step, divergent syntheses of fluorenes and styrenes was investigated with other enynes (Table 1).14 Aryl enynes with a variety of substitution patterns and functional groups were tolerated, demonstrating the power of this method to prepare multiply substituted arenes from simple starting materials (entries 1-13). Moreover, both electron-rich and electron-poor enynes 14 and 18 undergo the cyclopropanation and arene syntheses; however, commensurate with the expected nucleophilicities of the aryl group, they demonstrated diametric preferences for styrenes versus fluorene formation (entries 3,4). Alkyl-substituted enynes are also tolerated in both the cyclopropanation and annulation steps (entries 14-16).
Table 1.
Au(I)-Catalyzed Arene Synthesis: Enyne Scope
 | ||||||
|---|---|---|---|---|---|---|
| entry | enyne | cp yielda | conditions Ab | yieldc | conditions Bb | yieldc |
| 1 |
|
7 94% |
|
8 81% |
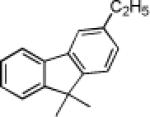
|
9 79% |
|
| ||||||
| 2 |
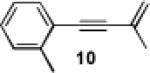
|
11 83% |
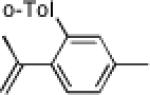
|
12 72% |
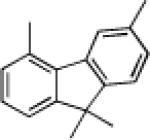
|
13 66% |
|
| ||||||
| 3 |
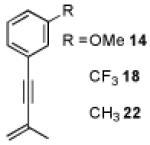
|
15 82% |
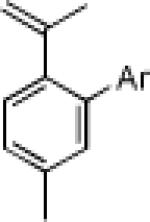
|
16 31% (+ 54% 17) |
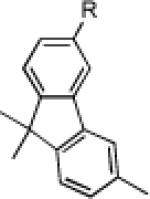
|
17 80% (15:1)d |
| 4 | 19 72% | 20 93% | 21 25% (1:1)d (+ 62% 20) | |||
| 5 | 23 75% | 24 60% | 25 85% (1.6:1)d | |||
|
| ||||||
| 6 |
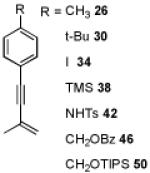
|
27 41% |

|
28 58% |
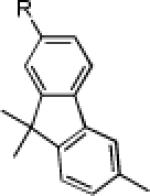
|
29 76% |
| 7 | 31 62% | 32 86% | 33 93% | |||
| 8 | 35 79% | 36 83% | 37 63% | |||
| 9 | 39 79% | 40 95% | 41 59% | |||
| 10 | 43 87% | 44 71% | 45 60% | |||
| 11 | 47 73% | 48 89% | 49 71% | |||
| 12 | 51 74% | 52 64% | 53 90% | |||
|
| ||||||
| 13 |
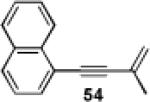
|
55 77% |
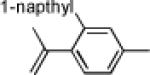
|
56 69% |
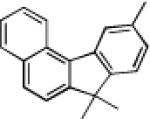
|
57 79% |
|
| ||||||
| 14 |

|
59 50% |
|
60 65% | N/Ae | |
| 15 | 62 62% | 63 53% | ||||
| 16 | 65 58% | 66 66% | ||||
Isolated yields of cis-cyclopropane Reactions run with 3:1 ratio of 1:enyne.
A: 5% AgOTf, 5% (ArO)3PAuCl, CH2Cl2. B: 5% AgSbF6, 5% (ArO)3PAuCl, CH2Cl2.
Isolated yields
Ratio of regioisomers.
Conditions B were not investigated with 58, 61 and 64.
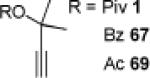
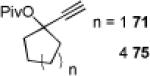
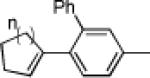
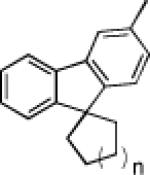
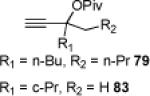
The carbene precursor was also varied (Table 2). The pivaloate ester provided the best selectivity in differentiating between the styrene and fluorene pathways (entries 1-3). [9,9]-Dibutyl-substituted fluorene 82 was prepared (entry 6); such hydrophobic solubilizing groups are often found in fluorenes designed for subsequent polymerization. Additionally, the use of unsymmetrical pivaloate 83 readily provided 85 or 86 (entries 7).
Table 2.
Au(I)-Catalyzed Arene Synthesis: Propargyl Ester Scope
 | ||||||
|---|---|---|---|---|---|---|
| entry | propargyl ester | cp yielda | conditions Ab | yieldc | conditions Bb | yieldc |
| 1 |
|
5 80% | 3 | 89% | 4 | 76% |
| 2 | 68 82% | 79% | 19% (+ 53% 3) | |||
| 3 | 70 60% | 81% | 57% (+ 32% 3) | |||
|
| ||||||
| 4 |
|
72 80% |
|
73 89% |
|
74 63% |
| 5 | 76 83% | 77 91% | 78 66% | |||
|
| ||||||
|
|
|
||||
| 6 | 80 59% | 81 77% (8.3:1)d | 82 84% | |||
| 7 | 84 79% | 85 72% | 86 84% | |||
Isolated yields of cis-cyclopropane. Reactions run with 3:1 ratio of propargyl ester:2
A: 5% AgOTf, 5% (ArO)3PAuCl, CH2Cl2. B: 5% AgSbF6, 5% (ArO)3PAuCl, CH2Cl2.
Isolated yields
E:Z ratio.
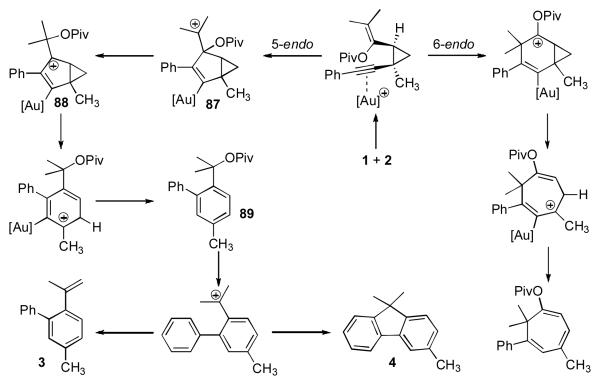
A mechanism accounting for the observed products begins with the formation of 5 by intermolecular cyclopropanation of enyne 2 via the gold carbenoid produced from rearrangement of propargyl ester 1. Following coordination of the cationic gold catalyst to the resulting alkyne, the pendant olefin can participate in either a 5-endo-dig or 6-endo-dig cyclization (Scheme 1). When tertiary propargyl esters are employed in the gold-catalyzed annulation, the 5-endo-dig cyclization to generate tertiary carbocation 87 dominates. Subsequent migration of the pivaloyloxy group gives allylic cation 88 that may be further stabilized by delocalization of the charge onto gold. Cyclopropyl ring opening via a pentadienyl cation leads to 89, which is most likely converted to 3 and 4 by E1 and SN1 mechanisms, respectively.15,16
Scheme 1.
Proposed Mechanism for the Formation of 3 and 4.
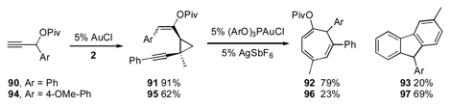
Use of secondary propargyl pivaloate 90 diverted the reaction pathway towards the 6-endo-dig cyclization and formation of cycloheptatriene 92 (eq 4). Selectivity for the 5-endo-dig pathway could be partially restored using 94, which predominantly provided the fluorene 97, suggesting that, for trisubstituted olefins, electronic factors govern the regioselectivity of the cycloisomerization.
 |
(4) |
In conclusion, readily available enynes and propargyl esters may be selectively transformed into styrenes or fluorenes under catalyst control via two new Au-catalyzed processes. Synthesized by a rarely investigated, highly selective cyclopropanation of 1,3 enynes, cis-vinyl-alkynyl-cyclopropanes undergo a novel cycloisomerization reaction, the outcome of which may be controlled simply through choice of catalyst counterion.
Supplementary Material
ACKNOWLEDGMENT
We gratefully acknowledge NIHGMS (RO1 GM073932), Merck Research Laboratories, Bristol-Myers Squibb, Amgen Inc., and Novartis for funding. D.J.G. thanks the ACS Organic Division (Merck) and Bristol Myers-Squibb for predoctoral fellowships. I.D.G.W. thanks NSERC for a postdoctoral fellowship.
Footnotes
Supporting Information Available Experimental procedures and compound characterization data. This material is available free of charges at http://pubs.acs.org
References
- 1.For a review, see: Saito S, Yamamoto Y. Chem. Rev. 2000;100:2901. doi: 10.1021/cr990281x. Intramolecular annulation of enynes and alkynes: Dunetz JR, Danheiser RL. J. Am. Chem. Soc. 2005;127:5776. doi: 10.1021/ja051180l. Danheiser RL, Gould AE, de la Pradilla RF, Helgason AL. J. Org. Chem. 1994;59:5514. Intramolecular annulation of arene-ynes and alkynes: Lian J-J, Chen P-C, Lin Y-P, Ting H-C, Liu R-S. J. Am. Chem. Soc. 2006;128:11372. doi: 10.1021/ja0643826. Rodriguez D, Martinez-Esperon MF, Navarro-Vazquez A, Castedo L, Dominguez D, Saa C. J. Org. Chem. 2004;69:3842. doi: 10.1021/jo0498213.
- 2.(a) Agenet N, Gandon V, Vollhardt KPC, Malacria M, Aubert C. J. Am. Chem. Soc. 2007;129:8860. doi: 10.1021/ja072208r. [DOI] [PubMed] [Google Scholar]; (b) Chopade PR, Louie J. Adv. Synth. Catal. 2006;348:2307. [Google Scholar]
- 3.(a) Rubina M, Conley M, Gevorgyan V. J. Am. Chem. Soc. 2006;128:5818. doi: 10.1021/ja060085p. [DOI] [PubMed] [Google Scholar]; (b) Saito S, Salter MM, Gevorgyan V, Tsuboya N, Tando K, Yamamoto Y. J. Am. Chem. Soc. 1996;118:3970. [Google Scholar]; (c) Gevorgyan V, Yamamoto Y. J. Organomet. Chem. 1999;576:232. [Google Scholar]
- 4.Rubin M, Rubina M, Gevorgyan V. Chem. Rev. 2007;107:3117. doi: 10.1021/cr050988l. [DOI] [PubMed] [Google Scholar]
- 5.Cyclopropanations of enynes are surprisingly undeveloped. Cyclopropanation with diazo-derived carbenes: Gmyzina RN, D'yakonov IA, Danilkina LP. Zhur. Obshch. Khimii. 1970;6:2168. Shapiro EA, Romanova TN, Dolgii IE, Nefedov OM. Izv. Akadem. Nauk SSSR, Seriya Khimicheskaya. 1985;11:2535. Kretschik O, Nieger M, Dotz KH. Chem. Ber. 1995;128:987. Simmons-Smith: Du H, Long J, Shi Y. Org. Lett. 2006;8:2827. doi: 10.1021/ol0609659.
- 6.(a) Gorin DJ, Dube P, Toste FD. J. Am. Chem. Soc. 2006;128:14480. doi: 10.1021/ja066694e. [DOI] [PubMed] [Google Scholar]; (b) Johansson MJ, Gorin DJ, Staben ST, Toste FD. J. Am. Chem. Soc. 2005;127:18002. doi: 10.1021/ja0552500. [DOI] [PubMed] [Google Scholar]; (c) Shi X, Gorin DJ, Toste FD. J. Am. Chem. Soc. 2005;127:5802. doi: 10.1021/ja051689g. [DOI] [PubMed] [Google Scholar]; (d) Lopez S, Herrero-Gomez E, Perez-Galan P, Nieto-Oberhuber C, Echavarren AM. Angew. Chem. Int. Ed. 2006;45:6029. doi: 10.1002/anie.200602448. [DOI] [PubMed] [Google Scholar]
- 7.(a) Miki K, Ohe K, Uemura S. Tetrahedron Lett. 2003;44:2019. [Google Scholar]; (b) Miki K, Ohe K, Uemura S. J. Org. Chem. 2003;68:8505. doi: 10.1021/jo034841a. [DOI] [PubMed] [Google Scholar]; (c) Ikeda Y, Murai M, Abo T, Miki K, Ohe K. Tetrahedron Lett. 2007;48:6651. [Google Scholar]
- 8.For recent reviews of gold-catalyzed reactions, see: Jimenez-Nunez E, Echavarren AM. Chem. Commun. 2007:333. doi: 10.1039/b612008c. Gorin DJ, Toste FD. Nature. 2007;446:395. doi: 10.1038/nature05592. Furstner A, Davies PW. Angew. Chem. Int. Ed. 2007;46:3410. doi: 10.1002/anie.200604335.
- 9.(a) Neher D. Macromol. Rapid Commun. 2001;22:1365. [Google Scholar]; (b) Scherf U, List E. Adv. Mater. 2002;14:477. [Google Scholar]; (c) Leclerc M. J. Polym Sci. A: Polym. Chem. 2001;39:2867. [Google Scholar]
- 10.The synthesis of substituted fluorenes is generally accomplished by [9,9]-dialkylation of fluorene followed by [2,7]-dibromination and subsequent functionalization. See, for example: Tsuie B, Reddinger JL, Sotzing GA, Soloducho J, Katritzky AR, Reynolds JR. J. Mater. Chem. 1999;9:2189.
- 11.For [3,3] rearrangements of cis-vinyl-alkynyl-cyclopropanes, see: metal-mediated: Ohe K, Yokoi T, Miki K, Nishino F, Uemura S. J. Am. Chem. Soc. 2002;124:526. doi: 10.1021/ja017037j. Thermal: Dolbier WR, Jr., Garza OT, Al-Sader BH. J. Am. Chem. Soc. 1975;97:5038.
- 12.For Au-catalyzed benzannulations, see: Hashmi ASK, Frost TM, Bats JW. J. Am. Chem. Soc. 2000;122:11553. Dankwardt JW. Tetrahedron Lett. 2001;42:5809. Asao N, Takahashi K, Lee S, Kasahara T, Yamamoto Y. J. Am. Chem. Soc. 2002;124:12650. doi: 10.1021/ja028128z. Asao N. Synlett. 2006;11:1645. Zhao J, Hughes CO, Toste FD. J. Am. Chem. Soc. 2006;128:7436. doi: 10.1021/ja061942s.
- 13.Other phosphine catalysts provided lower selectivity. For example, treatment of cis-5 with PPh3AuCl/AgSbF6 resulted in 63% 4 and 34% 3.
- 14.Optimization of the one-pot synthesis produced 4 in lower yield from 1 and 2. See supporting information.
- 15.89 was isolated and resubjected to the reaction conditions with and without the addition of triarylphosphitegold(I)chloride to afford the expected products, suggesting the possibility for silver or acid catalysis in the final step. Control experiments indicate that neither AgOTf nor HOTf catalyze the cycloisomerization of 5. See Supporting Information.
-
16.Further experiments (see supporting information) indicate that 3 readily isomerizes to 4 under strongly acidic conditions. A deuterium-labeling experiment suggested that this is not a major pathway for the formation of 4 under our reaction conditions:

Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.