Abstract
The present study reports elevated levels of endotoxin/lipopolysaccharide (LPS) concentrations in plasma from patients with sporadic amyotrophic lateral sclerosis (sALS) and Alzheimer’s (AD) as compared to healthy controls. Levels of plasma LPS showed a significant positive correlation with degree of blood monocyte/macrophage activation in disease groups and was most elevated in patients with advanced sALS disease. There was a significant negative relationship between plasma LPS and levels of monocyte/macrophage IL-10 expression in sALS blood. These data suggest that systemic LPS levels and activated monocyte/macrophages may play significant roles in the pathogenesis of sALS.
Keywords: Amyotrophic Lateral Sclerosis (ALS), lipopolysaccharide (LPS), monocyte/macrophage activation
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neuromuscular disorder characterized by the progressive loss of anterior-lateral horn spinal cord motor neurons leading to weakness and the eventual death of affected individuals. Although inflammatory mechanisms and immune activation have been considered as common components of the pathogenesis of ALS (Alexianu et al., 2001; Graves et al., 2004; Henkel et al., 2004; McGeer and McGeer, 2002; Simpson et al., 2004), the relevance of these processes to pathogenesis is unknown.
Endotoxin/lipopolysaccharide (LPS), is a potent inflammatory stimulus and immunostimulatory product (Takeda et al., 2003) and induces its effects through stimulation of CD14-bearing inflammatory cells (Flo et al., 2000; Tobias et al., 1999). LPS associated toxicity is mediated through systemic monocyte/macrophage and endothelial cell activation, and release of inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) (Beutler et al., 1985; Danner et al., 1991; Okusawa et al., 1988; Tracey et al., 1986). The effects of LPS are mediated primarily through Toll-Like Receptor 4 (TLR4).
Increased levels of proinflammatory cytokines, such as MCP-1 (Baron et al., 2005; Henkel et al., 2004; Simpson et al., 2004; Wilms et al., 2003; Zhang et al., 2006), and IL-6 (Ono et al., 2001; Sekizawa et al., 1998) have been reported in cerebral spinal fluid (CSF) and sera in patients with ALS. More recently, elevated levels of TNF-α have been observed in the blood of ALS patients (Babu et al., 2008; Cereda et al., 2008; Poloni et al., 2000). Our previous studies on blood specimens from patients with sporadic ALS (sALS) found elevated levels of abnormally activated monocyte/macrophages as compared to controls (Zhang et al., 2005). These data taken together suggested a potential role for LPS as a systemic monocyte activator in the pathogenesis of ALS. Therefore, the objectives of this study were: 1) to quantify the levels of plasma LPS in patients with sALS as compared to control groups, 2) to test whether levels of plasma LPS would correlate with monocyte activation and/or monocyte IL-10 (a regulator of activation) expression in blood, and 3) to determine if LPS levels in plasma correlated with clinical stage of disease in sALS.
2. Materials and Methods
2.1. Subjects
Twenty-three patients diagnosed with sALS (7 females and 16 males, mean age 59.2 ± 8.7 years) by El Escorial criteria (Brooks, 1994) at the Forbes Norris MDA/ALS Research Center (San Francisco, California, USA) had blood drawn in accordance with the CPMC (California Pacific Medical Center) and UCSF committees on human research guidelines, coordinated by the UCSF AIDS and Cancer Specimen Resource (ACSR) program and excluded patients with any evidence for intercurrent infection. The Revised ALS Functional Rating Scale (ALSFRS-R), scored 0–48, was used to evaluate overall patient functional status (Cedarbaum et al., 1999). All scores were updated within a month of blood testing. Demographic and medication information for sALS patients whose specimens were studied are shown in Table 1.
Table 1.
Clinical summary of sALS patients
| Patient ID | Patient age (years) |
Patient gender | Duration of illness (months) |
ALSFRS-R Score |
Therapy | |
|---|---|---|---|---|---|---|
| Riluzole a | NSAIDs b | |||||
| Patient 1 | 52 | M | 46 | 34 | yes | no |
| Patient 2 | 51 | F | 19 | 39 | yes | no |
| Patient 3 | 63 | M | 24 | 19 | yes | no |
| Patient 4 | 66 | M | 17 | 39 | yes | yes |
| Patient 5 | 48 | F | 8 | 33 | yes | no |
| Patient 6 | 72 | M | 17 | 37 | yes | no |
| Patient 7 | 45 | M | 16 | 33 | yes | no |
| Patient 8 | 52 | F | 40 | 29 | yes | yes |
| Patient 9 | 54 | M | 9 | 45 | yes | yes |
| Patient 10 | 52 | F | 24 | 29 | yes | no |
| Patient 11 | 70 | M | 17 | 36 | yes | yes |
| Patient 12 | 52 | M | 8 | 38 | yes | yes |
| Patient 13 | 59 | M | 11 | 42 | yes | no |
| Patient 14 | 70 | M | 12 | 35 | yes | yes |
| Patient 15 | 53 | M | 18 | 35 | yes | no |
| Patient 16 | 73 | F | 21 | 34 | yes | no |
| Patient 17 | 62 | M | 26 | 33 | yes | yes |
| Patient 18 | 52 | F | 20 | 42 | yes | no |
| Patient 19 | 74 | M | 29 | 31 | no | yes |
| Patient 20 | 63 | M | 6 | 37 | no | no |
| Patient 21 | 66 | M | 21 | 34 | yes | yes |
| Patient 22 | 54 | M | 22 | 37 | yes | no |
| Patient 23 | 58 | F | 7 | 37 | yes | yes |
Fifty milligrams twice daily.
NSAIDs, non-steroidal anti-inflammatory drugs (Aspirin, Ibuprofen, Motrin), standard dose.
Two control groups were used in the study. The first control group consisted of 18 age-matched healthy donors (6 females and 12 males, mean age 54.5 ± 8.4 years, Stanford University Blood Center). The second control group obtained from the memory clinic at Forbes Norris Center, had Alzheimer’s disease (AD). This group (11 females and 7 males, mean age 78.9 ± 8.3 years) was used as neurological disease controls and had no active infection at the time of blood donation into the study. All control blood specimens were processed in a similar manner to the sALS patient blood specimens.
2.2. Flow Cytometry
Blood immunophenotyping studies were performed as previously described (Zhang et al., 2005). Monocyte activation was evaluated by quantitating levels of HLA-DR on CD14 cells. IL-10 expression in CD14 cells was used as another marker for its function as a regulator of monocyte/macrophage activation.
2.3. Endotoxin Detection
Plasma from sALS, AD and healthy controls blood was obtained by Percoll gradient centrifugation, and was frozen at −70°C until assayed. Duplicate plasma LPS levels in all plasma specimens were quantified by the LAL (Limulus Amebocyte Lysate) Chromogenic Endpoint Assay (Cell Sciences Inc., Canton, MA, USA) according to the manufacture’s instructions.
2.4. Statistical analysis
Cut-off values for defining cell activation as “positive” and “negative” for sALS patients and disease controls were determined by comparison with values from healthy donors. Results are expressed as the mean ± SD. Statistical analysis of group distribution and differences, linear regression, and Pearson correlations were performed by GraphPad Prism 4.0 program (GraphPad Software, San Diego, California, USA). Between-group comparisons were made using One-Way ANOVA with Tukey’s multiple comparison tests. For all analyses, a value of p<0.05 was considered statistically significant.
3. Results
3.1 Plasma endotoxin LPS levels
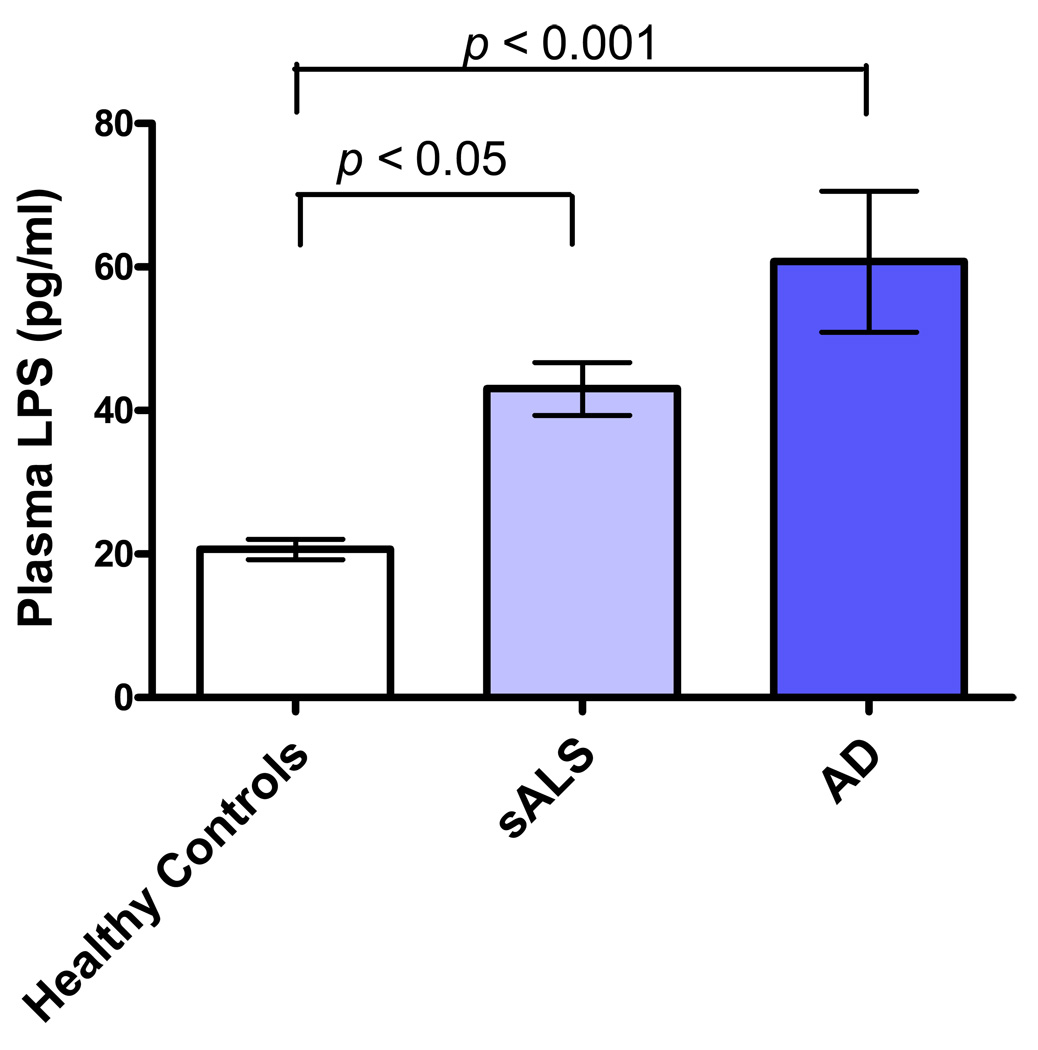
Compared to the healthy population, significantly higher levels of LPS were observed in sALS (p<0.05) and AD (p<0.001) plasma specimens (Figure 1). Plasma levels of LPS were similar between sALS and AD disease groups.
Figure 1.
Plasma levels of LPS in healthy controls (21 ± 6 pg/m, n = 18), AD (61 ± 42 pg/ml, n = 18), and sALS (43 ± 18 pg/ml, n = 23).
3.2 Monocyte activation and plasma LPS levels
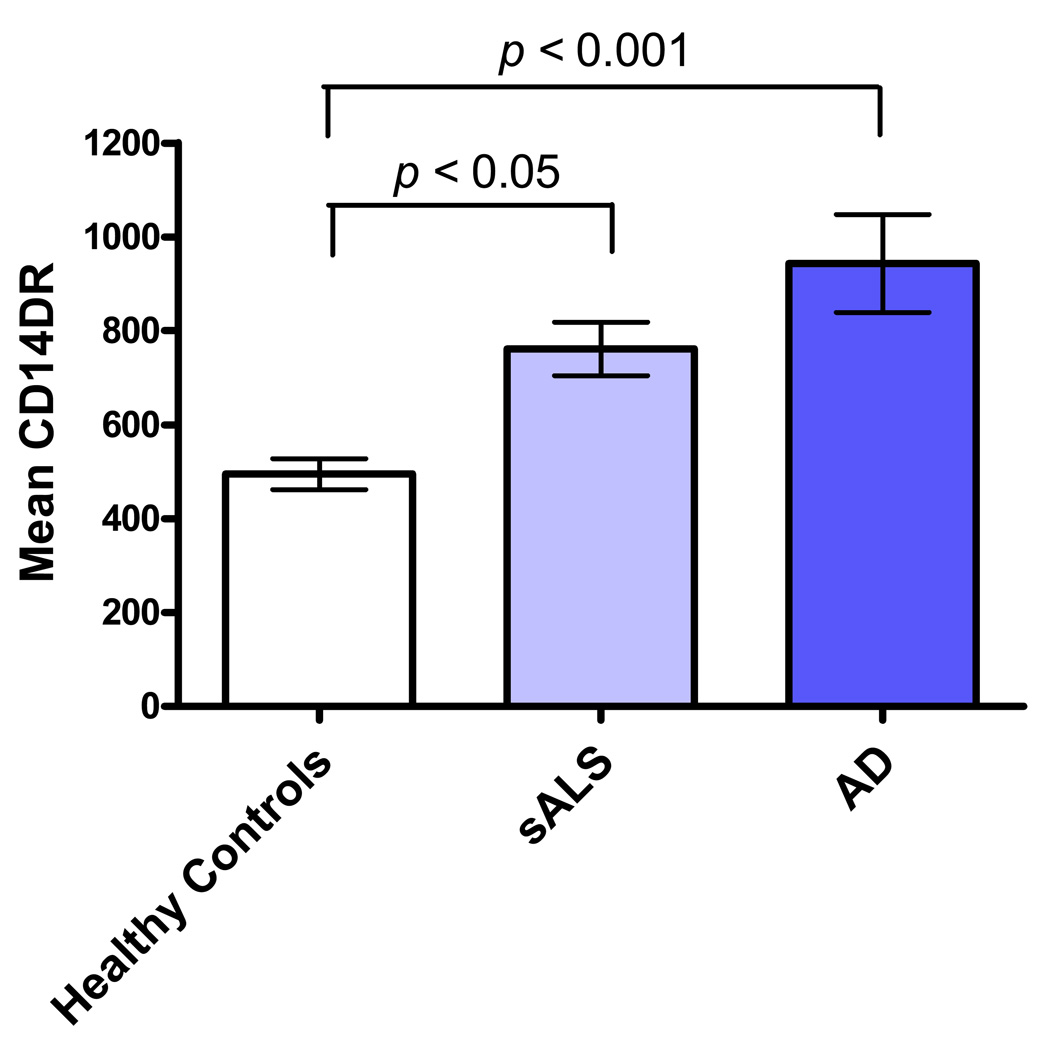
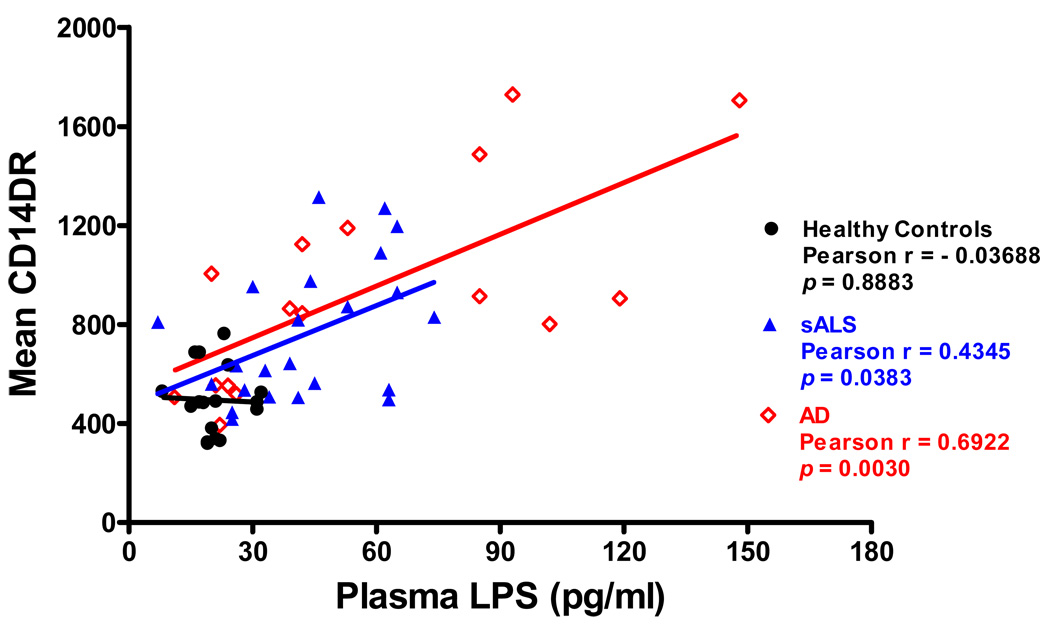
The levels of monocyte activation marker HLA-DR detected on sALS (p<0.05) and AD (p<0.001) was significantly higher than that detected on normal blood monocytes (Figure 2) and was directly related to level of plasma LPS. Figure 3 shows that monocyte HLA-DR varied in a direct relationship to plasma LPS levels in both sALS (r = 0.4345, p = 0.0383) and AD (r = 0.6922, p = 0.0030) groups. No correlation was found between degree of monocyte activation and plasma LPS levels in healthy group.
Figure 2.
Degree of monocyte HLA-DR expression in healthy controls (Mean CD14DR = 495 ± 136, n = 17), AD (Mean CD14DR = 943 ± 417, n = 17), and sALS (Mean CD14DR = 761 ± 274, n = 23).
Figure 3.
Correlations between monocyte activation and plasma LPS levels in healthy controls, AD, and sALS: Significantly positive correlation of the degree of monocyte HLA-DR expression with levels of plasma LPS in both sALS and AD.
3.3 Loss of monocyte IL-10 expression and plasma LPS levels in sALS
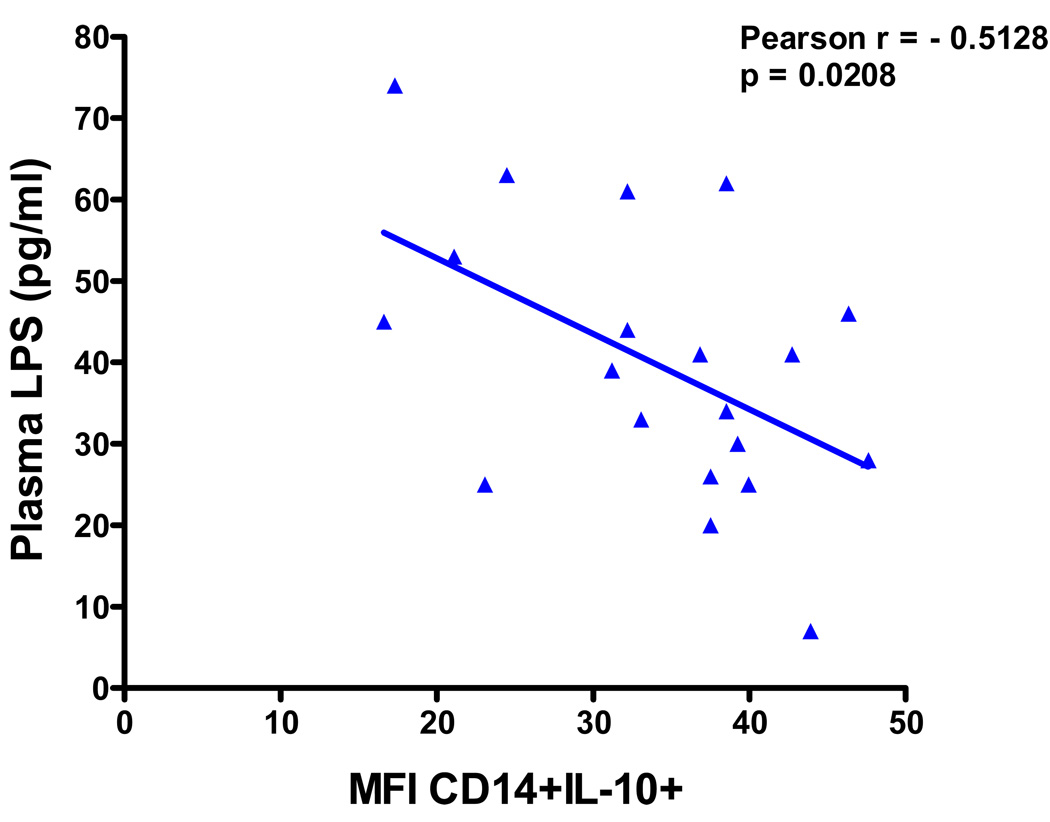
The levels of monocyte IL-10 expression (Median Fluorescence Intensity) were significantly higher in AD (49 ± 26, n = 17) compared to sALS (34 ± 9, n = 20, p<0.05) and healthy controls (33 ± 11, n = 17, p<0.05). No significant differences in monocyte IL-10 expression were observed between sALS and healthy controls. However, there was a significant inverse correlation between plasma LPS levels and degree of CD14 cell IL-10 expression in sALS blood (r = −0.5128, p = 0.0208), as showed in Figure 4, whereas no correlation was found between monocyte IL-10 expression and plasma LPS levels in AD and healthy controls (data not shown).
Figure 4.
Relationship of monocyte IL-10 expression to plasma LPS levels in sALS: Negative correlation of degree of IL-10 expression on CD14 cells with plasma LPS levels in sALS.
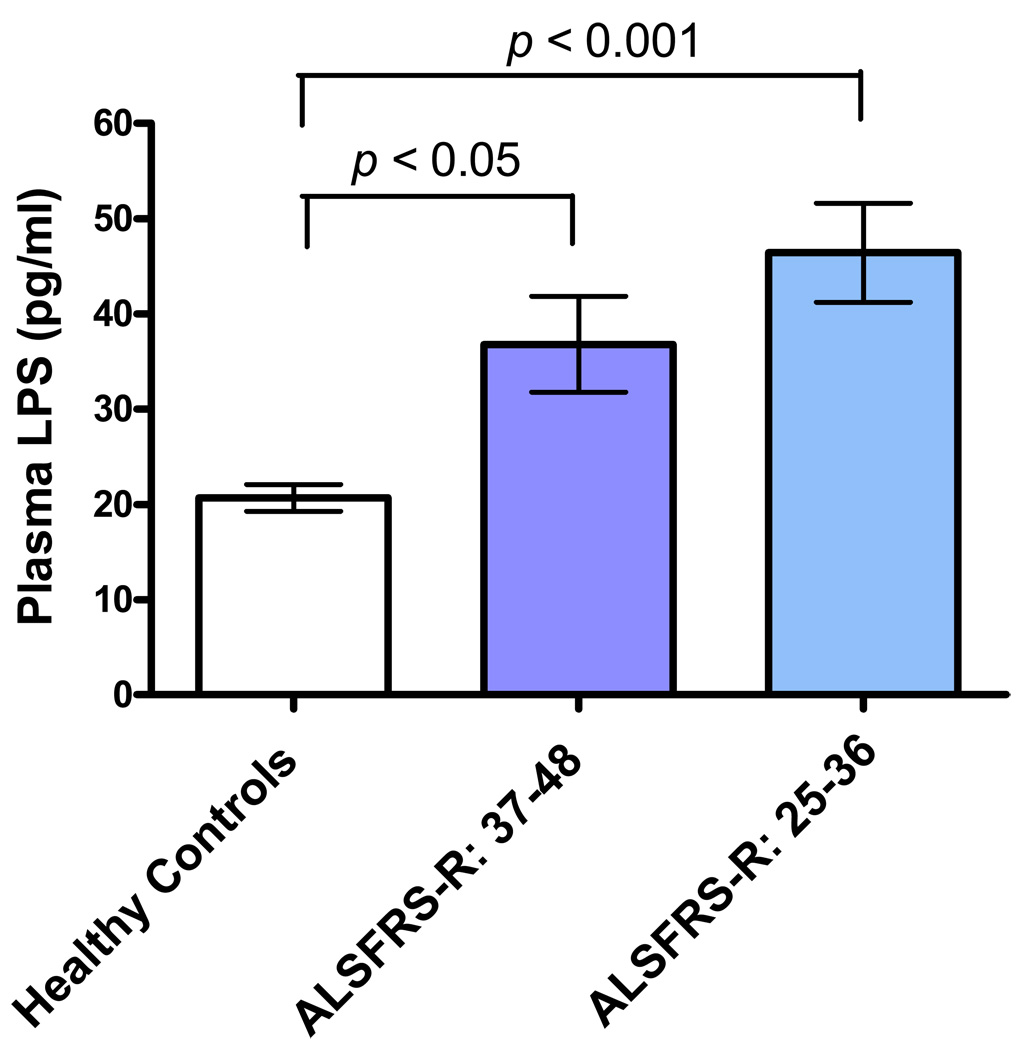
3.4 Plasma LPS levels and clinical disease status in sALS
To evaluate whether plasma LPS levels would be related to severity of disease, the plasma LPS results of patients with sALS from Figure 1 were compared with the clinical ALS values shown in Table 1. This analysis was performed by dividing sALS patients into two groups based on ALSFRS-R quartile scores. Those with moderate impairment (an ALSFRS-R score of 25–36, n = 12) were compared to those with early impairment (an ALSFRS-R score of 37–48, n = 10). As shown in Figure 5, increased levels of plasma LPS were highly significant in patients with moderate impairment as compared with healthy controls (p<0.001).
Figure 5.
Relationship of ALSFRS-R scores to plasma LPS levels in sALS patients. Increased LPS levels was highly significant in patients with moderate impairment (n = 12, p<0.001) compared to healthy controls (n = 18).
4. Discussion
In the current study, we show for the first time that plasma levels of LPS are elevated in patients with sALS and AD. Plasma LPS levels may also be related to clinical disease status in sALS. Early stage sALS patients had lower levels of plasma LPS than did patients with more advanced disease. Elevated levels of abnormally activated monocyte/macrophages defined by CD14 co-expression of HLA-DR were found in patients with sALS and AD both in the current and in our previous study (Zhang et al., 2005). Monocyte activation levels in the current study varied directly with plasma LPS levels. Moreover, the levels of plasma LPS were inversely correlated with monocyte IL-10 expression in sALS patients.
Recent studies on immune activation and disease have found that monocyte/macrophage activation in chronic HIV infection/AIDS (Brenchley et al., 2006) is associated with LPS levels from gut associated microbial translocation. Sources of plasma LPS include, but are not limited to, commensal and pathogenic bacteria, as well as subclinical opportunistic infections. None of our patients had any active infection at the time of the study. It seems that plasma LPS in sALS and AD might also be from gut associated microbial translocation like that observed in the chronic HIV infection (Brenchley et al., 2006). As a systemic macrophage activator, LPS administration leads to acute neuronal cell death(Cunningham et al., 2005) and chronic neuroninflammation and progressive neurondegeneration (Qin et al., 2007). More specific to ALS, the injection of LPS into SOD1(G37R) ALS mice (Nguyen et al., 2004) caused a dramatic shortening of their lifespan suggesting that LPS-mediated macrophage activation may exacerbate the pathogenesis of ALS in vivo. The current study suggests that circulating LPS, without specifying the source of LPS, may contribute to disease development in sALS.
Whereas LPS induces classical monocyte activation and production of inflammatory mediators, IL-10 is a cytokine with anti-inflammatory properties that may downregulate monocyte activation (de Waal Malefyt et al., 1991; Ramani et al., 1993). The expression of this endogenous IL-10 conferred significant protection from the harmful effects of LPS challenge and reduced expression of pro-inflammatory cytokines such as TNF-α, (Gerard et al., 1993; Marchant et al., 1994) and regulated leukocyte-endothelial cell interactions, and microvascular permeability (Hickey et al., 1998). In other experimental systems, IL-10 exhibited protective effects in models of local inflammation including brain or spinal cord injury (Bethea et al., 1999).
High LPS levels observed in plasma from patients with advanced sALS were associated with lower circulating monocyte IL-10 expression. It is unlikely that this decreased level of IL-10 was directly related to systemic exposure of monocytes to elevated levels of circulating LPS as AD patients also had high levels of plasma LPS, but no correlation was found with monocyte IL-10 expression. The loss of systemic monocyte IL-10 expression associated with high plasma LPS levels in sALS may be related to disease pathogenesis. It is possible that LPS associated IL-10 reductions may reduce the endogenous anti-inflammatory capability allowing neuroinflammatory disease progression. Accordingly, the balance between pro- and anti-inflammatory TLR4 signaling pathways triggered in response to bacterial products may influence the development of ALS disease. Systemic LPS levels and LPS activated monocyte/macrophage represent two new co-factors that may play significant roles in the pathogenesis of ALS and as such represent novel targets for therapeutic intervention in patients with ALS.
Acknowledgements
This work was funded in part by the National Institutes of Health’s (NIH) grant number U01-CA066529 (MSM), National Cancer Institute’s West Coast AIDS and Cancer Specimen Resource (ACSR) Consortium, University of California, San Francisco (UCSF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexianu ME, Kozovska M, Appel SH. Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology. 2001;57:1282–1289. doi: 10.1212/wnl.57.7.1282. [DOI] [PubMed] [Google Scholar]
- Babu GN, Kumar A, Chandra R, Puri SK, Kalita J, Misra UK. Elevated Inflammatory Markers in a Group of Amyotrophic Lateral Sclerosis Patients from Northern India. Neurochem Res. 2008 doi: 10.1007/s11064-007-9564-x. [DOI] [PubMed] [Google Scholar]
- Baron P, Bussini S, Cardin V, Corbo M, Conti G, Galimberti D, Scarpini E, Bresolin N, Wharton SB, Shaw PJ, Silani V. Production of monocyte chemoattractant protein-1 in amyotrophic lateral sclerosis. Muscle Nerve. 2005;32:541–544. doi: 10.1002/mus.20376. [DOI] [PubMed] [Google Scholar]
- Bethea JR, Nagashima H, Acosta MC, Briceno C, Gomez F, Marcillo AE, Loor K, Green J, Dietrich WD. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 1999;16:851–863. doi: 10.1089/neu.1999.16.851. [DOI] [PubMed] [Google Scholar]
- Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial "Clinical limits of amyotrophic lateral sclerosis" workshop contributors. J Neurol Sci. 1994;(124 Suppl):96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) J Neurol Sci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- Cereda C, Baiocchi C, Bongioanni P, Cova E, Guareschi S, Metelli MR, Rossi B, Sbalsi I, Cuccia MC, Ceroni M. TNF and sTNFR1/2 plasma levels in ALS patients. J Neuroimmunol. 2008;194:123–131. doi: 10.1016/j.jneuroim.2007.10.028. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner RL, Elin RJ, Hosseini JM, Wesley RA, Reilly JM, Parillo JE. Endotoxemia in human septic shock. Chest. 1991;99:169–175. doi: 10.1378/chest.99.1.169. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flo TH, Halaas O, Lien E, Ryan L, Teti G, Golenbock DT, Sundan A, Espevik T. Human toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J Immunol. 2000;164:2064–2069. doi: 10.4049/jimmunol.164.4.2064. [DOI] [PubMed] [Google Scholar]
- Gerard C, Bruyns C, Marchant A, Abramowicz D, Vandenabeele P, Delvaux A, Fiers W, Goldman M, Velu T. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993;177:547–550. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves MC, Fiala M, Dinglasan LA, Liu NQ, Sayre J, Chiappelli F, van Kooten C, Vinters HV. Inflammation in amyotrophic lateral sclerosis spinal cord and brain is mediated by activated macrophages, mast cells and T cells. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5:213–219. doi: 10.1080/14660820410020286. [DOI] [PubMed] [Google Scholar]
- Henkel JS, Engelhardt JI, Siklos L, Simpson EP, Kim SH, Pan T, Goodman JC, Siddique T, Beers DR, Appel SH. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann Neurol. 2004;55:221–235. doi: 10.1002/ana.10805. [DOI] [PubMed] [Google Scholar]
- Hickey MJ, Issekutz AC, Reinhardt PH, Fedorak RN, Kubes P. Endogenous interleukin-10 regulates hemodynamic parameters, leukocyte-endothelial cell interactions, and microvascular permeability during endotoxemia. Circ Res. 1998;83:1124–1131. doi: 10.1161/01.res.83.11.1124. [DOI] [PubMed] [Google Scholar]
- Marchant A, Bruyns C, Vandenabeele P, Abramowicz D, Gerard C, Delvaux A, Ghezzi P, Velu T, Goldman M. The protective role of interleukin-10 in endotoxin shock. Prog Clin Biol Res. 1994;388:417–423. [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26:459–470. doi: 10.1002/mus.10191. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, D'Aigle T, Gowing G, Julien JP, Rivest S. Exacerbation of motor neuron disease by chronic stimulation of innate immunity in a mouse model of amyotrophic lateral sclerosis. J Neurosci. 2004;24:1340–1349. doi: 10.1523/JNEUROSCI.4786-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okusawa S, Gelfand JA, Ikejima T, Connolly RJ, Dinarello CA. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 1988;81:1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S, Hu J, Shimizu N, Imai T, Nakagawa H. Increased interleukin-6 of skin and serum in amyotrophic lateral sclerosis. J Neurol Sci. 2001;187:27–34. doi: 10.1016/s0022-510x(01)00514-7. [DOI] [PubMed] [Google Scholar]
- Poloni M, Facchetti D, Mai R, Micheli A, Agnoletti L, Francolini G, Mora G, Camana C, Mazzini L, Bachetti T. Circulating levels of tumour necrosis factor-alpha and its soluble receptors are increased in the blood of patients with amyotrophic lateral sclerosis. Neurosci Lett. 2000;287:211–214. doi: 10.1016/s0304-3940(00)01177-0. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani M, Ollivier V, Khechai F, Vu T, Ternisien C, Bridey F, de Prost D. Interleukin-10 inhibits endotoxin-induced tissue factor mRNA production by human monocytes. FEBS Lett. 1993;334:114–116. doi: 10.1016/0014-5793(93)81693-t. [DOI] [PubMed] [Google Scholar]
- Sekizawa T, Openshaw H, Ohbo K, Sugamura K, Itoyama Y, Niland JC. Cerebrospinal fluid interleukin 6 in amyotrophic lateral sclerosis: immunological parameter and comparison with inflammatory and non-inflammatory central nervous system diseases. J Neurol Sci. 1998;154:194–199. doi: 10.1016/s0022-510x(97)00228-1. [DOI] [PubMed] [Google Scholar]
- Simpson EP, Henry YK, Henkel JS, Smith RG, Appel SH. Increased lipid peroxidation in sera of ALS patients: a potential biomarker of disease burden. Neurology. 2004;62:1758–1765. doi: 10.1212/wnl.62.10.1758. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Tobias PS, Tapping RI, Gegner JA. Endotoxin interactions with lipopolysaccharide-responsive cells. Clin Infect Dis. 1999;28:476–481. doi: 10.1086/515163. [DOI] [PubMed] [Google Scholar]
- Tracey KJ, Beutler B, Lowry SF, Merryweather J, Wolpe S, Milsark IW, Hariri RJ, Fahey TJ, 3rd, Zentella A, Albert JD, et al. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Wilms H, Sievers J, Dengler R, Bufler J, Deuschl G, Lucius R. Intrathecal synthesis of monocyte chemoattractant protein-1 (MCP-1) in amyotrophic lateral sclerosis: further evidence for microglial activation in neurodegeneration. J Neuroimmunol. 2003;144:139–142. doi: 10.1016/j.jneuroim.2003.08.042. [DOI] [PubMed] [Google Scholar]
- Zhang R, Gascon R, Miller RG, Gelinas DF, Mass J, Hadlock K, Jin X, Reis J, Narvaez A, McGrath MS. Evidence for systemic immune system alterations in sporadic amyotrophic lateral sclerosis (sALS) J Neuroimmunol. 2005;159:215–224. doi: 10.1016/j.jneuroim.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Zhang R, Gascon R, Miller RG, Gelinas DF, Mass J, Lancero M, Narvaez A, McGrath MS. MCP-1 chemokine receptor CCR2 is decreased on circulating monocytes in sporadic amyotrophic lateral sclerosis (sALS) J Neuroimmunol. 2006;179:87–93. doi: 10.1016/j.jneuroim.2006.06.008. [DOI] [PubMed] [Google Scholar]