Abstract
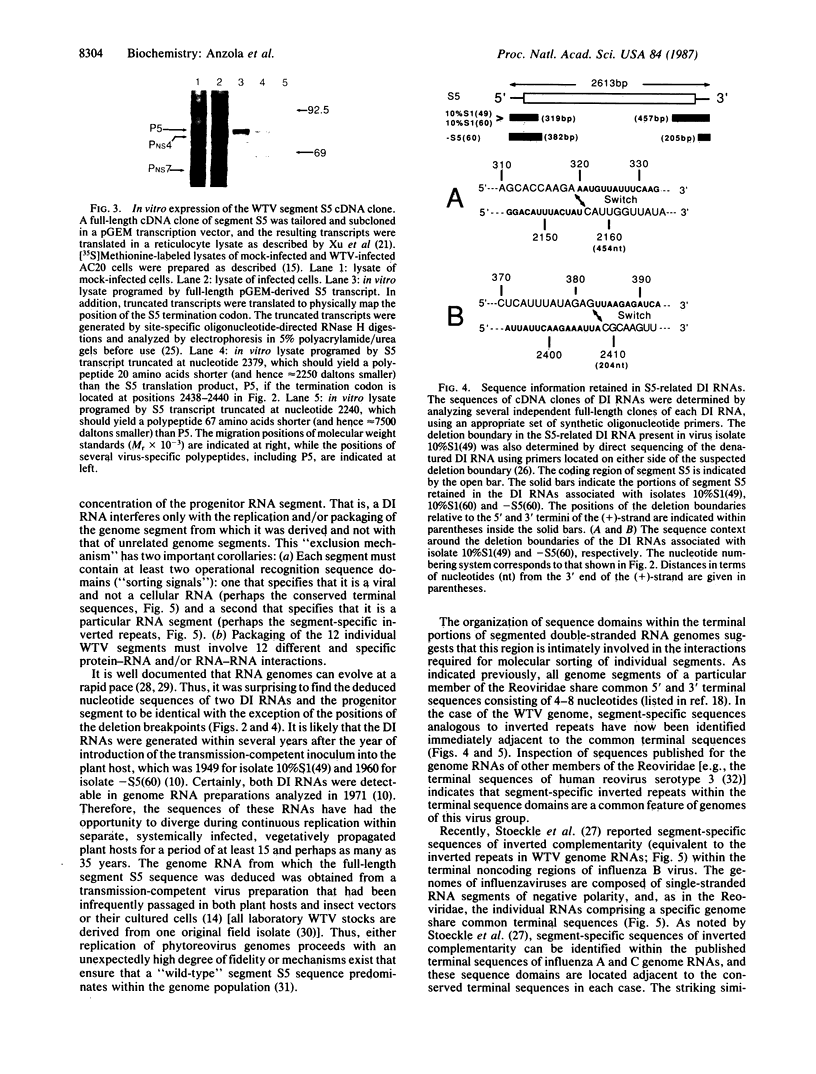
Defective interfering (DI) RNAs are often associated with transmission-defective isolates of wound tumor virus (WTV), a plant virus member of the Reoviridae. We report here the cloning and characterization of WTV genome segment S5 [2613 base pairs (bp)] and three related DI RNAs (587-776 bp). Each DI RNA was generated by a simple internal deletion event that resulted in no sequence rearrangement at the deletion boundaries. Remarkably, although several DI RNAs have been in continuous passage for more than 20 years, their nucleotide sequences are identical to that of corresponding portions of segment S5 present in infrequently passaged, standard, transmission-competent virus. The positions of the deletion breakpoints indicate that the minimal sequence information required for replication and packaging of segment S5 resides within 319 bp from the 5' end of the (+)-strand and 205 bp from the 3' end of the (+)-strand. The terminal portions of segment S5 were found to contain a 9-bp inverted repeat immediately adjacent to the conserved terminal 5'-hexanucleotide and 3'-tetranucleotide sequences shared by all 12 WTV genome segments. The presence of a 6- to 9-nucleotide segment-specific inverted repeat immediately adjacent to the conserved terminal sequences was found to be a feature common to all WTV genome segments. These results reveal several basic principles that govern the replication and packaging of a segmented double-stranded RNA genome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antczak J. B., Chmelo R., Pickup D. J., Joklik W. K. Sequence at both termini of the 10 genes of reovirus serotype 3 (strain Dearing). Virology. 1982 Sep;121(2):307–319. doi: 10.1016/0042-6822(82)90170-2. [DOI] [PubMed] [Google Scholar]
- Asamizu T., Summers D., Motika M. B., Anzola J. V., Nuss D. L. Molecular cloning and characterization of the genome of wound tumor virus: a tumor-inducing plant reovirus. Virology. 1985 Jul 30;144(2):398–409. doi: 10.1016/0042-6822(85)90281-8. [DOI] [PubMed] [Google Scholar]
- Bassel-Duby R., Spriggs D. R., Tyler K. L., Fields B. N. Identification of attenuating mutations on the reovirus type 3 S1 double-stranded RNA segment with a rapid sequencing technique. J Virol. 1986 Oct;60(1):64–67. doi: 10.1128/jvi.60.1.64-67.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay D., Banerjee A. K. Phosphorylation within a specific domain of the phosphoprotein of vesicular stomatitis virus regulates transcription in vitro. Cell. 1987 May 8;49(3):407–414. doi: 10.1016/0092-8674(87)90293-5. [DOI] [PubMed] [Google Scholar]
- DePolo N. J., Giachetti C., Holland J. J. Continuing coevolution of virus and defective interfering particles and of viral genome sequences during undiluted passages: virus mutants exhibiting nearly complete resistance to formerly dominant defective interfering particles. J Virol. 1987 Feb;61(2):454–464. doi: 10.1128/jvi.61.2.454-464.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo E., Sabo D., Taniguchi T., Weissmann C. Nucleotide sequence heterogeneity of an RNA phage population. Cell. 1978 Apr;13(4):735–744. doi: 10.1016/0092-8674(78)90223-4. [DOI] [PubMed] [Google Scholar]
- Elleman T. C., Hoyne P. A., Dyall-Smith M. L., Holmes I. H., Azad A. A. Nucleotide sequence of the gene encoding the serotype-specific glycoprotein of UK bovine rotavirus. Nucleic Acids Res. 1983 Jul 25;11(14):4689–4701. doi: 10.1093/nar/11.14.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Kimura I., Black L. M. The cell-infecting unit of wound tumor virus. Virology. 1972 Aug;49(2):549–561. doi: 10.1016/0042-6822(72)90506-5. [DOI] [PubMed] [Google Scholar]
- Levis R., Weiss B. G., Tsiang M., Huang H., Schlesinger S. Deletion mapping of Sindbis virus DI RNAs derived from cDNAs defines the sequences essential for replication and packaging. Cell. 1986 Jan 17;44(1):137–145. doi: 10.1016/0092-8674(86)90492-7. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D. P., Chambers T. M., Akkina R. K. Defective-interfering (DI) RNAs of influenza viruses: origin, structure, expression, and interference. Curr Top Microbiol Immunol. 1985;114:103–151. doi: 10.1007/978-3-642-70227-3_3. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Watanabe Y., Graham A. F. Defective virions of reovirus. J Virol. 1970 Aug;6(2):226–236. doi: 10.1128/jvi.6.2.226-236.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss D. L., Peterson A. J. Expression of wound tumor virus gene products in vivo and in vitro. J Virol. 1980 May;34(2):532–541. doi: 10.1128/jvi.34.2.532-541.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss D. L., Summers D. Variant dsRNAs associated with transmission-defective isolates of wound tumor virus represent terminally conserved remnants of genome segments. Virology. 1984 Mar;133(2):276–288. doi: 10.1016/0042-6822(84)90395-7. [DOI] [PubMed] [Google Scholar]
- Peterson A. J., Nuss D. L. Regulation of expression of the wound tumor virus genome in persistently infected vector cells is related to change in translational activity of viral transcripts. J Virol. 1986 Aug;59(2):195–202. doi: 10.1128/jvi.59.2.195-202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re G. G., Kingsbury D. W. Nucleotide sequences that affect replicative and transcriptional efficiencies of Sendai virus deletion mutants. J Virol. 1986 May;58(2):578–582. doi: 10.1128/jvi.58.2.578-582.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reanney D. C. The evolution of RNA viruses. Annu Rev Microbiol. 1982;36:47–73. doi: 10.1146/annurev.mi.36.100182.000403. [DOI] [PubMed] [Google Scholar]
- Reddy D. V., Black L. M. Deletion mutations of the genome segments of wound tumor virus. Virology. 1974 Oct;61(2):458–473. doi: 10.1016/0042-6822(74)90282-7. [DOI] [PubMed] [Google Scholar]
- Reddy D. V., Black L. M. Electrophoretic separation of all components of the double-stranded RNA of wound tumor virus. Virology. 1973 Aug;54(2):557–562. doi: 10.1016/0042-6822(73)90168-2. [DOI] [PubMed] [Google Scholar]
- Reddy D. V., Black L. M. Increase of wound tumor virus in leafhoppers as assayed on vector cell monolayers. Virology. 1972 Nov;50(2):412–421. doi: 10.1016/0042-6822(72)90393-5. [DOI] [PubMed] [Google Scholar]
- Reddy D. V., Black L. M. Isolation and replication of mutant populations of wound tumor virions lacking certain genome segments. Virology. 1977 Jul 15;80(2):336–346. doi: 10.1016/s0042-6822(77)80009-3. [DOI] [PubMed] [Google Scholar]
- Reddy D. V., MacLeod R. Polypeptide components of wound tumor virus. Virology. 1976 Apr;70(2):274–282. doi: 10.1016/0042-6822(76)90270-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spendlove R. S., McClain M. E., Lennette E. H. Enhancement of reovirus infectivity by extracellular removal or alteration of the virus capsid by proteolytic enzymes. J Gen Virol. 1970 Aug;8(2):83–94. doi: 10.1099/0022-1317-8-2-83. [DOI] [PubMed] [Google Scholar]
- Stoeckle M. Y., Shaw M. W., Choppin P. W. Segment-specific and common nucleotide sequences in the noncoding regions of influenza B virus genome RNAs. Proc Natl Acad Sci U S A. 1987 May;84(9):2703–2707. doi: 10.1073/pnas.84.9.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z. K., Anzola J. V., Nuss D. L. Tailored removal of flanking homopolymer sequences from cDNA clones. DNA. 1987 Oct;6(5):505–513. doi: 10.1089/dna.1987.6.505. [DOI] [PubMed] [Google Scholar]