Abstract
Background
Excessive alcohol drinking continues to be an important health problem. Recent studies from our laboratory and others have demonstrated that animal models of ethanol dependence and relapse can contribute to understanding factors that contribute to excessive drinking. In the current study, we tested the hypothesis that the amount and duration of ethanol exposure is critical for promoting the escalation in drinking by mice given access to ethanol in a limited access paradigm.
Methods
We used several methods of chronic intermittent ethanol exposure in male C57BL/6J mice that would vary in the amount and duration of exposure to ethanol as indicated by blood ethanol concentrations (BEC). After establishing baseline drinking in the mice using a 2 hour, 2 bottle choice drinking paradigm, each study involved alternating between periods of ethanol exposure and periods of limited access to ethanol (1 cycle) for a total of 3 cycles. In study 1, mice were allowed extended access (16 hrs) to ethanol for oral consumption or remained in the home cage. In study 2, the ethanol exposure consisted of intragastric gavage of increasing doses of ethanol or isocaloric sucrose as the control. Study 3 compared intragastric gavage combined with pyrazole, an aldehyde dehydrogenase inhibitor, with vapor inhalation of ethanol using procedures known to lead to increased drinking in mice. Finally, Study 4 was a retrospective review of several studies conducted in our laboratory using inhalation procedures. The retrospective review encompassed a range of post-vapor chamber BEC values and ethanol intakes that would allow a relationship between increased drinking and BEC to be examined.
Results
Allowing mice to drink for longer periods of time did not cause increased drinking in subsequent limited access sessions. Likewise, gastric intubation of ethanol which produced high BECs (>300 mg/dl) with or without pyrazole did not increase drinking. Only the vapor inhalation procedure, which was associated with sustained BECs above 175 mg/dl for the entire exposure period resulted in increased drinking. The retrospective study provided further evidence that sustained BEC levels above 175 mg/dl was critical to the escalation in drinking.
Conclusions
We found that the intensity (amount) and duration of ethanol exposure, indexed by BEC, is critical to produce increased drinking in mice. Specifically, blood ethanol concentrations must regularly exceed 175 mg/dl for the escalation in drinking to occur. Future studies will examine neurobiological adaptations that may underlie the increased drinking behavior caused by chronic intermittent ethanol exposure.
Keywords: dependence, excessive drinking, alcohol, mouse
Introduction
Excessive alcohol drinking remains a major public health problem. Prolonged heavy drinking can lead to the development of dependence that further feeds into the compulsive and relapsing nature of the disease. Ethanol dependent individuals often attempt to stop or reduce their drinking, only to find themselves reverting to patterns of excessive use again. Ethanol dependence and relapse are thought to reflect a state of dysregulated homeostatic brain mechanisms that ultimately contribute to the perpetuation of excessive drinking even in the face of adverse consequences (Koob, 2003; Koob and Le Moal, 2001).
Animal models of ethanol dependence and relapse have played a key role in advancing knowledge about underlying neurobiological mechanisms and factors that influence escalation of drinking to harmful levels. Several rodent models have been developed that link dependence models involving inhalation exposure with self-administration procedures. For example, studies from our laboratory have shown that repeated cycles of chronic ethanol exposure delivered by inhalation results in an escalation of voluntary ethanol drinking in mice (Becker and Lopez, 2004; Lopez and Becker, 2005). Others have reported similar results using inhalation procedures in mice (Dhaher et al., 2008; Finn et al., 2007) and in rats (Rimondini et al., 2002; Rimondini et al., 2003; Sommer et al., 2008). Likewise, studies using operant procedures have demonstrated increased ethanol self-administration in mice (Chu et al., 2007; Lopez et al., 2008) and rats (O’Dell et al., 2004; Roberts et al., 1996; Roberts et al., 2000) with a history of repeated chronic ethanol exposure and withdrawal experience.
Studies also have demonstrated that escalation of ethanol self-administration is facilitated when dependence is induced by delivery of chronic ethanol vapor exposure in an intermittent rather than continuous fashion (Lopez and Becker, 2005; O’Dell et al., 2004). Additionally, we reported that increased number of cycles of chronic intermittent ethanol exposure by inhalation resulted in greater and longer lasting enhancement of voluntary ethanol drinking (Lopez and Becker, 2005). This effect apparently was specific to ethanol because repeated cycles of chronic intermittent ethanol exposure did not produce alterations in sucrose intake (Becker and Lopez, 2004). Further, more detailed analysis of the pattern of ethanol consumption revealed that dependent mice not only consumed a greater overall amount of ethanol compared to non-dependent mice, but also the rate of consumption was faster and progressively increased over successive withdrawal test periods, which led to significantly higher and more sustained blood and brain ethanol levels (Griffin et al., 2009). Collectively, these studies demonstrate escalation of ethanol self-administration following a history of dependence.
Although other methods for dependence induction have been used (Gehlert et al., 2007) most studies demonstrating enhanced ethanol drinking following repeated cycles of chronic ethanol exposure and withdrawal have employed inhalation procedures to induce dependence (Finn et al., 2007; Lopez and Becker, 2005; Rimondini et al., 2003; Sommer et al., 2008). The inhalation procedure offers many advantages, including the fact that dependence can be induced fairly rapidly with relatively minimal health-related problems, the dose and duration of exposure (both onset of intoxication and timing of withdrawal) can be precisely controlled, and the level of intoxication (blood ethanol levels) can be maintained relatively stable during the entire course of exposure as well as from one cycle of exposure to another (Becker, 2000). Nevertheless, parameters for chronic intermittent ethanol exposure that are optimal for engendering escalation of drinking have not been systematically examined. The present studies were conducted to evaluate different methods of chronic ethanol delivery as well as exposure parameters that best favor enhanced drinking in dependent mice.
Methods
Subjects
Male C57BL/6J mice (10 weeks of age) obtained from Jackson Laboratories (Bar Harbor, ME) were individually housed in polycarbonate pans with wood shavings and stainless steel wire lids, and maintained in a temperature- and humidity-controlled AAALAC accredited animal facility under a 12 hr light cycle (lights on 0200 hr). Mice had free access to food and water at all times during experimental procedures. All experimental protocols were approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina and consistent with guidelines of the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23, revised 1996). Mice were acclimated to the vivarium for a minimum of two weeks prior to the start of the experiments. Separate groups of mice were used in each experiment.
Experimental Design
The general experimental design for all studies first involved training mice to drink 15% (v/v) ethanol using a limited access (2 hr/day) 2-bottle choice paradigm (described below). After establishing stable baseline intake (~2–4 Weeks), mice received chronic intermittent ethanol exposure (or appropriate control condition) over several days delivered by different methods (described below). A 72 hr forced abstinence period was imposed immediately after the chronic intermittent ethanol treatment, and then mice were given the opportunity to drink ethanol for 5 consecutive days under limited access conditions. This pattern of weekly chronic intermittent ethanol exposure followed by a week of limited access drinking test sessions was repeated for 3 cycles in each of the studies. Details of the methods for delivering chronic intermittent ethanol exposure in each study are described below.
Study 1
This study evaluated whether extending oral access to ethanol for 16 hrs over several days would subsequently result in elevated ethanol intake during limited access (2 hr/day) test sessions. After establishing stable baseline drinking in the limited access paradigm using a sucrose-fading procedure previously described (Becker and Lopez, 2004), ethanol exposed mice (EtOHPO, n= 12) were allowed access to their ethanol bottle for 16 hr/day for 4 days before returning to the limited access paradigm the next week. This exposure regimen of chronic intermittent oral ethanol access in the 2-bottle choice paradigm followed by 5-day limited access drinking test sessions was repeated for 3 cycles. Control mice (CTL, n= 12) did not have access to ethanol during the exposure week but did receive access to 2 bottles of water when the EtOHPO mice were given ethanol. Water was freely available to both EtOHPO and CTL groups at all times during the study.
Study 2
This study evaluated the effects of chronic intermittent ethanol exposure delivered by daily intragastric gavage (IG). After establishing stable baseline drinking in the limited access paradigm (~4 Weeks), mice (EtOHIG, n= 8) were exposed to ethanol by intragastric gavage once daily for 5 days during each exposure cycle. Ethanol was administered using curved, blunt tipped feeding needles (20 gauge, Popper & Sons #7910). The ethanol dose was increased with each exposure cycle; i.e., daily gavage doses during the first, second, and third exposure cycles were 4.0, 4.5, and 5.0 g/kg ethanol (15–20% v/v), respectively. Control mice (CTLIG, n= 6) were gavaged with isocaloric sucrose solutions. Blood samples were collected 1 and 4 hours after gavage on Tuesday and Thursday of each exposure cycle for analysis of blood ethanol levels (see below). Blood samples were collected only once daily from each mouse.
Study 3
This study compared the effects of repeated IG delivery of ethanol with our previously published inhalation procedures on subsequent voluntary limited access drinking. Baseline intake was established using a sucrose-fading procedure as in Study 1. For mice given IG ethanol (EtOHIG3, n= 15), the daily dose was held constant at 3 g/kg and administered along with daily intraperitoneal injections of the alcohol dehydrogenase inhibitor pyrazole (1 mmol/kg) across the 3 exposure cycles. Control mice (CTLIG0, n= 8) were administered isocaloric sucrose and pyrazole. Separate groups of mice received either chronic intermittent exposure to ethanol vapor (CTLIH, n= 15) or air (EtOHIH, n= 8) in inhalation chambers (details of inhalation procedures are described below). Blood samples were collected at 1, 4, 8 and 16 hrs after gavage or entry into the inhalation chambers to measure ethanol concentrations during each exposure cycle (Wednesday). Blood samples were collected only once from each mouse.
Study 4
A fourth study involved analysis of a data set collected by retrospective review of studies conducted in our laboratory employing the model of dependence and relapse involving chronic intermittent ethanol exposure by inhalation. The goals of these studies were different but several aspects of methodology were pertinent to the basic research question being addressed in this paper. These methodological characteristics included: use of male subjects, use of limited access drinking as the primary dependent variable, minimum of 3 exposure cycles, and assessment of blood ethanol concentrations (BEC) associated with the repeated cycles of chronic intermittent ethanol vapor exposure. These criteria identified 38 EtOHIH mice from our records that could be included for further analysis of the relationship between BEC (mg/dl) during ethanol exposure cycles and increased drinking relative to baseline levels of intake. Nineteen CTL mice were selected from the same database based on similar baseline ethanol drinking for comparison with the 38 EtOHIH mice. None of these data have been previously published except in abstract form.
Limited Access Procedures
Mice were given limited access to drinking solutions in the home cage, as previously described (Becker and Lopez, 2004; Griffin et al., 2009; Lopez and Becker, 2005). Briefly, at 30 min before the beginning of the dark cycle (1330 hr), water bottles were removed from the home cage and replaced with 15 ml graduated bottles, one containing ethanol (15% v/v) and the other tap water. Following a 2 hr access period, the graduated bottles were removed from the home cage and replaced with water bottles. The position of the water and ethanol bottles was alternated daily to avoid side preferences. Food and water were always freely available throughout the experiments.
Chronic Ethanol Inhalation Procedures
Chronic ethanol (or air) vapor exposure was delivered in Plexiglas inhalation chambers, as previously described (Becker and Hale, 1993; Becker and Lopez, 2004; Lopez and Becker, 2005). Briefly, ethanol (95%) was volatilized, mixed with fresh air and delivered to the chambers at a rate of 10 l/min to maintain consistent ethanol concentrations (15–20 mg/l air) in the chamber. Prior to entry into the ethanol chambers for each 16 hr exposure period, EtOH mice were administered ethanol (1.6 g/kg; 8% w/v) and the alcohol dehydrogenase inhibitor pyrazole (1 mmol/kg) by intraperitoneal injection in a volume of 20 ml/kg body weight. CTL mice were handled similarly, except they received injections of saline and pyrazole and received air exposure. The housing conditions in the inhalation chambers were identical to those in the colony room. Finally, chamber ethanol concentrations were monitored daily (described below) and air flow was adjusted to maintain ethanol concentrations within the specified range.
Ethanol Assays
Blood ethanol concentrations in samples taken from the retro-orbital sinus were determined using the Analox© method as previously described (Lopez and Becker, 2005). Ethanol concentrations within the inhalation chambers were collected daily and assayed as previously described (Lopez and Becker, 2005).
Data Analysis
Because there was no significant daily variation, measures of ethanol intake (g/kg) were averaged over the 5-day limited access sessions during baseline and each of the 3 test cycles for each subject. These data were analyzed by Analysis of Variance (ANOVA), with Group (EtOH vs. CTL) as a between-subjects factor and Test Cycle as a repeated measure. Further analyses of significant main effects and interaction terms were performed by post hoc comparisons using Bonferroni’s corrections. For all analyses, significance levels were set at p< 0.05.
Results
Study 1: Chronic intermittent ethanol exposure by extending oral access to ethanol
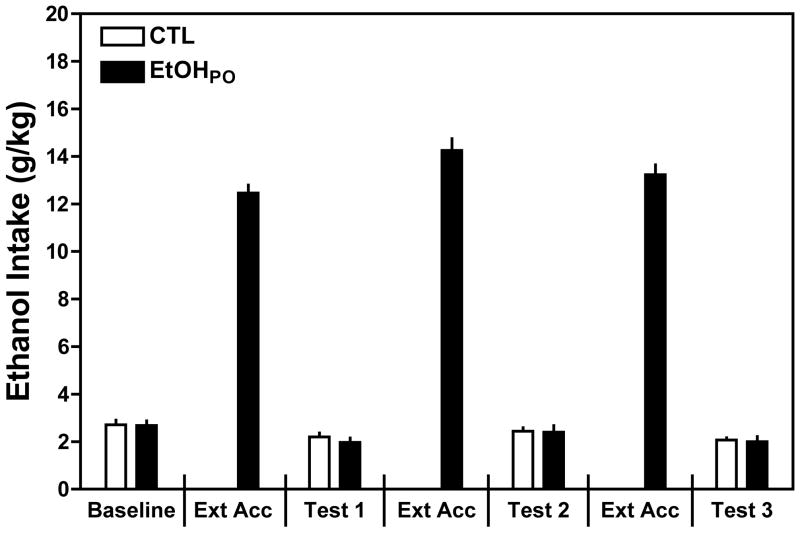
As summarized in Fig. 1, when given the opportunity to drink for 16 hours, EtOHPO mice consumed approximately 6 times more ethanol than when they had 2-hr access drinking sessions. This observation was supported by a significant one-way ANOVA on ethanol intake by EtOHPO [F (6,77)= 329.10, p< 0.001], with post hoc analysis indicating significantly greater ethanol intake during the 3 extended (16-hr) access periods compared to intake during the 2-hr access periods representing the baseline and Tests 1–3. However, the large amount of ethanol consumption by EtOHPO mice did not yield increased ethanol consumption during subsequent 2-hr access drinking sessions. ANOVA did not reveal a significant Group × Test Cycle interaction indicating that ethanol consumption in both groups was similar over baseline and the 3 test cycles [F (3,66)= 0.29. p=0.830]. There was a slight decline in ethanol intake for both groups across test cycles, as indicated by a significant main effect of Test Cycle [F(3,66)= 13.26, p< 0.001]. Additionally, this analysis also indicates that the CTL mice, despite a week without access to ethanol, did not evidence a deprivation effect because their ethanol intake remained relatively unchanged throughout the study. Thus, allowing mice extended oral access to ethanol did not increase drinking in subsequent limited access test sessions.
Figure 1.
Extended oral access to ethanol. EtOHPO mice received repeated weekly cycles of extended access to ethanol (16 hr/day) while CTL mice were maintained on water. EtOHPO mice consumed approximately 6 times more ethanol than baseline 2 hr limited access intake. However, extending oral access to ethanol did not increase ethanol intake during subsequent 2 hr drinking test sessions. Values are means ± S.E.M.
Study 2: Chronic intermittent ethanol exposure by intragastric gavage of ethanol
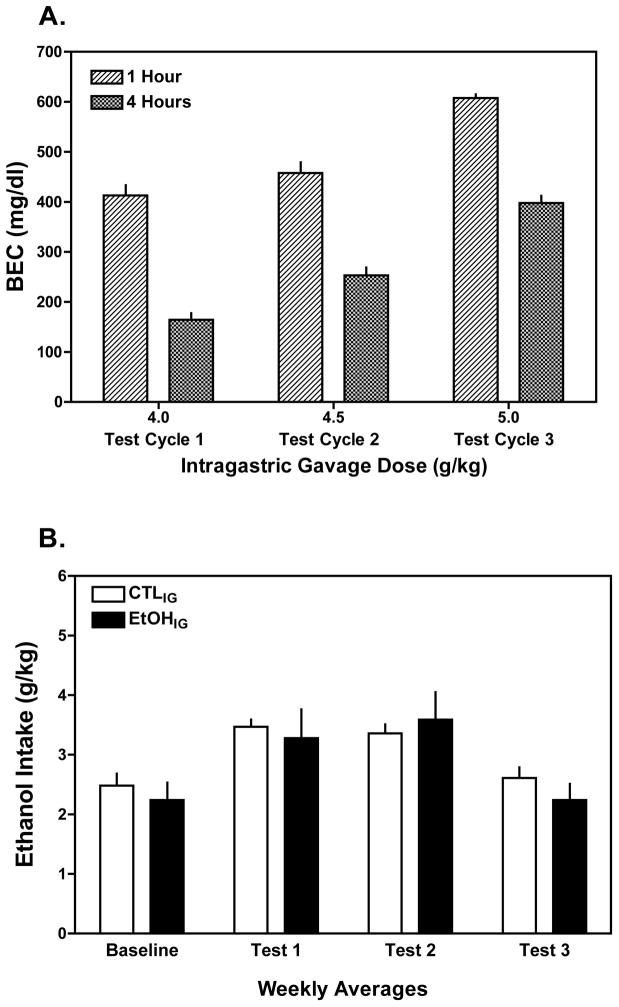
As illustrated in Fig. 2A, repeated daily intragastric administration of ethanol during exposure cycles produced very high levels of exposure, as indexed by blood ethanol levels measured at 1 and 4 hours after gavage. Further, blood ethanol levels in EtOHIG mice increased slightly with each increase in dose across the exposure cycles. Linear extrapolation of the BEC data suggested that BEC values would be at or near zero about 10 hours after gavage of the 5 g/kg dose, suggesting a long exposure to relatively high levels of ethanol.
Figure 2.
Intragastric gavage of ethanol. A) Ethanol gavage (4.0, 4.5, and 5.0 g/kg over successive exposure cycles, respectively) produced high peak blood ethanol concentrations (BEC >400 mg/dl) at 1 and 4 hours post-gavage. Linear extrapolation of these data indicated that ethanol would remain in circulation for several hours, falling to negligible levels at about 10 hr post-gavage. B) Despite attaining high BECs, albeit for a relatively short period of time, ethanol drinking during subsequent limited access test sessions did not significantly differ between EtOHIG and CTLIG groups of mice. Values are means ± S.E.M.
Still, repeated gavage of high ethanol doses in EtOHIG mice did not produce an increase in ethanol drinking compared to CTLIG mice gavaged with isocaloric sucrose (Fig. 2B). This impression is supported by the lack of a Group × Test Cycle interaction [F (3,36)= 1.13, p= 0.351]. Both groups (EtOHIG and CTLIG) evidenced a slight, but similar increase in drinking during Test Cycles 1 and 2, as indicated by a significant main effect of Test Cycle [F(3,36)= 23.48, p< 0.001]. Thus, despite repeated exposure to relatively high levels of ethanol by gavage, voluntary drinking during subsequent limited access test sessions did not increase as a function of this ethanol exposure regimen.
Study 3: Comparison of chronic intermittent ethanol exposure by intragastric gavage and inhalation
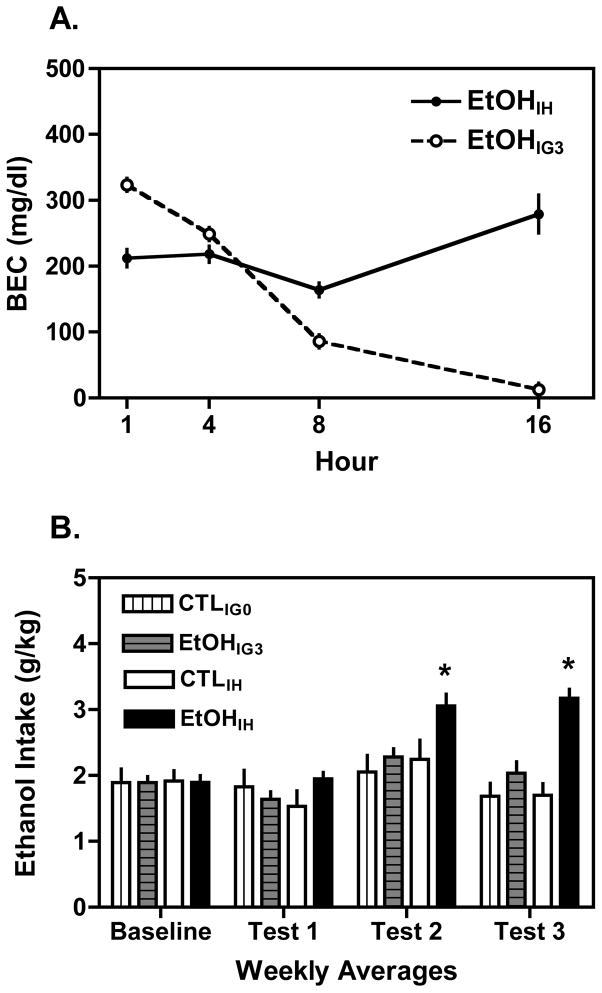
To better compare the two methods of chronic intermittent ethanol exposure, the alcohol dehydrogenase inhibitor pyrazole was administered simultaneously with ethanol given by gavage in order to retard metabolism and sustain exposure (a similar strategy is used in the inhalation procedure). The BEC values shown in Fig. 3A indicate that 3 g/kg ethanol gavage in combination with 1 mmol/kg pyrazole produced peak ethanol levels of 323.4 ± 9.1 mg/dl. Although these peak ethanol levels were lower than for Study 2, the inclusion of pyrazole maintained blood ethanol levels at ~100 mg/dl at the 8 hour time point, with ethanol concentrations declining to negligible levels at 16 hours. In contrast, while not producing as high a peak blood ethanol level (212.4 ± 3.4 mg/dl), ethanol exposure by vapor inhalation did produce sustained levels above 175 mg/dl for the entire 16-hour exposure period. This relatively stable level of exposure throughout the exposure period is a hallmark feature of the inhalation procedure.
Figure 3.
Comparison of intragastric gavage (IG) and inhalation procedures (IH). A) Intragastric gavage of 3 g/kg ethanol in combination with pyrazole produced high peak BECs (>300 mg/dl) that were maintained >100 mg/dl for 8 hr, with levels declining to near zero at 16 hr post-gavage. In contrast, inhalation exposure (with pyrazole) produced somewhat lower peak BECs, but this exposure intensity (>175 mg/dl) was sustained for the entire 16 hr period. B) Sustained exposure to ethanol by inhalation (EtOHIH), but not by gavage (EtOHIG3) significantly increased ethanol drinking during subsequent limited access test sessions compared to the corresponding CTL groups. Values are means ± S.E.M. (* p< 0.05 between groups and compared to baseline).
The drinking data collected for Study 3 are summarized in Fig. 3B. Consistent with our previous reports (Becker and Lopez, 2004; Griffin et al., 2009; Lopez and Becker, 2005), repeated cycles of chronic intermittent ethanol exposure by the inhalation route yielded a substantial increase in voluntary ethanol consumption. However, repeated exposure to ethanol by intragastric gavage combined with pryrazole did not subsequently increase ethanol drinking during limited access test sessions. These observations are supported by ANOVA, which revealed a significant Group × Exposure × Test Cycle interaction [F (3,135)= 3.48, p= 0.018] and a significant Group × Exposure interaction [F(1,45)= 4.83, p= 0.033]. Separate analysis of mice treated by inhalation indicated a significant Group × Test Cycle interaction [F(3,66)= 11.07, p< 0.001], and post hoc analysis revealed EtOHIH mice consumed significantly more ethanol than controls (CTLIH mice) during Test Cycles 2 and 3 (ps< 0.05). In contrast, repeated intubations of ethanol (EtOHIG3 mice) did not significantly alter ethanol intake compared to their respective controls (CTLIG0 mice) across all test phases of the study (Group × Test Cycle interaction: [F(3,66)= 2.45, p= 0.071]).
Study 4: Retrospective data analysis of the inhalation model
We identified 38 EtOHIH and 19 CTLIH mice for analysis to examine the relationship between chronic intermittent ethanol exposure and escalation of voluntary drinking. As shown in Table 1, ethanol drinking in the EtOHIH mice (n= 38) increased with each Test Cycle compared to their own baseline and with the CTLIH mice (n= 19). This observation is supported by a significant Group × Test interaction [F(3,165)= 8.03, p< 0.0001]. Post-hoc analysis indicated that ethanol consumption in CTL mice did not change over test cycles, but drinking in EtOHIH mice increased during each test cycle compared to their baseline level of intake (ps< 0.05). Additionally, ethanol consumption was significantly greater in EtOH compared to CTL mice during Test Cycle 3 (p< 0.05). Because increased ethanol drinking in the EtOHIH mice was most robust during Test Cycle 3 (intake difference relative to baseline= ~1.2 g/kg increase), these data were analyzed further to examine the relationship between this intake difference (g/kg) and post-inhalation BEC (Fig. 4A). Analysis indicated a positive correlation between the g/kg intake difference (Baseline to Test Cycle 3) and BEC (measured immediately following the 3rd inhalation exposure cycle) [Pearson’s r= 0.55 (r2= 0.3), p= 0.0003]. This significant positive correlation indicates that higher intensity of chronic ethanol exposure, indexed by BEC, generally predicts greater increase in ethanol intake relative to baseline level of drinking.
Table 1.
| Group (n) | Baseline | Test 1 | Test 2 | Test 3 |
|---|---|---|---|---|
| CTLIH (19) | 2.54 ± 0.16 | 2.39 ± 0.15 | 2.48 ± 0.15 | 2.59 ± 0.17 |
| EtOHIH (38) | 2.39 ± 0.13 | 2.77 ± 0.18* | 2.78 ± 0.20* | 3.31 ± 0.20*^ |
Values are mean g/kg ± S.E.M.
p<0.05 within group compared to baseline,
p<0.05 between groups
Figure 4.
Escalation of voluntary ethanol intake during limited access Test Cycles as a function of BECs maintained during each of the inhalation exposure cycles. A) Increase in drinking during Test Cycle 3 (intake difference from baseline) plotted as a function of post-inhalation BEC (exposure cycle 3) for ethanol-exposed subjects (N= 38). Analysis indicated a significant positive correlation between the increase in drinking and the intensity of ethanol exposure (p< 0.05). The median intake difference was 0.98 g/kg, and BEC values were 231.17 ± 20.10 and 173.62 ± 16.63 mg/dl for mice above (black circles) and below (gray circles) the median intake difference. B) Based on this information, data shown in Table 1 were stratified according to whether chronic intermittent ethanol exposure consistently yielded BECs either ≥175 mg/dl or <175 g/dl during each exposure cycle. EtOHIH mice (N= 19) with BECs maintained ≥175 mg/dl during all three exposure periods evidenced a significant increase in ethanol intake during each Test Cycle compared to their baseline level of intake, as well as the amount consumed by a separate EtOHIH group (with lower BEC exposure) during Test Cycle 3. In contrast, EtOHIH mice (N= 19) with BECs maintained <175 mg/dl during each exposure cycle increased ethanol consumption above their baseline only during Test Cycle 3. Ethanol consumption in CTLIH mice (N= 19) did not significantly change during the course of the study. Values are means ± S.E.M. (* p< 0.05 within groups comparison to baseline; ^ p< 0.05 between groups, Test Cycle 3).
The distribution of intake differences during Test Cycle 3 was examined more closely to elucidate the role of ethanol exposure intensity in the escalation of drinking. The median increase in ethanol intake relative to baseline drinking was 0.98 g/kg in the EtOHIH mice in Test Cycle 3. Separating the EtOHIH mice into two groups based on this median intake difference, mice falling below the median (depicted as grey circles in Fig. 4A) and mice above the median value (depicted as black circles in Fig. 4A) evidenced mean post-inhalation BEC values for the 3rd exposure cycle of 173.62 ± 16.63 and 231.17 ± 20.10 mg/dl, respectively. These data suggest that chronic ethanol exposure that produces a minimum BEC of 175 mg/dl is associated with significant increases in ethanol drinking.
On the basis of this information, data shown in Table 1 were re-examined by sorting the EtOHIH mice into two groups based on whether BEC values were consistently above or below 175 mg/dl for all 3 exposure cycles. For the EtOHIH group with BECs <175 mg/dl (n=19), baseline ethanol intake averaged 2.50 ± 0.12 g/kg and baseline drinking for the EtOHIH group with BECs >175 mg/dl (n=19) was 2.28 ± 0.13 g/kg. Baseline intake for CTLIH mice (n=19) was 2.54 ± 0.16 g/kg. One-way ANOVA indicated no significant difference in baseline intake across groups [F(2,54)= 0.37, p> 0.05]. As can be seen in Fig. 4B, escalation in drinking in EtOHIH depended on the intensity of exposure (BEC level) maintained during the exposure cycles. That is, ethanol consumption was significantly elevated (relative to baseline) during all 3 test cycles for EtOHIH mice with BEC >175 mg/dl. On the other hand, for mice with BEC values less than 175 mg/dl during all 3 exposure cycles, ethanol consumption only increased above baseline levels during Test Cycle 3. As expected, ethanol drinking in CTLIH mice did not change over test cycles. These observations were supported by a significant Group × Test Cycle interaction [F(3,162)= 7.89, p< 0.001]. Post-hoc analysis revealed that EtOHIH mice with BEC >175 mg/dl evidenced significant increases in ethanol consumption during all 3 test cycles compared to their own baseline level of intake, as well as that for the other EtOHIH group and the CTLIH mice. Further, the EtOHIH >175 BEC group consumed significantly more ethanol than the EtOHIH <175 BEC group during Test Cycle 3 (ps< 0.05). In contrast, EtOHIH mice with BEC <175 mg/dl did not escalate their ethanol drinking until Test Cycle 3. Taken together, these findings suggest that an apparent minimum intensity (amount) of chronic ethanol exposure (indexed by blood ethanol concentrations) needs to be sustained over repeated cycles of exposure in order to observe a significant and robust escalation in ethanol consumption. Mice exposed to lower intensity chronic ethanol exposure (i.e., <175 mg/dl BEC) may require a greater number of exposure cycles to demonstrate increased ethanol intake over baseline levels.
Discussion
The principle finding from these studies is that the intensity (amount) and duration of chronic ethanol exposure critically determines whether an escalation in ethanol consumption will subsequently occur in mice. Specifically, we demonstrated that repeated cycles of chronic intermittent ethanol exposure that produced blood ethanol levels of at least 175 mg/dl and, importantly, were sustained for at least 16 hrs during each exposure period is critical for escalation of drinking. Simply achieving high peak blood ethanol levels, but for relatively brief periods of time, was not sufficient to drive an increase in drinking. Additionally, providing mice the opportunity to drink ethanol for 16 hr/day did not result in elevation of subsequent voluntary drinking. Collectively, these findings suggest that a threshold level of chronic ethanol exposure is required to produce escalation of drinking.
Results from the present study confirm our previous work demonstrating that repeated cycles of chronic intermittent delivery of ethanol by inhalation produces robust increases in voluntary drinking (Lopez and Becker, 2005). To determine whether other modes of chronic intermittent ethanol delivery can produce similar results several different methods and regimens of ethanol exposure were examined. First, we examined the effects of allowing mice extended oral access to ethanol for 16 hr/day over repeated weekly cycles. Despite a nearly 6-fold increase in consumption during the extended access drinking periods (12–15 g/kg/16 hr), this did not produce enhanced ethanol drinking during subsequent limited access test sessions. We did not measure blood ethanol concentrations in this study. However, we previously reported that non-dependent mice attain BECs of approximately 60 mg/dl after 2 hours of drinking (intake: ~2.3–2.7 g/kg)(Becker and Lopez, 2004). Thus, even assuming that similar intake and BEC levels were maintained for the entire 16 hour access periods in the present study, the exposure to ethanol in this procedure was still far less than that achieved by intragastric gavage and inhalation procedures. Moreover, prior studies conducted with C57BL/6 mice have indicated that intake is not uniform over extended access periods and, although cumulative ethanol intake (g/kg) increases over time, the relationship between intake and resultant blood ethanol levels diminishes indicating different patterns of intake between mice across time (Middaugh et al., 2003). Therefore, repeatedly providing free oral access to ethanol over an extended period of time (16 hr) does not ensure stable levels of exposure that are comparable across subjects and, more importantly, sufficiently high enough ethanol exposure to favor subsequent escalation of drinking.
Two studies examined the effects of chronic intermittent ethanol exposure delivered by daily intragastric gavage. In one case, ethanol dosage was increased over the three weekly exposure cycles (4.0, 4.5, and 5.0 g/kg, respectively) and, while this treatment regimen produced very high peak BEC levels (400–550 mg/dl), voluntary ethanol drinking during 2-hr test sessions was not significantly altered relative to controls (Fig. 2). Since ethanol intake similarly increased in both ethanol and control groups during the first two test cycles, stress associated with the gavage procedure might be related to this non-selective increase in ethanol intake. However, this does not seem likely in that a follow-up study (Study 3) involving the same route of administration (gavage) did not produce significant changes in ethanol consumption for ethanol-treated or control mice. In this separate gavage study, a moderate dose (3 g/kg) was held constant across exposure cycles, but administered with the alcohol dehydrogenase inhibitor pyrazole. This produced peak blood ethanol levels that exceeded 300 mg/dl and sustained BEC >100 mg/dl for at least 8 hr. However, voluntary ethanol drinking during limited access test sessions did not increase as a result of this treatment condition (Fig. 3). This is in contrast to results obtained using the inhalation route for delivery of chronic intermittent ethanol exposure. Although this exposure method produced lower peak BEC values (~200 mg/kg), this level of exposure was sustained for the entire 16 hr exposure period. Further, only the inhalation treatment regimen produced increased voluntary drinking in the mice (Fig. 3), emphasizing that obtaining high peak blood ethanol levels is less important than the overall amount and duration of ethanol exposure in this model of dependence and relapse drinking.
In the current studies we examined ethanol drinking during the course of three cycles of chronic intermittent ethanol exposure. Because we previously reported a positive relationship between the number of cycles of chronic intermittent ethanol exposure by inhalation and degree of enhanced drinking (Lopez and Becker, 2005), it is possible that increasing the number of cycles of ethanol exposure by extended oral access or gavage (with or without pyrazole) may result in increased subsequent drinking. Additionally, more frequent administration of ethanol by gavage in a manner that maintains elevated BECs over an extended period of time may favor escalation of subsequent drinking. Consistent with this, use of a chronic ethanol treatment regimen that involves repeated gavage (Majchrowicz, 1975) was recently reported to maintain BEC values above 175 mg/dl for 12 hr and support enhanced ethanol self-administration in rats (Gehlert et al., 2007). Further, our retrospective analysis (Fig. 4) also supports the idea that a sustained level of exposure is necessary for enhanced drinking. The EtOHIH group that received chronic intermittent ethanol exposure in which BEC levels were maintained at >175 mg/dl over repeated exposure cycles showed significant increases in ethanol intake during each of three drinking test cycles. In contrast, the EtOHIH group that experienced less intense exposure (BEC <175 mg/dl) displayed an increase in voluntary drinking only after the third exposure cycle. Collectively, these results support the notion that regardless of route of administration, repeated cycles of chronic intermittent ethanol exposure that maintain BECs above a threshold level (e.g., 175 mg/dl) over an extended period of time is critical for facilitating escalation of drinking.
Implicit in the use of animal models that link chronic ethanol exposure regimens with self-administration procedures is the idea that dependence (and withdrawal experience) drive excessive drinking through altered motivational processes (e.g., negative reinforcement). Although we did not directly assess withdrawal responses in the present studies, we previously reported this inhalation model (and similar exposure parameters) to produce dependence, as indexed by a variety of withdrawal symptoms (Becker, 1999). The fact that a certain threshold of chronic ethanol exposure appears critical in favoring increased self-administration suggests that a certain degree of dependence (and withdrawal intensity) may be required to produce such escalation in drinking. In this vein, it is noteworthy that delivery of chronic ethanol exposure in an intermittent pattern (and of sufficient intensity that involves repeated withdrawal experience) has been shown to facilitate escalation of voluntary ethanol intake in both mice (Lopez and Becker, 2005) and rats (O’Dell et al., 2004).
In both mice and rats, enhanced ethanol self-administration following repeated cycles of chronic ethanol exposure and withdrawal was associated with significantly higher resultant blood ethanol levels compared with the levels achieved by non-dependent animals (Becker and Lopez, 2004; Roberts et al., 2000). Additionally, we recently reported that greater voluntary ethanol consumption in dependent mice produced brain ethanol concentrations that approximated those levels experienced during the chronic intermittent ethanol exposure (which rendered the animals dependent in the first place) (Griffin et al., 2009). Although it is tempting to speculate that dependent animals increase voluntary ethanol drinking to attain blood and brain ethanol levels in a range consistent with sustaining dependence (and preventing withdrawal), additional studies are needed to more directly address this issue.
In summary, results from this series of studies demonstrate that repeated cycles of chronic intermittent ethanol exposure result in escalation of voluntary drinking in mice, but the effect is dependent on the intensity and duration of the exposure. Delivery of chronic ethanol exposure in a manner that produces sustained high blood ethanol concentrations over repeated cycles of exposure appears to facilitate robust escalation of drinking. As indicated by the retrospective analysis of our dependence and drinking model, more moderate chronic ethanol exposure that yields lower blood ethanol levels may also produce escalation of drinking, but a greater number of exposure cycles may be needed to observe such an effect. Thus, both intensity and duration of ethanol exposure (i.e., number of cycles) play a significant role in contributing to escalation of drinking in dependent animals. While a great deal of caution should be used in extrapolating results from this animal model to the clinical situation, results from the present study may have important clinical implications. Specifically, findings from the present study appear to align with recent clinical studies that emphasize the importance of quantity and frequency measures of alcohol consumption in refining diagnostic validity and accuracy, as well as predicting numerous adverse medical, psychiatric, and social consequences (e.g., Keyes et al., 2009; Li et al., 2007a; 2007b). Frequent at-risk drinking (defined by NIAAA guidelines as 5+ drinks for men and 4+ drinks for women during a drinking occasion) that commonly results in substantially exceeding legal limit of intoxication is reported to be predictive of alcohol-related negative consequences. Further, criteria reflecting physical dependence and loss of control/compulsion were shown to strongly influence an overall diagnostic alcohol use disorder severity continuum (Saha et. al., 2007). To the extent that repeated cycles of episodic ethanol exposure in our mouse model of dependence relates to binge-like or “bender” patterns of alcohol consumption in humans, quantity and frequency dimensions of exposure appear to play an important role in promoting and/or modulating escalation of drinking to excessive (harmful) levels in both preclinical and clinical domains. Future studies aimed at further characterization of our mouse model of ethanol dependence and relapse drinking may reveal additional factors (e.g., level of baseline drinking) that significantly influence and/or contribute to enhanced vulnerability to relapse and excessive drinking associated with alcohol dependence.
Acknowledgments
This work was supported by NIH grants AA10716, AA14095 and AA13885. The authors acknowledge the expert technical assistance of Melissa Overstreet, Laura Ralston and Kay Fernandes in conducting these studies.
References
- Becker HC. Alcohol withdrawal: Neuroadaptation and sensitization. CNS Spectrums. 1999;4(1):38–65. [Google Scholar]
- Becker HC. Animal models of alcohol withdrawal. Alcohol Res Health. 2000;24(2):105–13. [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Hale RL. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal “kindling”. Alcohol Clin Exp Res. 1993;17(1):94–8. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28(12):1829–38. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav. 2007;86(4):813–21. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaher R, Finn D, Snelling C, Hitzemann R. Lesions of the extended amygdala in C57BL/6J mice do not block the intermittent ethanol vapor-induced increase in ethanol consumption. Alcohol Clin Exp Res. 2008;32(2):197–208. doi: 10.1111/j.1530-0277.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist d-Phe-CRF(12–41) Alcohol Clin Exp Res. 2007;31(6):939–49. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl- imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27(10):2718–26. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology (Berl) 2009;201(4):569–80. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Geier T, Grant BF, Hasin DS. Influence of a drinking quantity and frequency measure on the prevalence and demographic correlates of DSM-IV alcohol dependence. Alcohol Clin Exp Res. 2009;22(5):761–71. doi: 10.1111/j.1530-0277.2009.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27(2):232–43. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Li TK, Hewitt BG, Grant BK. The alcohol dependence syndrome, 30 years later: a commentary. Addiction. 2007a;102:1522–30. doi: 10.1111/j.1360-0443.2007.01911.x. [DOI] [PubMed] [Google Scholar]
- Li TK, Hewitt BG, Grant BF. Is there a future for quantifying drinking in the diagnosis, treatment, and prevention of alcohol use disorders? Alcohol Alcohol. 2007b;42:57–63. doi: 10.1093/alcalc/agl125. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Anderson RI, Becker HC. Repeated cycles of chronic intermittent ethanol exposure increase both self-administration and the reinforcing value of ethanol in C57BL/6J mice. Alcohol Clin & Exp Res. 2008;32(6):163A. [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology. 2005;181(4):688–96. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43(3):245–54. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Middaugh L, Szumlinski KK, Van Patton Y, Marlow AB, Kalivas PW. Chronic ethanol consumption by C57BL/6 mice promotes tolerance to its interoceptive cues and increases extracellular dopamine, an effect blocked by naltrexone. Alcohol Clin Exp Res. 2003;27(12):1892–1900. doi: 10.1097/01.ALC.0000099264.36220.48. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28(11):1676–82. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Sommer W, Heilig M. Effects of tiagabine and diazepam on operant ethanol self-administration in the rat. J Stud Alcohol. 2002;63(1):100–6. [PubMed] [Google Scholar]
- Rimondini R, Sommer W, Heilig M. A temporal threshold for induction of persistent alcohol preference: behavioral evidence in a rat model of intermittent intoxication. J Stud Alcohol. 2003;64(4):445–9. doi: 10.15288/jsa.2003.64.445. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20(7):1289–98. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22(6):581–94. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Saha TD, Stinson FS, Grant BF. The role of alcohol consumption in future classifications of alcohol use disorders. Drug Alcohol Depend. 2007;89:82–92. doi: 10.1016/j.drugalcdep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63(2):139–45. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]