Abstract
An intriguing question is how epidermal pattern formation processes are established, and which are the molecular mechanisms involved in these events. The establishment of the pattern is concomitant with the formation of ectodermal appendages, which involves complex interactions between the epithelium and the underlying mesenchyme. Among ectodermal appendages, hair follicles are the ‘mini organs’ that produce hair shafts. Several developmental and structural features are common to all hair follicles and to the hair shaft they produce. However, many different hair types are produced in a single organism. Also, different characteristics can be observed depending on the part of the body where the hair follicle is formed. Here, we review the mechanisms involved in the patterning of different hair types during mouse embryonic development, as well as the influence of the body axes on hair patterning.
Keywords: mouse development, epidermal patterning, hair placode induction, hair shaft development, guard, awl, auchene, zigzag
INTRODUCTION
In mammals, the development of skin appendages such as hair, teeth and mammary glands involves complex interactions between the epidermis and the underlying mesenchyme through established hierarchical morphogenetic processes. Hair follicles are produced from a series of interactions between specific sites in the ectoderm and the underlying mesoderm. Although there are developmental and structural features that are common to all hair follicles and to the hair shaft they produce, there are many different types of hair produced in a single organism. The normal hair pattern results from the development of different types of hair that commence to form at distinct embryonic stages. Hair follicles are found in a regular distribution, with precise spacing between the large primary hair follicles and the smaller follicles. Specifically, mice develop a coat containing four distinct types of hair in addition to several specialized hairs such as vibrissae and tail hair. The generation of such diversity of hair types is due to signaling pathways that drive the patterning and induction in a specific spatial and temporal manner. The analysis of natural or engineered mouse mutants with hair defects has helped elucidate some of the mechanisms leading to the specification of the different types of hair during mouse embryonic development. We will summarize the current knowledge on the regulatory factors and pathways that demarcate the sites in the skin where hair follicles will develop and that determine their different structures and shapes. We will not discuss, in detail, the molecular basis of hair shaft differentiation or hair cycling.
PATTERNING, GROWTH AND CYCLING OF HAIR FOLLICLES
Pattern formation
Regional specificity implies that different areas of the skin have different characteristics. Hair follicles are found in specific arrays, with large follicles being interspersed by smaller follicles throughout the skin. To explain how, from a seemingly homogenous epidermis, the regular pattern and anterior-posterior orientation of the hair follicles is accomplished, Turing proposed a reaction-diffusion (R-D) model (Turing, 1952) in which a morphogenetic field starts with a homogenous distribution of cells, activator(s) and inhibitor(s). Fluctuations and interactions between the activator(s) and inhibitor(s) initiate the patterning process with the final distribution depending on the ratio of activators and inhibitors, and the size and shape of the pattern field.
Suggestions as to which signaling pathways were crucial for the initiation of hair follicle development came from reports showing that ectopic expression of the Wnt/βcatenin inhibitor Dkk1, causes blockage of hair follicle induction, while the constitutive activation of Wnt/βcatenin results in stimulation (Andl et al., 2002; Gat et al., 1998). Two recent publications have addressed the establishment of hair follicle distribution and orientation (Sick et al., 2006; Wang et al., 2006). Sick et al., utilize the established Wnt and Dkk developmental regulatory factors and mathematical predictions to establish models that support an R-D mechanism involving Wnt and Dkk in the determination of hair follicle spacing (Sick et al., 2006). On the other hand, Wang and collaborators studied aberrant hair patterns in the coats of Frizzled6 knockout mice to predict how planar cell polarity signaling directs the orientation of hair follicles (Wang et al., 2006).
Hair morphogenesis
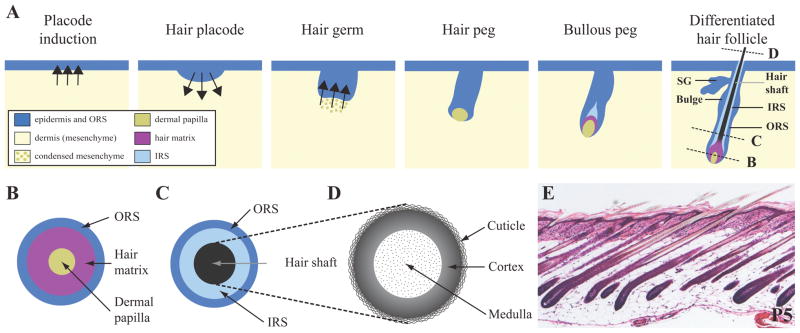
The development of the hair follicle starts with the morphological thickening of the epidermis (hair placode) in response to a signal from the dermis (Figure 1). A signal from the hair placode to the underlying mesenchyme then induces the condensation of the dermal cells that are adjacent to the placode, and this leads to the formation of the hair germ. The epidermal part of the hair germ proliferates, elongates and invaginates into the dermis to form the hair peg in which the condensed mesenchyme is progressively engulfed by epidermal cells, becoming the dermal papilla. Signals from the dermal papilla to the adjacent epidermal cells induce the differentiation of the inner root sheath (IRS) that will form a rigid tube in which the future hair shaft will develop and grow. The IRS is itself surrounded by the epidermal-derived outer root sheath (ORS), which is a continuation of the basal layer of the epidermis. When the IRS is formed, the epidermal cells surrounding the dermal papilla, known as the hair matrix cells, start differentiating into distinct lineages to form the different structures of the hair shaft that will grow inside the IRS and eventually protrude through the epidermis. From the outside to the inside, the hair shaft consists of the following three layers: the cuticle forming the hair surface, the cortex where almost all hair keratins are accumulated, and the medulla characterized by the presence of cells that differentiate and produce air spaces that are arranged into one or several columns. Several signaling pathways (Wnt/βCatenin, Shh/Gli2, BMP, Notch1/Delta1, Noggin, Eda/Edar, FGF) and transcription factors (GATA3, HoxC13, Cutl1, Msx1, Msx2, WHN, p63 and Dlx3) have been established as critical for hair follicle development (Millar, 2002; Schmidt-Ullrich et al., 2006).
Figure 1. Hair follicle morphogenesis and structure of the hair shaft.
A) Schematic representation of the major stages of hair follicle morphogenesis. B–D) Cross views of mature hair follicle at three different levels along the proximo-distal axis. Section plans are shown in A with dashed lines (last panel). E) Skin section at postnatal day 5 (P5) stained with hematoxylin and eosin. IRS, inner root sheath; ORS, outer root sheath; SG: sebaceous gland.
Hair cycling
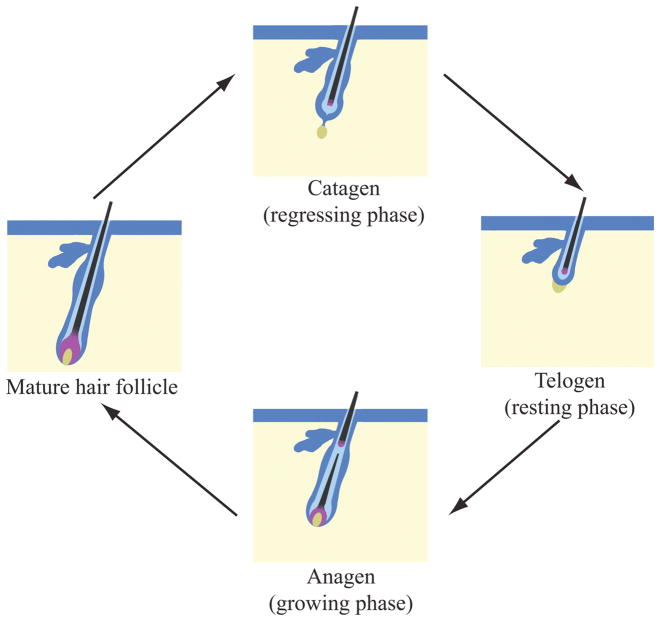
The development of the hair follicle is not limited to the morphogenesis phase. Indeed, after the initial morphogenesis, throughout life, the hair follicle cycles through a series of stages (Figure 2), including a growing phase (anagen), a regressing phase (catagen), and a resting phase (telogen) (Alonso and Fuchs, 2006) (Figure 2). In mice, the hair coat has a defined length. A report on targeted and spontaneous mutations of FGF5 demonstrated the essential role of this factor as a regulator of hair cycle growth. FGF5, which is expressed in the ORS, is required for the progression of the hair cycle from anagen to the catagen stage. In the angora mouse mutant and in the FGF5 knockout, the follicles remain in anagen and hair shaft continues to grow (Hebert et al., 1994). Wnt3 is also involved in the limits of hair growth. Wnt3 is expressed in the hair matrix cells that become the medulla of the hair shaft. Overexpression of Wnt3 in transgenic mice causes a short-hair phenotype with altered differentiation of the hair shaft. Overexpression of Dishevelled-2, an effector of Wnt3 signaling, present in a subset of cells of the ORS and in precursor cells of the cortex and cuticle, also leads to the short hair phenotype observed when Wnt3 is overexpressed (Millar et al., 1999).
Figure 2. Hair cycling.
Schematic representation of the stages of hair follicle cycling: the regressing phase (catagen), resting phase (telogen) and growing phase (anagen).
PATTERNING OF THE MOUSE COAT: SUCCESSIVE WAVES OF HAIR FOLLICLE INDUCTION LEAD TO FOUR DIFFERENT TYPES OF HAIR
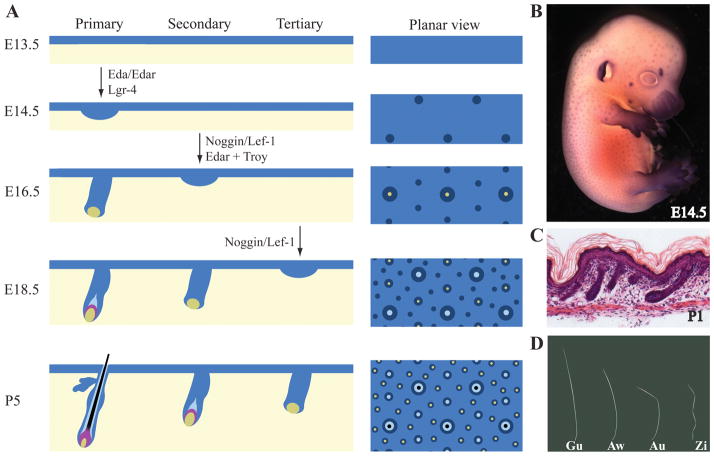
The mouse produces a coat containing a mix of four different types of hair: guard, awl, auchene and zigzag, as well as specialized hairs such as vibrissae and tail hair (Sundberg, 1994). The generation of the coat involves three successive waves of hair follicle induction together with specific patterning mechanisms, which leads to different hair structures (Figure 3A and D). The features, such as length, number of medulla columns and presence of hair shaft bends, which allow for distinction between the four hair types, are summarized in Table I. The first wave of hair follicle induction (primary hair placodes) occurs around embryonic day 14 (E14), and whole mount alkaline phosphatase staining can be used to visualize this process (Figure 3B). Primary hair placodes gives rise to guard hairs that are straight, contain two columns of medulla cells and are relatively long. They represent 1–3% of the total number of hair and protrude above the coat. Guard hairs are also known as tylotrich hair due to their unique sensory function and form two sebaceous glands per follicle. The second wave of hair follicle induction (secondary hair placodes) happens around E16–E17 and produces awl hairs. Awl hairs represent 30% of the coat, are about half to two-third the size of guard hairs, are straight and contain two to four columns of medulla cells. The third wave of hair follicle induction (tertiary hair placodes) occurs close to birth (E18-P1) and generates zigzag hairs. Zigzag hairs, also known as undercoat hair, are the most abundant hair in the coat (65–70%). Zigzag hairs are about the same size as awl hairs, and contain only one column of medulla cells. As their name indicates they present several constrictions along the hair shaft forming three to four bends alternately pointing in opposite directions. The fourth hair type are auchene hairs that are present at very low frequency (around 0.1%). They share common features with both awl and zigzag hairs. Indeed, they contain two to four columns of medulla cells, like awl hairs, but have only one bend due to a unique local constriction of the hair shaft. It is not clear whether they are formed during the second wave of hair placodes or with the third. Different hair types at distinct stages of morphogenesis can be observed in mouse skin at postnatal day 1 (P1) (Figure 3C).
Figure 3. Coat hair development: three waves of hair follicle induction forming four different hair types.
A) Schematic representation of the three waves of hair follicle induction (primary, secondary and tertiary) involved in the formation of the mouse coat. Genes known to be essential for the induction of specific waves are indicated. The planar view on the right shows the pattern distribution of the successive waves of hair follicles. B) Alkaline phosphatase staining of mouse embryo at embryonic day 14.5 (E14.5) showing the distribution of primary hair follicles. C) Cross section of mouse back skin at postnatal day 1 (P1) showing the presence of hair follicles at different stages of their morphogenesis. D) Four different hair types found in the mouse coat: guard (Ga), awl (Aw), auchene (Au) and zigzag (Zi) hairs. Characteristics of these four hair types are summarized in Table I.
Table I.
Characteristics of the different hair types in the mouse coat
| Guard | Awl | Auchene | Zigzag | ||
|---|---|---|---|---|---|
| Tylotrich | Non-tylotrich | ||||
| Time of placode induction | E14 | E16–17 | E18-P1 | ||
| Primary placodes | Secondary placodes | Terciary placodes | |||
| Size (relative to guard hair) | 1 (around 1 cm) | 1/2 to 2/3 | 1/2 to 2/3 | 1/2 to 2/3 | |
| Number of medulla columns | 2 | 2–4 | 2–4 | 1 | |
| Number of bends | 0 (straight hair) | 0 | 1 | 3–4 | |
| Frequency | 1–3% | 30% | 0.1% | 65–70% | |
In the following section, we summarize the main mechanisms that have been shown to be involved in the induction or patterning of specific hair types in the mouse coat. We will also discuss the influence of the body axis on the patterning of the coat. Although the composition of the coat is basically the same throughout the body, there is evidence of differential regulations of hair follicle formation along the anteroposterior and dorsoventral axes.
Molecular mechanisms involved in the formation of specific types of coat hair
Induction of primary hair follicles
Among the pathways involved in the induction of primary hair follicles, the Eda/Edar pathway has been the most studied and well characterized (Mikkola, 2008; Mikkola and Thesleff, 2003). Eda, also called ectodysplasin, is a member of the tumor necrosis factor (TNF) family that binds to the receptor Edar. There are two isoforms of ectodysplasin: Eda-A1 and Eda-A2. Even though they only differ by two amino acids, they each bind a distinct receptor: Eda-A1 binds only to Edar while Eda-A2 binds only to another TNF receptor called Xedar. Since several observations suggest that the Eda-A2/Xedar signaling is not essential during hair follicle development (Newton et al., 2004; Srivastava and Amla, 2002), we will focus on the Eda-A1/EdaR signaling (referred to as Eda/Edar). At the stage of primary hair placode formation, Edar is expressed in the placode itself while Eda is expressed in the non-placodal ectoderm. Later on, Eda and Edar are both expressed in the hair bulb (Laurikkala et al., 2002). The Eda/Edar signaling pathway involves the Edar-binding death domain adaptor protein Edaradd and activates the transcription factor NF-kB (nuclear factor kappaB) (Koppinen et al., 2001). In humans, mutations in EDA, EDAR or EDARADD lead to hypohydrotic ectodermal dysplasia (HED) characterized by sparse hair, missing and malformed teeth, absence of sweat glands, and defects in other glands including mammary glands (Headon et al., 2001; Kere et al., 1996; Monreal et al., 1999). Several spontaneous mouse mutants with a similar phenotype were shown to carry mutations in the same pathway: Eda is mutated in Tabby mice lacking both Eda isoforms (Ferguson et al., 1997; Srivastava et al., 1997), Edar is mutated in downless mice (Headon and Overbeek, 1999) and Edaradd is mutated in crinckled mice (Yan et al., 2002). In all these mutants, the primary hair placodes do not form (Laurikkala et al., 2002), resulting in the absence of guard hairs in the adult mice (Headon and Overbeek, 1999). The transgenic expression of a degradation-resistant IkB mutant, results in the constitutive inhibition of the NF-kB signaling pathway, leading to a phenotype that is similar to Eda and Edar mutant mice (Schmidt-Ullrich et al., 2001). Thus, the Eda/Edar pathway is required for the induction of primary hair follicles.
The adhesion molecule MadCAM-1 has been proposed to be a target of the Eda/Edar pathway and to be involved in placode formation (Nishioka et al., 2002). Two recent and independent microarray analyses, which utilized different strategies to compare Eda induced and Eda null skin, identified the Wnt antagonist Dkk4 as an Eda target (Cui et al., 2006; Fliniaux et al., 2008). The induction of Dkk4 suggests a complex regulation of hair placode formation by Eda, which induces both activators and repressors of hair placode formation (Fliniaux et al., 2008; Mikkola, 2008). Eda inhibits BMP activity and induces the expression of Shh, which was demonstrated to be essential for the downgrowth of the hair germ (Pummila et al., 2007). A recent study showed that the knockout (total and skin-specific) of Lgr4, a leucine-rich repeat G-protein coupled receptor, leads to a hair phenotype similar to Eda/Edar mutants, including the absence of primary hair placodes (Mohri et al., 2008). The relation between Lgr4 and the Eda/Edar pathway remains to be elucidated.
Although the secondary and tertiary hair placodes are formed in mice mutated in the Eda/Edar pathway, they produce only abnormal awl-like hairs (Sundberg, 1994). This suggests a secondary role for the Eda/Edar pathway in hair patterning. Interestingly, the transgenic expression in Tabby mice of Eda-A1, and not Eda-A2, almost completely restores normal development of hair, teeth and glands (Cui et al., 2003; Srivastava et al., 2001). The same effect was observed in Tabby mice that were administered recombinant Eda-A1 during embryonic development (Gaide and Schneider, 2003). However, although primary hair placodes form normally and differentiate into guard hairs, rescued Tabby mice still do not form zigzag hairs (Gaide and Schneider, 2003). Surprisingly, the transgenic overexpression of Eda-A1 in wild-type mice also resulted in the absence of zigzag hair formation, although it does not affect the total number of hair (Cui et al., 2003; Mustonen et al., 2003).
Induction of secondary and tertiary hair follicles
The BMP2/4 antagonist Noggin, which is expressed in the mesenchyme of all developing hair follicles (condensed mesenchyme and dermal papilla) and antagonizes the inhibitory effect of BMP-2/4 on hair follicle development (Botchkarev et al., 1999), was the first factor shown to be required for the induction of secondary hair follicles (Botchkarev et al., 2002). This observation was made by analyzing late embryonic stages of noggin knockout mice that die before birth, and by grafting E18.5 skin from these mice onto SCID mice. Although primary hair follicle induction normally occurs at E14.5 in noggin knockout embryos, the secondary and tertiary waves of hair follicle induction are missing. Moreover, the grafting approach revealed that while primary hair follicles were induced, their development did not progress further. Thus, even though noggin is not required for the induction of primary hair follicles, it is required for differentiation (Botchkarev et al., 2002). The downstream effects of Noggin involve the Lymphoid enhancer-binding factor 1 (Lef-1) which is downregulated in noggin knockout mice (Botchkarev et al., 1999). Lef-1 knockout mice form primary hair placodes at E14.5, exhibit a reduced number of hair follicles at subsequent stages and produce only rudimentary hair which become visible around day 9 after birth but are progressively lost (van Genderen et al., 1994). Lef-1 activity involves the formation of a complex with βcatenin, an essential component of the canonical Wnt signaling pathway. In response to Wnt signaling, βcatenin is protected from degradation and translocated to the nucleus where it forms a complex with members of the Lef/Tcf family and controls the transcription of target genes. Interestingly, while nuclear βcatenin can be detected in all hair placodes at E16.5, nuclear Lef-1 is only present in secondary hair placodes, which is consistent with the fact that primary hair placodes do not require Lef-1 expression (Jamora et al., 2003). On the other hand, the Wnt/βcatenin pathway seams to be involved the induction of all hair follicles (Andl et al., 2002; Huelsken et al., 2001), implying that βcatenin interacts with another member of Lef/Tcf family during the formation of primary placodes.
A recent study of Troy, a TNF receptor homologous to Edar, revealed its implication in the induction of secondary hair follicles (Pispa et al., 2008). Even though the expression pattern of Troy in developing hair follicles is similar to that of Edar, mice lacking Troy have no hair defects. However, mice lacking both Edar and Troy did not develop either primary or secondary hair follicles. These double knockout mice lack all types of hair follicles on the skin covering the cranium, demonstrating that Troy is also involved in tertiary hair follicle induction in the cranial skin (Pispa et al., 2008).
Dominant mutations in the Sox18 transcription factor have been correlated with the ragged phenotype in mice. The hair coat of these mutant mice is characterized by a reduced number of hairs due to the absence of auchene and zigzag hair types (Pennisi et al., 2000b). However, Sox18 knockout mice exhibit a milder phenotype, with only a reduction in the number of zigzag hairs and defects in pigmentation (Pennisi et al., 2000a).
Differentiation of tertiary hair follicles: what controls hair bending?
All hair follicles have an anteroposterior polarity that is established at the beginning of hair placode formation (Devenport and Fuchs, 2008) and is maintained throughout development and in the adult mouse. In mature hair follicles, Sonic hedgehog (Shh) marks hair polarity by its restricted expression in a group of matrix cells on the posterior part of the follicle (Gat et al., 1998). In addition to the polarity that is applied to all hair follicles, zigzag hairs contain a series of bends in alternating orientations, which must involve cyclic mechanisms that are still poorly understood.
Several mouse mutants do not form zigzag hairs although they do form tertiary hair follicles, suggesting that they are affected not in the induction of this hair type but in the patterning of the characteristic zigzag shape. As introduced earlier, in addition to not forming primary hair placodes and consequently lacking guard hairs, mice with mutations in the Eda/Edar signaling pathway do not have auchene or zigzag hairs while the induction of the secondary and tertiary hair placodes does not seem to be affected (Headon et al., 2001; Headon and Overbeek, 1999; Srivastava et al., 1997). The transgenic expression of Eda-A1 (under K14 promoter or involucrin promoter) in the epidermis of wild-type mice also results in the absence of zigzag hairs while the total number of hair is not affected (Cui et al., 2003; Mustonen et al., 2003; Zhang et al., 2003). The use of an inducible system, allowing to turn on and off the expression of the transgene, revealed that the formation of zigzag hairs was not restored when the expression of Eda-A1 was maintained during development and turned off in adult mice (Cui et al., 2003). This irreversibility suggests that misexpression of Eda-A1 is not only disturbing the asymmetric and cyclic expression of the genes controlling hair shaft bending but is irreversibly compromising the establishment of the molecular mechanism responsible for hair bend formation. It is likely that the Eda/Edar pathway has specific target genes at different stages of hair follicle development. In a recent study, the lymphotoxin-b pathway was identified as a target of Eda involved primarily in hair differentiation (Cui et al., 2006). Indeed, lymphotoxin-b knockout mice form primary hair placodes but produce abnormal hair shafts including zigzag hairs with no bends.
Ectopic overexpression, controlled by the involucrin promoter, of the insulin-like growth factor IGF-1 (normally expressed in the dermal papilla) in the IRS and the medulla of the hair follicle, leads to the absence of zigzag hairs (Weger and Schlake, 2005). The insulin-like growth factor binding protein IGFBP5 was the first protein identified as a molecular marker of zigzag hairs (Schlake, 2005). The expression pattern of IGFBP5 in zigzag hair follicles is highly dynamic, switches from being expressed in the dermal papilla to being asymetrically distributed in the hair matrix and the medulla, and seems to correlate with hair shaft bending (Schlake, 2005). It has been proposed that IGFBP5 is regulated by Krox-20, a zinc finger transcription factor with an intriguing expression pattern during hair development (Gambardella et al., 2000; Schlake, 2006). However, no hair phenotype has been reported on IGFBP-5 knockout mice, even when IGFBP-3 and 4 are also knocked out (Ning et al., 2006).
Wnt signaling was also shown to play a role in the patterning of zigzag hairs (Hammerschmidt and Schlake, 2007). This was demonstrated by transgenic expression of the Wnt antagonist Dkk1 in the hair cortex under the control of the Foxn1 promoter (Foxn1-Dkk1). In these transgenic mice, the Wnt signaling is locally inhibited in the hair bulb after hair follicles have begun to form. Foxn1-Dkk1 mice exhibit several features of Tabby mice, including the absence of zigzag hairs. In both Foxn1-Dkk1 and Tabby mice, IGFBP5 expression was down-regulated (Hammerschmidt and Schlake, 2007). The same observation was made for Shh expression that exhibits asymmetric and dynamic distribution in the hair follicle of wild-type mice (Gambardella et al., 2000; Hammerschmidt and Schlake, 2007). These observations suggest that Eda/Edar and Wnt signaling control the molecular machinery allowing tertiary hair follicles to produce zigzag hairs.
Members of the Runx family of transcription factors are also involved in the determination of hair structure (Raveh et al., 2005; Raveh et al., 2006). Runx1 is dynamically expressed both in the dermal papilla and the epidermal part of all developing hair follicles (Raveh et al., 2006). When Runx1 is specifically inactivated in the epidermis, most zigzag hairs form less pronounced bends that are often misoriented and fragile (Raveh et al., 2006). The knockout of Runx3, which is expressed in the dermal papilla of all hair follicles, leads to a change in the composition of the coat: the proportion of zigzag hairs is reduced to 55% instead of 70%, their length is significantly reduced, and they exhibit two bends rather than three to five (Raveh et al., 2005). Thus, even though they are expressed in all hair follicles, both Runx1 and Runx3 seem to be specifically required for the normal hair shaft structure of bent hair types.
The zinc finger transcription factor Miz1, which is expressed in the basal layer of the epidermis and the ORS of the hair follicle, is involved in the regulation of hair cycling and affects the proportion and structure of zigzag hairs (Gebhardt et al., 2007). Miz1 knockout mice exhibit a rough fur containing only 40% zigzag hairs, which do not form bends.
Influence of the body axes on patterning of the coat
Although the general structure of the skin is the same throughout the body, its embryologic origin varies along the anteroposterior and dorsoventral axes. How dermal specificity and epidermal competence are established has not been determined. It is not known why certain regions of the human body have hair while other regions do not. Could this be a result of regionalization of the dermis? While the epidermis is derived from the relatively homogenous non-neural ectoderm, the dermis has various origins: the dorsal dermis is derived from the dermomyotome, the ventral dermis is derived from the somatopleure (external layer of the lateral plate mesoderm), the craniofacial dermis is cranial neural crest-derived.
Although it is clear that dermal fibroblasts have distinct inductive properties depending on their localization (Chang et al., 2002), the mechanisms involved in the differential patterning of the skin along the body axes is still poorly documented. A HOX code has been proposed, where different combinations of HOX gene expression may be the basis of skin regional specificity. Indeed, there are spatiotemporally defined HOX expression patterns in the dermal cells derived from different topological regions in humans (Chang et al., 2002). Other transcription factors that characterize specific areas of the skin are: engrailed, ventral paw (no hair) versus dorsal paw (with hair) (Loomis et al., 1996); and TBX4 and 5, which establish scale versus feather forming dermis (Rodriguez-Esteban et al., 1999). These results support the idea that a combination of molecular codes specify regional skin characteristics. Below, we summarize what is currently known in the differential patterning of the coat along the body axes.
Dorsoventral patterning of the coat
The most obvious evidence of the existence of different mechanisms governing the patterning of dorsal and ventral skin is the difference in coat color observed in several strains. The difference in coat color is governed by complex allelic variations of the Agouti gene. Agouti is secreted by the dermal papilla and, through paracrine action, causes neighboring melanocytes to produce yellow pigments (pheomelanosomes) instead of black pigments (eumelanosomes). Two predominant Agouti mRNA isoforms differing by their transcriptional initiation site and promoter region have been identified: a “hair cycle-specific” isoform and a “ventral specific” isoform (Bultman et al., 1994; Vrieling et al., 1994). The “hair cycle-specific” isoform is transiently expressed at a specific stage of hair development and cycling resulting in the presence of a band of yellow pigment in a black hair. This isoform is expressed in both dorsal and ventral skin. The “ventral specific” isoform is expressed throughout the growing phase of the hair but only in ventral skin. The presence of these two isoforms with different distribution patterns, together with the allelic variation of the Agouti gene, result in large diversity of coat colors that may or may not differ along the dorsoventral axis. For example, mice with the black-and-tan genotype at/at form ventral hairs that are completely yellow and dorsal hairs that are completely black.
The T-box transcription factor Tbx15 is involved in the establishment of the boundary between dorsal and ventral skin (Candille et al., 2004). At E11.5, the expression of Tbx15 is complementary to the expression of agouti and restricted to the dorsolateral mesenchyme, before being expanded ventrally. Mice mutated in Tbx15 (knockout mice or the spontaneous mutant Droopy ear) exhibit a dorsal expansion of the yellow belly hair in agouti black-and-tan mice. Pigmentation is not the only difference between ventral and dorsal hair. Although the four hair types are present in dorsal and ventral skin, ventral hair are significantly shorter than dorsal hair with a slightly increased proportion of zigzag hairs (Candille et al., 2004). Interestingly, the mechanisms defining the boundary between ventral and dorsal coat are similar to the mechanisms involved in the dorsoventral positioning of the limbs and in mammary gland positioning, although they happen at different times during development. In all cases, the establishment of the boundary seems to involve the antagonistic interaction between BMP4, which is expressed in the ventral epithelium, and Tbx15 which is expressed in the dorsal mesenchyme (Cho et al., 2006).
Specificity of cranial hair patterning
Several mouse models exhibit particular defects in localized areas of their cranial coat. In addition to not forming guard hairs and zigzag hairs throughout their coat, Tabby mice do not have hair behind the ears (focal alopecia). This phenotype demonstrates that, in this area of the body, the Eda/EdaR signaling pathway is required for the differentiation of all hair types (Mikkola and Thesleff, 2003). This phenotype is rescued by the transgenic expression of Eda-A1 in Tabby mice (Cui et al., 2003; Srivastava et al., 2001). The local inhibition of Wnt signaling in the hair follicle, by transgenic expression of the Wnt antagonist Dkk1 under control of the Foxn1 promoter (Foxn1-Dkk1), also leads to formation of bald patches behind the ears (Hammerschmidt and Schlake, 2007). Sparse head hair and focal alopecia behind the ears was also observed in mice lacking Lgr4 (Mohri et al., 2008). Interestingly, both Foxn1-Dkk1 mice and Lgr4-/- mice share more than one feature with Tabby mice, including the absence of primary hair placodes. As introduced earlier, while mice lacking the TNF receptor Troy have no hair defects, mice lacking both Eda and Troy lack not only primary hair follicles but also secondary hair follicles (Pispa et al., 2008). These double knockout mice lack all types of hair follicles on the cranium in addition to the bald spots observed behind the ears. The mechanisms leading to differential function of Eda/Edar, Troy, Lgr4 and Wnt in cranial skin remain to be elucidated.
PATTERNING OF SPECIALIZED TYPES OF HAIR
Vibrissae
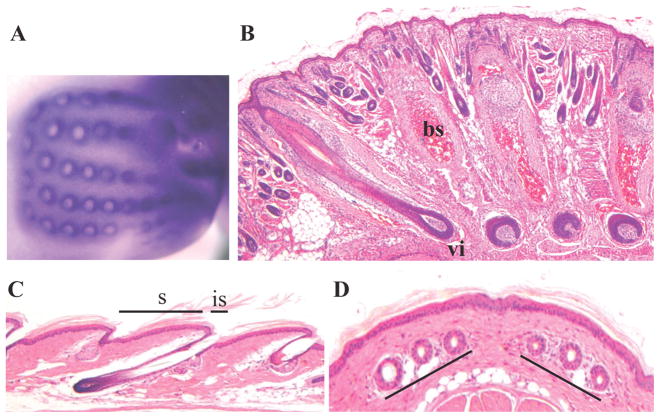
Vibrissae, also called whiskers, are very large long hairs extending from the snout of the mouse. They are highly specialized and serve as tactile sense organs. They develop within characteristic blood sinuses and are highly innervated. There are three types of vibrissae (primary, secondary and supernumerary) that are organized in a very specific pattern in the snout region (Van Exan and Hardy, 1980; Yamakado and Yohro, 1979) (Figure 4A). Interestingly, the pattern of vibrissae distribution is faithfully projected to the somato-sensory cortex of the telencephalon where it forms a characteristic neuronal aggregation termed “cortical barrel”. In mice, the development of vibrissae follicles starts at E12.5, and at birth, vibrissae hair shafts have already emerged (Figure 4B).
Figure 4. Two specialized hair types: vibrissae and tail hair.
A) Alkaline phosphatase staining of whisker pads of mouse embryo at embryonic day 13.5 (E13.5) showing the characteristic pattern distribution of vibrissae. bs, blood sinus; vi, vibrissae. B) Cross section of whisker follicles at postnatal day 7 (P7). C) Longitudinal section of adult mouse tail. D) Cross section of adult mouse tail. s, scale region; is, interscale region.
Interestingly, mice mutated in the Eda/Edar pathway form vibrissae at E12.5, while they do not form primary coat hair placodes at E14.5 (Headon and Overbeek, 1999). On the other hand, mice mutated in the Noggin/Lef1 pathway do not form vibrissae at E12.5, while they form primary coat hair placodes at E14.5 but do not form secondary hair placodes at E16.5 (Botchkarev et al., 2002). Thus, even though vibrissae are induced prior to the initiation of coat hair development, the mechanisms involved in vibrissae development seem to be more closely related to the induction of secondary coat hair placodes than to the induction of primary coat hair placodes.
Tail hairs
The epidermis of the tail is thicker than the trunk epidermis and has a very specific structure (Schweizer and Marks, 1977). Parallel rings of scale-like structures form along the axis of the tail (Figure 4C). These structures are separated by inter-scale regions characterized by a unique type of keratinization. Each row contains a certain number of scales, each of which face the junction between two scales of the next row (opposite phase). A neatly patterned group of three hairs develop under each scale and emerge through the interscale region (Figure 4D). During embryonic development, hair placode induction occurs at E16.5 in the tail (Sofaer, 1973). Beneath each scale, the three hairs forming are not induced simultaneously. The central hair, that is usually the most developed, is induced first while the development of the two other hairs is slightly delayed (Schweizer and Marks, 1977). Although absence of tail hair formation or defects in their development have been observed in many mouse models, the mechanisms involved specifically in the development of this type of hair have been poorly addressed.
As mentioned, tail hairs develop at the same time as the secondary wave of coat hair (E16.5). Tabby mice do not form hair on their tail, while they do form secondary and tertiary but not primary coat hair follicles (Headon and Overbeek, 1999). As for the first wave of coat hair placode induction, the induction of tail hair placodes is rescued by transgenic expression of Eda-A1 in Tabby mice (Cui et al., 2003; Gaide and Schneider, 2003; Srivastava et al., 2001). These observations suggests that the function of the Eda/Edar pathway in tail hair placode induction at E16.5 is more related to its function in primary coat hair placode induction at E14.5 than to its function in secondary coat hair placode induction at E16.5. This is further supported by the fact that mice mutated in the Noggin/Lef-1 pathway seem to form tail hair. Noggin-/- mice die before birth and tail hair was not analyzed at embryonic stages (Botchkarev et al., 2002). However, Lef-1-/- mice are viable, lack coat hair (secondary and tertiary) and vibrissae, but do form tail hair (van Genderen et al., 1994). Thus, the Noggin/Lef-1 pathway, that is required for the secondary hair placode induction at E16.5, is not required for the induction of tail hair at the same stage.
CONCLUSION
Much has been learned in the last decade on the regulatory mechanisms and essential factors that control hair formation. Although many questions remain, recent work has begun to elucidate what is the specific combinatorial signaling that is required to generate the variability in hair shafts. Mechanisms determining the regional specificity (head, back of the ears, etc.) remain to be completely understood and have mostly been investigated in the mouse model. However, the determination of regulatory programs in the different hair types in humans is of great clinical and cosmetic relevance. In humans, it is unclear which are the mechanisms that underlie the generation of patterns of specific types of hair follicles in localized areas of the body (scalp, axilla, body, etc.). Interestingly, a condition characterized by disruption of the pattern leading to altered hair growth and distribution more typical of furry mammals is the congenital generalized hypertrichosis (Baumeister et al., 1993). Identification of a gene associated with a form of congenital universal hypertrichosis that is inherited in an apparent X-linked recessive manner and is involved in the growth of human hair in certain regions of the body has been linked to the Xq24-Xq27.1 region (Tadin-Strapps et al., 2003).
The better characterization of a large number of mutant mice with hair defects, the identification of the locus mutated in each phenotype and reassessment of the results in published studies that have not provided detailed description of the hair phenotype (i.e. delay in hair growth without analyzing the timing of hair follicle induction) will allow for better understanding of hair follicle biology. The identification of the molecular markers controlling hair follicle morphogenesis and patterning will provide unlimited possibilities for therapeutic approaches.
Acknowledgments
We thank members of the Morasso laboratory, especially Katherine Maddox and Joonsung Hwang. Our research is supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases at NIH.
References
- Alonso L, Fuchs E. The hair cycle. Journal of Cell Science. 2006;119(Pt 3):391–393. doi: 10.1242/jcs.02793. [DOI] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Developmental Cell. 2002;2(5):643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Baumeister FA, Egger J, Schildhauer MT, Stengel-Rutkowski S. Ambras syndrome: delineation of a unique hypertrichosis universalis congenita and association with a balanced pericentric inversion (8) (p11.2; q22)[see comment] Clinical Genetics. 1993;44(3):121–128. doi: 10.1111/j.1399-0004.1993.tb03862.x. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, Herzog W, Lindner G, McMahon JA, Peters C, Lauster R, McMahon AP, Paus R. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nature Cell Biology. 1999;1(3):158–164. doi: 10.1038/11078. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Sharov AA, Funa K, Huber O, Gilchrest BA. Modulation of BMP signaling by noggin is required for induction of the secondary (nontylotrich) hair follicles. Journal of Investigative Dermatology. 2002;118(1):3–10. doi: 10.1046/j.1523-1747.2002.01645.x. [DOI] [PubMed] [Google Scholar]
- Bultman SJ, Klebig ML, Michaud EJ, Sweet HO, Davisson MT, Woychik RP. Molecular analysis of reverse mutations from nonagouti (a) to black-and-tan (a(t)) and white-bellied agouti (Aw) reveals alternative forms of agouti transcripts. Genes & Development. 1994;8(4):481–490. doi: 10.1101/gad.8.4.481. [DOI] [PubMed] [Google Scholar]
- Candille SI, Van Raamsdonk CD, Chen C, Kuijper S, Chen-Tsai Y, Russ A, Meijlink F, Barsh GS. Dorsoventral patterning of the mouse coat by Tbx15. Plos Biology. 2004;2(1):E3. doi: 10.1371/journal.pbio.0020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, Brown PO. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(20):12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KW, Kim JY, Song SJ, Farrell E, Eblaghie MC, Kim HJ, Tickle C, Jung HS. Molecular interactions between Tbx3 and Bmp4 and a model for dorsoventral positioning of mammary gland development. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(45):16788–16793. doi: 10.1073/pnas.0604645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui CY, Durmowicz M, Ottolenghi C, Hashimoto T, Griggs B, Srivastava AK, Schlessinger D. Inducible mEDA-A1 transgene mediates sebaceous gland hyperplasia and differential formation of two types of mouse hair follicles. Human Molecular Genetics. 2003;12(22):2931–2940. doi: 10.1093/hmg/ddg325. [DOI] [PubMed] [Google Scholar]
- Cui CY, Hashimoto T, Grivennikov SI, Piao Y, Nedospasov SA, Schlessinger D. Ectodysplasin regulates the lymphotoxin-beta pathway for hair differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(24):9142–9147. doi: 10.1073/pnas.0509678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles.[see comment] Nature Cell Biology. 2008;10(11):1257–1268. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson BM, Brockdorff N, Formstone E, Ngyuen T, Kronmiller JE, Zonana J. Cloning of Tabby, the murine homolog of the human EDA gene: evidence for a membrane-associated protein with a short collagenous domain. Human Molecular Genetics. 1997;6(9):1589–1594. doi: 10.1093/hmg/6.9.1589. [DOI] [PubMed] [Google Scholar]
- Fliniaux I, Mikkola ML, Lefebvre S, Thesleff I. Identification of dkk4 as a target of Eda-A1/Edar pathway reveals an unexpected role of ectodysplasin as inhibitor of Wnt signalling in ectodermal placodes. Developmental Biology. 2008;320(1):60–71. doi: 10.1016/j.ydbio.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Gaide O, Schneider P. Permanent correction of an inherited ectodermal dysplasia with recombinant EDA. Nature Medicine. 2003;9(5):614–618. doi: 10.1038/nm861. [DOI] [PubMed] [Google Scholar]
- Gambardella L, Schneider-Maunoury S, Voiculescu O, Charnay P, Barrandon Y. Pattern of expression of the transcription factor Krox-20 in mouse hair follicle. Mechanisms of Development. 2000;96(2):215–218. doi: 10.1016/s0925-4773(00)00398-1. [DOI] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95(5):605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Gebhardt A, Kosan C, Herkert B, Moroy T, Lutz W, Eilers M, Elsasser HP. Miz1 is required for hair follicle structure and hair morphogenesis. Journal of Cell Science. 2007;120(Pt 15):2586–2593. doi: 10.1242/jcs.007104. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt B, Schlake T. Localization of Shh expression by Wnt and Eda affects axial polarity and shape of hairs. Developmental Biology. 2007;305(1):246–261. doi: 10.1016/j.ydbio.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Headon DJ, Emmal SA, Ferguson BM, Tucker AS, Justice MJ, Sharpe PT, Zonana J, Overbeek PA. Gene defect in ectodermal dysplasia implicates a death domain adapter in development. Nature. 2001;414(6866):913–916. doi: 10.1038/414913a. [DOI] [PubMed] [Google Scholar]
- Headon DJ, Overbeek PA. Involvement of a novel Tnf receptor homologue in hair follicle induction.[see comment] Nature Genetics. 1999;22(4):370–374. doi: 10.1038/11943. [DOI] [PubMed] [Google Scholar]
- Hebert JM, Rosenquist T, Gotz J, Martin GR. FGF5 as a regulator of the hair growth cycle: evidence from targeted and spontaneous mutations. Cell. 1994;78(6):1017–1025. doi: 10.1016/0092-8674(94)90276-3. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105(4):533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development.[see comment][erratum appears in Nature. 2003 Aug 21;424(6951):974] Nature. 2003;422(6929):317–322. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kere J, Srivastava AK, Montonen O, Zonana J, Thomas N, Ferguson B, Munoz F, Morgan D, Clarke A, Baybayan P, Chen EY, Ezer S, Saarialho-Kere U, de la Chapelle A, Schlessinger D. X-linked anhidrotic (hypohidrotic) ectodermal dysplasia is caused by mutation in a novel transmembrane protein.[see comment] Nature Genetics. 1996;13(4):409–416. doi: 10.1038/ng0895-409. [DOI] [PubMed] [Google Scholar]
- Koppinen P, Pispa J, Laurikkala J, Thesleff I, Mikkola ML. Signaling and subcellular localization of the TNF receptor Edar. Experimental Cell Research. 2001;269(2):180–192. doi: 10.1006/excr.2001.5331. [DOI] [PubMed] [Google Scholar]
- Laurikkala J, Pispa J, Jung HS, Nieminen P, Mikkola M, Wang X, Saarialho-Kere U, Galceran J, Grosschedl R, Thesleff I. Regulation of hair follicle development by the TNF signal ectodysplasin and its receptor Edar. Development. 2002;129(10):2541–2553. doi: 10.1242/dev.129.10.2541. [DOI] [PubMed] [Google Scholar]
- Loomis CA, Harris E, Michaud J, Wurst W, Hanks M, Joyner AL. The mouse Engrailed-1 gene and ventral limb patterning. Nature. 1996;382(6589):360–363. doi: 10.1038/382360a0. [DOI] [PubMed] [Google Scholar]
- Mikkola ML. TNF superfamily in skin appendage development. Cytokine & Growth Factor Reviews. 2008;19(3–4):219–230. doi: 10.1016/j.cytogfr.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Mikkola ML, Thesleff I. Ectodysplasin signaling in development. Cytokine & Growth Factor Reviews. 2003;14(3–4):211–224. doi: 10.1016/s1359-6101(03)00020-0. [DOI] [PubMed] [Google Scholar]
- Millar SE. Molecular mechanisms regulating hair follicle development. Journal of Investigative Dermatology. 2002;118(2):216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- Millar SE, Willert K, Salinas PC, Roelink H, Nusse R, Sussman DJ, Barsh GS. WNT signaling in the control of hair growth and structure. Developmental Biology. 1999;207(1):133–149. doi: 10.1006/dbio.1998.9140. [DOI] [PubMed] [Google Scholar]
- Mohri Y, Kato S, Umezawa A, Okuyama R, Nishimori K. Impaired hair placode formation with reduced expression of hair follicle-related genes in mice lacking Lgr4. Developmental Dynamics. 2008;237(8):2235–2242. doi: 10.1002/dvdy.21639. [DOI] [PubMed] [Google Scholar]
- Monreal AW, Ferguson BM, Headon DJ, Street SL, Overbeek PA, Zonana J. Mutations in the human homologue of mouse dl cause autosomal recessive and dominant hypohidrotic ectodermal dysplasia.[see comment] Nature Genetics. 1999;22(4):366–369. doi: 10.1038/11937. [DOI] [PubMed] [Google Scholar]
- Mustonen T, Pispa J, Mikkola ML, Pummila M, Kangas AT, Pakkasjarvi L, Jaatinen R, Thesleff I. Stimulation of ectodermal organ development by Ectodysplasin-A1. Developmental Biology. 2003;259(1):123–136. doi: 10.1016/s0012-1606(03)00157-x. [DOI] [PubMed] [Google Scholar]
- Newton K, French DM, Yan M, Frantz GD, Dixit VM. Myodegeneration in EDA-A2 transgenic mice is prevented by XEDAR deficiency. Molecular & Cellular Biology. 2004;24(4):1608–1613. doi: 10.1128/MCB.24.4.1608-1613.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning Y, Schuller AG, Bradshaw S, Rotwein P, Ludwig T, Frystyk J, Pintar JE. Diminished growth and enhanced glucose metabolism in triple knockout mice containing mutations of insulin-like growth factor binding protein-3, -4, and -5. Molecular Endocrinology. 2006;20(9):2173–2186. doi: 10.1210/me.2005-0196. [DOI] [PubMed] [Google Scholar]
- Nishioka E, Tanaka T, Yoshida H, Matsumura K, Nishikawa S, Naito A, Inoue J, Funasaka Y, Ichihashi M, Miyasaka M, Nishikawa S. Mucosal addressin cell adhesion molecule 1 plays an unexpected role in the development of mouse guard hair. Journal of Investigative Dermatology. 2002;119(3):632–638. doi: 10.1046/j.1523-1747.2002.01851.x. [DOI] [PubMed] [Google Scholar]
- Pennisi D, Bowles J, Nagy A, Muscat G, Koopman P. Mice null for sox18 are viable and display a mild coat defect. Molecular & Cellular Biology. 2000a;20(24):9331–9336. doi: 10.1128/mcb.20.24.9331-9336.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi D, Gardner J, Chambers D, Hosking B, Peters J, Muscat G, Abbott C, Koopman P. Mutations in Sox18 underlie cardiovascular and hair follicle defects in ragged mice. Nature Genetics. 2000b;24(4):434–437. doi: 10.1038/74301. [DOI] [PubMed] [Google Scholar]
- Pispa J, Pummila M, Barker PA, Thesleff I, Mikkola ML. Edar and Troy signalling pathways act redundantly to regulate initiation of hair follicle development. Human Molecular Genetics. 2008;17(21):3380–3391. doi: 10.1093/hmg/ddn232. [DOI] [PubMed] [Google Scholar]
- Pummila M, Fliniaux I, Jaatinen R, James MJ, Laurikkala J, Schneider P, Thesleff I, Mikkola ML. Ectodysplasin has a dual role in ectodermal organogenesis: inhibition of Bmp activity and induction of Shh expression. Development. 2007;134(1):117–125. doi: 10.1242/dev.02708. [DOI] [PubMed] [Google Scholar]
- Raveh E, Cohen S, Levanon D, Groner Y, Gat U. Runx3 is involved in hair shape determination. Developmental Dynamics. 2005;233(4):1478–1487. doi: 10.1002/dvdy.20453. [DOI] [PubMed] [Google Scholar]
- Raveh E, Cohen S, Levanon D, Negreanu V, Groner Y, Gat U. Dynamic expression of Runx1 in skin affects hair structure. Mechanisms of Development. 2006;123(11):842–850. doi: 10.1016/j.mod.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Esteban C, Tsukui T, Yonei S, Magallon J, Tamura K, Izpisua Belmonte JC. The T-box genes Tbx4 and Tbx5 regulate limb outgrowth and identity.[see comment] Nature. 1999;398(6730):814–818. doi: 10.1038/19769. [DOI] [PubMed] [Google Scholar]
- Schlake T. Segmental Igfbp5 expression is specifically associated with the bent structure of zigzag hairs. Mechanisms of Development. 2005;122(9):988–997. doi: 10.1016/j.mod.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Schlake T. Krox20, a novel candidate for the regulatory hierarchy that controls hair shaft bending. Mechanisms of Development. 2006;123(8):641–648. doi: 10.1016/j.mod.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Aebischer T, Hulsken J, Birchmeier W, Klemm U, Scheidereit C. Requirement of NF-kappaB/Rel for the development of hair follicles and other epidermal appendices. Development. 2001;128(19):3843–3853. doi: 10.1242/dev.128.19.3843. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Tobin DJ, Lenhard D, Schneider P, Paus R, Scheidereit C. NF-kappaB transmits Eda A1/EdaR signalling to activate Shh and cyclin D1 expression, and controls post-initiation hair placode down growth. Development. 2006;133(6):1045–1057. doi: 10.1242/dev.02278. [DOI] [PubMed] [Google Scholar]
- Schweizer J, Marks F. A developmental study of the distribution and frequency of Langerhans cells in relation to formation of patterning in mouse tail epidermis. Journal of Investigative Dermatology. 1977;69(2):198–204. doi: 10.1111/1523-1747.ep12506298. [DOI] [PubMed] [Google Scholar]
- Sick S, Reinker S, Timmer J, Schlake T. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism.[see comment] Science. 2006;314(5804):1447–1450. doi: 10.1126/science.1130088. [DOI] [PubMed] [Google Scholar]
- Sofaer JA. Hair follicle initiation in reciprocal recombinations of downless homozygote and heterozygote mouse tail epidermis and dermis. Developmental Biology. 1973;34(2):289–296. doi: 10.1016/0012-1606(73)90358-8. [DOI] [PubMed] [Google Scholar]
- Srivastava AK, Durmowicz MC, Hartung AJ, Hudson J, Ouzts LV, Donovan DM, Cui CY, Schlessinger D. Ectodysplasin-A1 is sufficient to rescue both hair growth and sweat glands in Tabby mice. Human Molecular Genetics. 2001;10(26):2973–2981. doi: 10.1093/hmg/10.26.2973. [DOI] [PubMed] [Google Scholar]
- Srivastava AK, Pispa J, Hartung AJ, Du Y, Ezer S, Jenks T, Shimada T, Pekkanen M, Mikkola ML, Ko MS, Thesleff I, Kere J, Schlessinger D. The Tabby phenotype is caused by mutation in a mouse homologue of the EDA gene that reveals novel mouse and human exons and encodes a protein (ectodysplasin-A) with collagenous domains. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(24):13069–13074. doi: 10.1073/pnas.94.24.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R, Amla DV. Molecular characteristics of glnA linked mutations in the nitrogen-fixing cyanobacterium Nostoc muscorum. Current Microbiology. 2002;44(2):94–101. doi: 10.1007/s00284-001-0057-x. [DOI] [PubMed] [Google Scholar]
- Sundberg . Hair types and subtypes in the laboratory mouse. In: JPS, editor. Handbook of Mouse Mutations with Skin and Hair Abnormalities: Animal Models and Biochemical Tools. Boca Raton, FL: CRC Press; 1994. [Google Scholar]
- Tadin-Strapps M, Salas-Alanis JC, Moreno L, Warburton D, Martinez-Mir A, Christiano AM. Congenital universal hypertrichosis with deafness and dental anomalies inherited as an X-linked trait. Clinical Genetics. 2003;63(5):418–422. doi: 10.1034/j.1399-0004.2003.00069.x. [DOI] [PubMed] [Google Scholar]
- Turing A. The chemical basis of morphogenesis. Phil Trans Royal Soc Lond B. 1952;237:37–72. doi: 10.1098/rstb.2014.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Exan RJ, Hardy MH. A spatial relationship between innervation and the early differentiation of vibrissa follicles in the embryonic mouse. Journal of Anatomy. 1980;131(Pt 4):643–656. [PMC free article] [PubMed] [Google Scholar]
- van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8(22):2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- Vrieling H, Duhl DM, Millar SE, Miller KA, Barsh GS. Differences in dorsal and ventral pigmentation result from regional expression of the mouse agouti gene. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(12):5667–5671. doi: 10.1073/pnas.91.12.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Badea T, Nathans J. Order from disorder: Self-organization in mammalian hair patterning. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(52):19800–19805. doi: 10.1073/pnas.0609712104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weger N, Schlake T. Igf-I signalling controls the hair growth cycle and the differentiation of hair shafts.[see comment] Journal of Investigative Dermatology. 2005;125(5):873–882. doi: 10.1111/j.0022-202X.2005.23946.x. [DOI] [PubMed] [Google Scholar]
- Yamakado M, Yohro T. Subdivision of mouse vibrissae on an embryological basis, with descriptions of variations in the number and arrangement of sinus hairs and cortical barrels in BALB/c (nu/+; nude, nu/nu) and hairless (hr/hr) strains. American Journal of Anatomy. 1979;155(2):153–173. doi: 10.1002/aja.1001550202. [DOI] [PubMed] [Google Scholar]
- Yan M, Zhang Z, Brady JR, Schilbach S, Fairbrother WJ, Dixit VM. Identification of a novel death domain-containing adaptor molecule for ectodysplasin-A receptor that is mutated in crinkled mice. Curr Biol. 2002;12(5):409–413. doi: 10.1016/s0960-9822(02)00687-5. [DOI] [PubMed] [Google Scholar]
- Zhang M, Brancaccio A, Weiner L, Missero C, Brissette JL. Ectodysplasin regulates pattern formation in the mammalian hair coat. Genesis: the Journal of Genetics & Development. 2003;37(1):30–37. doi: 10.1002/gene.10230. [DOI] [PubMed] [Google Scholar]