Abstract
The human fetal heart develops arrhythmias and conduction disturbances in response to ischemia, inflammation, electrolyte disturbances, altered load states, structural defects, inherited genetic conditions, and many other causes. Yet sinus rhythm is present without altered rate or rhythm in some of the most serious electrophysiological diseases, which makes detection of diseases of the fetal conduction system challenging in the absence of magnetocardiographic or electrocardiographic recording techniques. Life-threatening changes in QRS or QT intervals can be completely unrecognized if heart rate is the only feature to be altered. For many fetal arrhythmias, echocardiography alone can assess important clinical parameters for diagnosis. Appropriate treatment of the fetus requires awareness of arrhythmia characteristics, mechanisms, and potential associations. Criteria to define fetal bradycardia specific to gestational age are now available and may allow detection of ion channelopathies, which are associated with fetal and neonatal bradycardia. Ectopic beats, once thought to be entirely benign, are now recognized to have important pathologic associations. Fetal tachyarrhythmias can now be defined precisely for mechanism-specific therapy and for subsequent monitoring of response. This article reviews the current and future diagnostic techniques and pharmacologic treatments for fetal arrhythmia.
Introduction
The final common pathway to death in humans of all ages occurs through arrhythmia or asystole. In the case of fetal death, little is known about the electrophysiological state of the heart immediately before demise. The fetus is presumed to die from an inalterable cascade of cardiac ischemia and ventricular dysfunction brought on by impaired blood flow through the placenta or umbilical cord. Yet, evidence supports in some cases a mechanism completely separate from this process. Potential causes of fetal death include genetic diseases affecting ion channel conduction as a result of spontaneous mutations1–7 or inherited predisposition,8 or acquired fetal diseases producing malignant arrhythmias that lead to sudden cardiac arrest in the prenatal or postnatal period, similar to those seen in adults and children of all ages. Paroxysmal arrhythmias, such as torsades de pointes (Figure 1) and junctional and ventricular tachycardia, may cause adverse prenatal or postnatal outcomes.1,3,9,10 Other complications of pregnancy, such as fetal growth restriction, might induce fetal cardiac instability that is undetected by present methods of diagnosis. Thus, improvements in diagnostic, genetic assessment and monitoring technologies for arrhythmias are required to determine the extent to which these diseases account for fetal or neonatal death.
Figure 1.
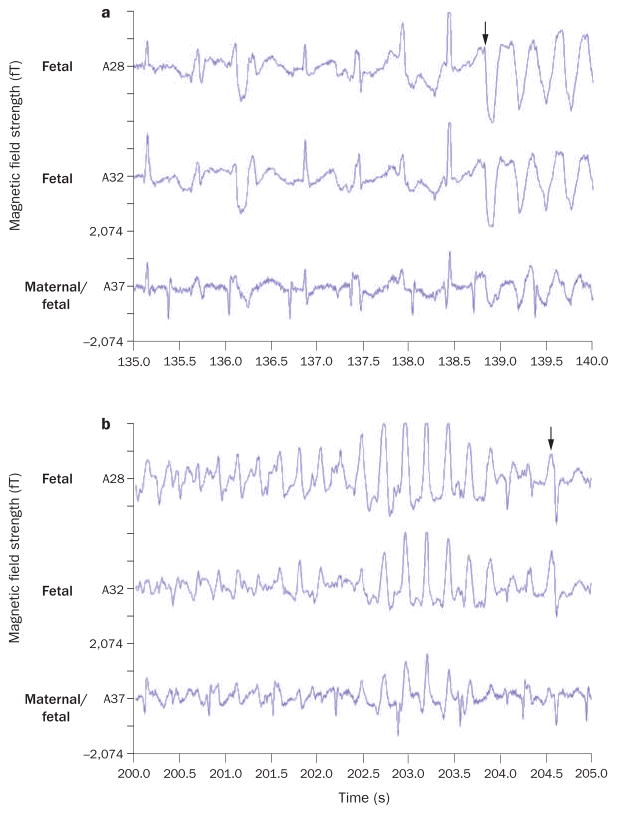
Two fetal magnetocardiography recordings from a 30-week fetus with long QT syndrome 3.1 a | Onset of torsades de pointes ventricular tachycardia occurs after 138.5 s (arrow). b | Termination of torsades de pointes occurs at 204.5 s (arrow). Arrhythmia was triggered by use of amiodarone to treat what was initially considered, on the basis of findings from echocardiography, to be drug-refractory fetal ventricular tachycardia. Permission obtained from Elsevier Ltd.
© Cuneo, B. F. et al. Am. J. Cardiol. 91, 1395–1398 (2003).
Nonlethal arrhythmias in the fetus generally create rhythm disturbances that clinicians more-readily recognize as abnormal than they do most potentially fatal arrhythmias. The study of fetal arrhythmias is often broadly divided into bradyarrhythmias (heart rate <110 bpm when adjusted for gestational age), fetal tachycardia (heart rate >180 bpm), and fetal ectopy (irregular rhythm due to early contractions). These broad categories notably exclude electrophysiological abnormalities in QRS or QT intervals caused by ion channelopathies, where rate or rhythm alterations are absent. These genetic disorders perhaps comprise the most lethal group of abnormalities. In this Review we present data on the currently available diagnostic techniques and pharmacologic treatments for fetal arrhythmia. We also discuss directions required for improvements in the future.
Current diagnostic modalities
Echocardiography is the only widely used method of assessing fetal cardiac function. Over the past three decades, this modality has been instrumental in making fetal arrhythmias one of the most successful areas of fetal treatment. Rein and colleagues demonstrated that fetal tissue Doppler echocardiography (also called kinetocardiography) provides accurate localization of many arrhythmias, but remains limited in its ability to assess fetal rhythm because it measures the mechanical consequence of the arrhythmia rather than the arrhythmia or conduction itself.11,12 Other limitations of echo-cardiography relate to the inability to record rhythms over long periods of time, to assess trends in heart rate during tachycardia or bradycardia, and to assess QRS duration or QT interval.
Fetal heart rate monitoring (electronically interpreted cardiotocography) is widely available, but its utility is limited by fetal movement leading to signal loss before 30 weeks’ gestation and by data displays that poorly characterize abnormal rhythms. In addition, the relationship between fetal movement and heart rate acceleration is attenuated at these early gestation times. Direct electrocardiographic assessment of rhythm (fetal electrocardiography, fECG) is also of limited use at present, possibly owing to the poor quality of recording obtained via maternal skin electrodes.13
Fetal magnetocardiography (fMCG), although limited in its availability at present, provides high-quality electro-physiological cardiac signals that enable diagnosis of previously unrecognized fetal pathologies. Although fMCG and fECG are similar in many respects, fMCG achieves much higher quality recordings owing to advantageous signal-transmission properties, being much less affected by the poor electrical conductivity of fetal skin, vernix caseosa, and surrounding maternal tissues. fMCG can be performed in many fetuses after 20 weeks’ gestation, but is less reliable before this gestational age. Recordings are usually performed over 1–2 h in 5–10 min epochs. Although signal-processing techniques vary by laboratory, heart rate trend analysis, signal-averaged complexes, and rhythm tracings are readily obtainable in most fetuses. The fetal position determines the orientation of the signal and—unlike neonatal electrocardiography—the directional components of the signal (P, QRS, T) must be referenced to each other, rather than to a standard lead orientation. In aggregate, the P, QRS, and T polarities are usually concordant during normal sinus rhythm.
Fetal bradyarrhythmias
Fetal sinus and low atrial bradycardias
In some fetuses, persistent sinus or low atrial bradycardia may be present and the extent to which these types of arrhythmia are associated with ion channel diseases is not yet known. Acherman and colleagues described a number of fetal and maternal conditions that predispose to fetal bradycardia with 1:1 atrioventricular conduction.14 Fetal sinus bradycardia was defined as a heart rate <100 bpm, but in 2009 this threshold was revised to <110 bpm by the American College of Obstetrics and Gynecology in response to population data.15 A short PR interval or a short echocardiographic mechanical PR interval in the presence of bradycardia usually denotes a low atrial rhythm. Low atrial mechanisms are seen in left atrial isomerism due to the absence of the sinus node.
Few large, systematic studies have been conducted on heart rates in normal fetuses during early gestation, and use of cardiotocography before 30 weeks’ gestation is limited. Indeed, in many prenatal records for low-risk fetuses of any gestational age, the heartbeat is merely checked as being present without documentation of its rate. Serra and colleagues examined electronic cardiotocographic records from 4,412 healthy singleton fetuses, and derived percentiles for fetal heart rates from 25 weeks’ gestation onward.16 On average, ventricular rates were below the fifth percentile if they were <130 bpm at 25 weeks of gestation, <120 bpm at 30 weeks’ gestation, or <110 bpm at term. Robinson and colleagues17 have published data on heart rates in healthy fetuses from 15 weeks’ gestation onwards—a time that is not represented in the study by Serra and co-workers.
While mild cases of fetal bradycardia were initially believed by many obstetricians to be benign, links have been identified between uncharacterized fetal bradycardia and long QT syndrome,8,18 SSA/Ro or SSB/La antibody isoimmunization,19 noncompaction syndrome,20 and other life-threatening diseases.21,22 Fetal sinus bradycardia and low atrial bradycardia usually demonstrate heart-rate reactivity even in the presence of serious underlying conditions (Supplementary Figure 1); however, this reactivity may be blunted due to a reduction in autonomic influences. These disease associations warrant improved screening and monitoring of all such fetuses throughout gestation, especially those noted to have persistent fetal bradycardia.23
Neither sinus nor low atrial bradycardia require specific treatment, and are not generally associated with hemodynamic compromise at the time of birth; however, neonatal electrocardiographic screening for long QT syndrome is recommended by some clinicians for babies affected by sinus bradycardia23,24 and those of families affected by sudden infant death syndrome or unexplained stillbirths.25,26 Prenatal electrocardiographic screening of both parents may also be warranted when fetal sinus bradycardia is noted, given the autosomal-dominant transmission of common forms of long QT syndrome, especially if a family history of premature sudden death is present. In several cases, a diagnosis of familial long QT syndrome has been established after a fetus presented with sinus bradycardia. Isoimmunization with or without heart block can be associated with sinus bradycardia and can imply increased severity of disease and a poor prognosis.19,27
Fetal atrioventricular block
Congenital atrioventricular block can be classified as block related to structural defects that anatomically displace the distal conduction system, block related to the presence of maternal SSA/Ro or SSB/La antibodies that produce an inflammatory myocardial response, and block of unclear etiology (Table 1). Fetal atrioventricular block is classified according to its severity as first-degree, second-degree (incomplete) or third-degree (complete block).
Table 1.
Studies of prognosis in congenital fetal atrioventricular block
| Study | Number of fetuses | |||||
|---|---|---|---|---|---|---|
| Total | LAI | TOP | IUFD | NND | Survival to 28 days (%) | |
| Structural heart defects and atrioventricular block | ||||||
| Berg27 | 32 | 31 | 22 | 3 | 3 | 4 (12.5) |
| Lopes31 | 59 | 40 | 1 | 23 | 20 | 15 (25.0) |
| Zhao42 | 8 | 6 | 0 | 0 | 5 | 3 (37.5) |
| Schmidt111 | 29 | 17 | 5 | 24*‡ | NR | 4 (14.0) |
| Jaeggi112 | 24 | 18 | 8 | 7 | 5 | 4 (17.0) |
| Eronen113 | 4 | NR | 0 | 2 | 3‡ | NR |
| Machado114 | 21 | 18 | 9 | 4 | 5 | 3 (14.0) |
| Gembruch115 | 17 | 6 | NR | NR | NR | 3 (18.0) |
| Maeno116 | 17 | 14 | 3 | 1 | 3 | 10 (59.0) |
| Isoimmune atrioventricular block | ||||||
| Berg27 | 20 | NA | 2 | 1 | 1 | 16 (80.0) |
| Lopes31 | 41 | NA | 0 | 6 | 7‡ | 32 (78.0) |
| Friedman36 | 40 | NA | 4 | NR | NR | 36 (90.0) |
| Zhao42 | 18 | NA | 1 | 0 | 0 | 17 (94.0) |
| Fesslova63 | 28 | NA | 0 | 2 | 2 | 24 (86.0) |
| Schmidt111 | 19 | NA | NR | NR | NR | NR |
| Jaeggi112 | 35 | NA | 2 | 4 | 4‡ | 25 (71.0) |
| Eronen113 | 14 | NA | 0 | 2 | 3 | 13 (72.0)§ |
| Machado114 | 16 | NA | 1 | 3 | 0 | 12 (75.0) |
| Maeno116 | 22 | NA | 2 | NR | 2‡ | NR |
| Idiopathic isolated atrioventricular block | ||||||
| Baruteau7 | 23 | NA | NR | NR | NR | 23 (100) |
| Berg27 | 8 | NA | 1 | 2 | 2 | 3 (43.0) |
| Lopes31 | 16 | NA | NR | NR | NR | 12 (75.0) |
| Schmidt111 | 7 | NA | NR | NR | NR | NR |
| Jaeggi112 | 4 | NA | NR | NR | NR | NR |
| Maeno116 | 9 | NA | NR | NR | NR | NR |
Total for both IUFD and NND.
Could include patients with isoimmune atrioventricular block as well as idiopathic isolated atrioventricular block.
Survival for total group.
Abbreviations: IUFD, intrauterine fetal demise; LAI, left atrial isomerism; NA, not applicable; NND, neonatal death; NR, not reported; TOP, termination of pregnancy.
Atrioventricular block and structural defects
The prognosis for fetal atrioventricular block related to congenital heart defects remains extremely poor (Table 1), with a combined fetal and neonatal mortality of >80%.28 This rate is notably higher than that for complex fetal structural heart disease without heart block (30–55%).29 Left atrial isomerism confers the highest mortality in relation to atrioventricular block, at 50% to ≥90%, but anecdotal experience suggests the outlook with levo-transposition of the great arteries and with atrioventricular canal defects might be slightly better.29 The reason for the poor prognosis of all these conditions is not entirely clear. In a fetus with atrioventricular block and congenital heart defects who survives into the neonatal period, the response to hemodynamic compromise from cardiac or noncardiac surgical procedures and to stress is abnormal and often leads to abrupt, profound, and ultimately lethal changes in clinical status. This outcome seems to occur with or without pacing; indeed, pacemaker implantation itself may initiate a downhill spiral in the baby’s condition. Attempts to alter this natural history by early delivery, pacing at a reduced ventricular rate, use of dual-chamber pacing, and other supportive therapies have been largely ineffective. Research into congenital heart disease with atrioventricular block is needed to understand the basis of the poor prognosis associated with this condition, and to outline new, early treatments.
Isolated atrioventricular block
Isolated, complete atrioventricular block without SSA/Ro antibody positivity seems to have the best long-term prognosis.7,30 The etiology of this type of block is not clear. Lopes et al. reported that spontaneous regression of atrioventricular block in utero is possible in fetuses whose mothers remained seronegative for antinuclear antibodies throughout pregnancy.31 Baruteau et al. reported interim results from the French Multicenter Study7 that showed a distinct survival benefit for this subgroup of patients, with nearly 100% survival in 120 children, 23 of whom presented in utero or in the first month of life. Pacemaker insertion was required in 102 children (85%), among whom 16 (13%) were within their first year. Although the final results from this study have not yet been published, isolated, incomplete nonisoimmune atrioventricular block (as was initially seen in 37% of this group of children) frequently progressed to third-degree atrioventricular block during childhood.7
Isoimmune atrioventricular block
Isoimmune atrioventricular block develops as a result of placental transfer of autoantibodies produced by the mother that target ribonucleoproteins of unknown function. Autoantibodies that target SSA/Ro and SSB/La are termed ‘antinuclear antibodies’, but ~70% of these ribonucleoproteins are actually situated in the cytoplasmic compartment. Antibodies produced against the 48 kDa SSB/La peptide and the two 52 kDa and 60 kDa SSA/Ro peptides seem to be the most damaging to the fetal and neonatal conduction system.32 Complete atrioventricular block occurred in female mice immunized with recombinant proteins or antigens.33 In isolated perfused fetal hearts from their progeny, both initial functional block and subsequent permanent damage to the conduction system were observed. An initially reversible alteration in L-type calcium channels was present, followed by apoptosis and cell death.33 This damage targets, but is not confined to, the conduction system. Presumably, reversal can be facilitated during this period of functional block. Buyon and Clancy proposed that the damage that occurs in utero is followed with adaptations to this damage that occur after birth, when the heart’s load state is increased.34 These adaptations lead to changes in both conduction and function in the neonatal period and beyond.34 Hence, while most patients have complete atrioventricular block, some infants progress from second-degree to third-degree atrioventricular block or develop ventricular dysfunction. These events may occur even after autoantibody levels have dropped substantially, which suggests that a remodeling process rather than ongoing postnatal inflammation is the etiology of the worsening conduction system disease. While SSA/Ro autoantibodies can be present in breast milk, they are generally inactivated by enzymes in the gut, and hence are not thought to be responsible for postnatal progression of the disease.35 Friedman and colleagues have reported the results of a large series of antenatally monitored cases of atrioventricular block in the multi-institutional PRIDE (PR Interval Dexamethasone) study.36 This study delineated markers for severe disease and assessed late progression of incomplete atrioventricular block. First-degree and second-degree atrioventricular block have brief windows of reversibility, and early detection is important to enable treatment, but third-degree atrioventricular block is considered irreversible.37,38 Reversal of second-degree block has been achieved antenatally;31,36 for example Friedman et al. reported regression of second-degree atrioventricular block in two of six fetuses treated with dexamethasone, but despite this treatment three of the fetuses (50%) progressed to complete atrioventricular block (one prenatally, two postnatally).36,40
Detecting the onset of fetal heart block in SSA/Ro-positive mothers is a tremendous challenge. Initially, weekly echocardiograms were thought to be adequate to detect a gradual PR prolongation and eventual block; however, some fetuses with normal PR intervals can develop complete atrioventricular block in a matter of days (Figure 2) with no preceding first-degree block.40 By contrast, when first-degree atrioventricular block is seen, it rarely progresses to more substantial block.39,40,41 This feature is a highly specific marker for SSA/Ro or SSB/La exposure, although it is poorly sensitive.42 Motta and colleagues evaluated 51 newborn babies exposed to maternal SSA/Ro or SSB/La autoantibodies and found that first-degree atrioventricular block was present in five (10%) of them, but in none of 50 control (unexposed) infants.39 In other series, the most prevalent initial presentation was complete atrioventricular block.42,43 Echo-bright regions of the fetal myocardium with poor ventricular function, which are considered to be the fetal echocardiographic equivalent of endocardial fibroelastosis,36,40 are often seen in conjunction with fetal atrioventricular block and confer a poor prognosis (mortality 69%).44–48
Figure 2.
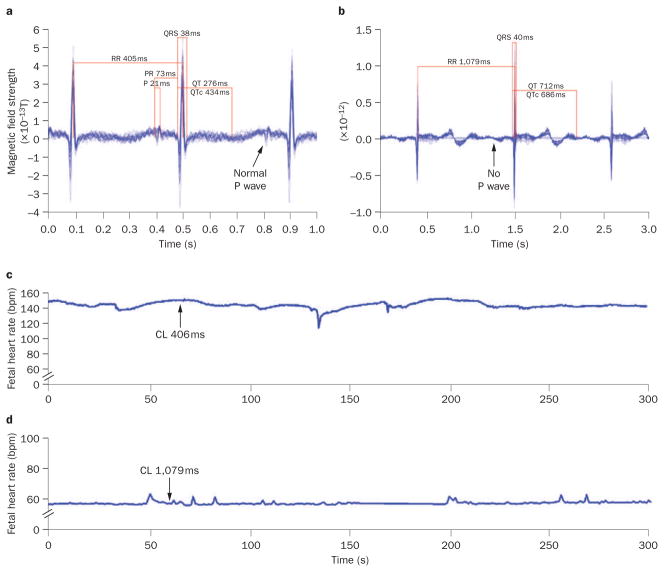
Signal-averaged electrograms of fetal magnetocardiography recordings from a fetus with maternal SSA/Ro autoantibody isoimmunization. a | Sinus rhythm is present at 19 weeks of gestation. b | At 20 weeks, complete atrioventricular block had developed with a prolonged QTUc interval of 686 ms. The P wave appears absent because it is signal-averaged away when not stably associated with the QRS interval. c | At 19 weeks, the fetal heart rate trend over 5 min (arrow) documents stable sinus rhythm at ~145 bpm. d | At 20 weeks, the fetal heart rate trend over 5 min (arrow) shows third-degree atrioventricular block at 55 bpm. Abbreviation: CL, cycle length.
In about 2% of pregnancies among patients with SSA/ Ro or SSB/La autoantibodies, the fetus will develop atrioventricular block.38 Given the low prevalence of rheumatic diseases such as systemic lupus erythematosus (which are associated with increased positivity for these autoantibodies) and the fact that only a small proportion of asymptomatic pregnant women are positive for SSA/Ro or SSB/La autoantibodies, screening with serial echocardiography can be a costly option, especially since such screening cannot prevent the development of atrioventricular block in most fetuses. The risk of recurrence for atrioventricular block when a prior fetus has been affected is 19%.40 In this group, therefore, close observation with serial echocardiography is warranted.
Perhaps the most serious presentation, seen with or without atrioventricular block, is the presence of repolarization abnormalities.9 In our series of 19 fetuses with isoimmune atrioventricular block, 14 (73%) had QT intervals (corrected according to the Bazett formula49) of ≥0.5 s. Postnatal QT interval prolongation has also been reported, and probably contributes to the increased incidence of sudden cardiac arrest recognized in this group of patients.50 Determination of maternal vitamin D status, which can be suboptimal in patients with systemic lupus erythematosus because of their limited sun exposure, is important because low levels of this vitamin may contribute to an altered calcium balance, which can affect fetal QT intervals in our experience. The incidence of intrauterine fetal death in mothers with systemic lupus erythematosus has declined to reflect that of the general population provided that pregnancy is avoided during active disease or in the presence of renal and other organ dysfunction.51
In a patient with SSA/Ro-related fetal cardiac manifestations, follow-up with fMCG is advantageous to evaluate depolarization and repolarization abnormalities, verify the degree of block, and assess myocardial hypertrophy;42,43,52–58 however, this technique is still not readily available to clinicians in most centers. If fMCG is unavailable, such pregnancies should be monitored closely by fetal echocardiography to assess mechanical PR interval11 and to look for evidence of endocardial fibroelastosis, tricuspid valve regurgitation, cardiac dysfunction, or ectopy mimicking atrioventricular block.19,36 Since repolarization abnormalities (such as a prolonged QT interval) cannot be recognized without fECG or fMCG, and since these abnormalities are usually seen in association with other cardiac manifestations such as junctional tachycardia that typically have an onset at 18–25 weeks’ gestation, avoidance of medications that prolong the QT interval is prudent in these women. Optimizing general nutrition throughout pregnancy can prevent development of fetal arrhythmia-triggering conditions, such as hypomagnesemia and hypocalcemia.
First-degree atrioventricular block, however, is not always seen before onset of second-degree or third-degree atrioventricular block (Figure 2). In addition, we have observed a lack of progression from second-degree to third-degree atrioventricular block in utero, and we believe that some cases of presumed second-degree block are actually third-degree block with nonsustained junctional or ventricular tachycardia (which is seen in 30% of fetuses with early isoimmune atrioventricular block), or with stable ventricular ectopy, which is seen in 70% of fetuses with this condition (Figure 3).42 Cases of true second-degree block are usually associated with fetal ventricular rates >70 bpm. Fetuses seem to progress through adaptive phases in the evolution of atrioventricular block. The first phase is instability directly following the onset of block, where the fetus is adapting to a sudden drop in heart rate. This stage is characterized by frequent junctional and ventricular tachyarrhythmias in up to 30% of cases. Instability is followed by a compensated phase and, finally, possible further instability near the time of delivery in fetuses with low ventricular rates.59
Figure 3.
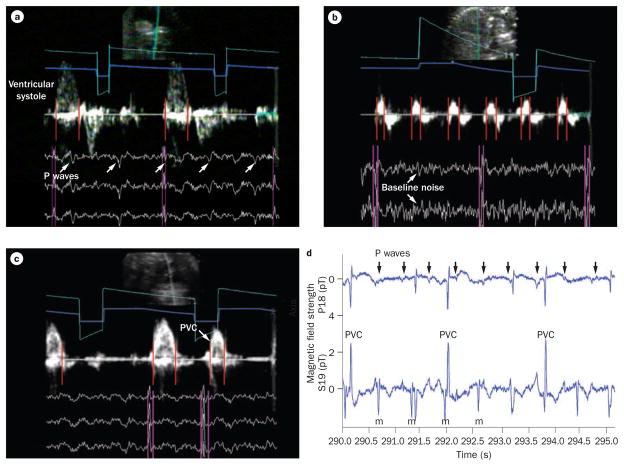
Simultaneous fetal magnetocardiography and Doppler echocardiography recordings from a fetus with isoimmune third-degree atrioventricular block at 23 weeks and 4 days of gestation. The two data types (echocardiographic and magnetocardiographic) were superimposed using timing marker channels (gray and turquoise lines) to provide a single image during postprocessing. QRS complexes (pink vertical lines) precede mechanical atrial wall and ventricular systolic velocities (red vertical lines), which enables interpretation of the electromechanics of the fetal heart. a | P waves are indicated by arrows and are not conducting. b | Baseline noise (arrows) results from Doppler echocardiography frequencies that must be removed during signal processing. Atrial wall velocities (red lines) and QRS complexes indicate atrioventricular dissociation. c | Ventricular systolic Doppler events, including a PVC (arrow). d | A fetal magnetocardiography recording from this fetus shows third-degree atrioventricular block and a narrow QRS duration with frequent ventricular ectopy. P waves (arrows) are not conducting. Abbreviations: m, maternal; PVC, premature ventricular contraction.
Postnatal manifestations of isoimmunization are evident in addition to those of atrioventricular block. These manifestations include neonatal lupus rash or hepatic dysfunction, development of dilated cardiomyopathy, sinus bradycardia, a prolonged corrected QT interval, and cardiac malformations, such as patent ductus arteriosus or atrial septal defect, endocardial fibroelastosis, rupture of the cordae tendonae, and systemic growth restriction among others.45,60–62 For these reasons, early assessment of the fetus and long-term cardiac follow-up of the affected baby is necessary and, in the mother’s subsequent pregnancies, close management and prenatal counseling should be employed. Since the characteristics and prognoses of atrioventricular block seem to differ by etiology, mechanism-based evaluation and counseling is important when the disorder is detected in utero.
Impact of hydrops fetalis on survival
An important distinction in terms of the fetal prognosis is whether hydrops fetalis is present. This condition, in the presence of atrioventricular block, is almost uniformly fatal. Pericardial effusion related to the inflammatory process early in the presentation of isoimmune heart block may mimic hydrops, but in such cases ascites or pleural effusions are absent. Fetuses with third-degree atrioventricular block and nonreactive ventricular rates of <55 bpm (Figure 2) are at the greatest risk of hydops fetalis or neonatal cardiac failure. Terbutaline has been used to augment the fetal heart rate,55 but at present, few treatments are available.
Intervention
The management of fetal atrioventricular block has undergone a number of advances. In utero, terbutaline has been used to raise the heart rate to >55 bpm, or in fetuses with signs of hydrops.55 This treatment has not, however, been proven to alter the risk of fetal or neonatal death. The addition of a beta-agonist agent in the setting of a low heart rate could trigger fetal tachyarrhythmias, and should initially be used with close echocardiographic monitoring.
The use of steroids to treat fetal atrioventricular block is controversial and at present no clear standard of care exists. Owing to the irreversibility of third-degree atrioventricular block, steroids are infrequently used to treat it, except when myocardial dysfunction or hydrops fetalis are also present. Rein and colleagues suggested that mothers who had previously had a fetus with atrioventricular block should receive steroid treatment in subsequent pregnancies if fetal mechanical PR intervals ≥150 ms develop.11 Our practice is not to treat first-degree fetal atrioventricular block with steroids. Second-degree fetal atrioventricular block may be reversible, and use of steroids in this setting is less controversial. Still, concerns regarding growth restriction and maternal adverse effects warrant further research to deduce the true benefit of steroid treatment.60,63 Pacing techniques and equipment for neonatal treatment after identification of atrioventricular block during gestation continue to improve. The strongest predictor of need for pacing is the presence of a nonreactive fetal heart rate (which is nearly always seen when fetal rates are <55 bpm). Fetuses can deteriorate from a reactive to a nonreactive pattern during the second or third trimesters of pregnancy.42 Standard guidelines for neonatal pacemaker implantation have been published.64 Nearly all fetuses with congenital heart disease and those with nonreactive heart rates warrant early pacing.27,42,65 Attempts at fetal temporary pacing have been described in two studies.66,67 In the case reported by Carpenter and colleagues, only 4 h of pacing was achieved.66 In the other report by Walkinshaw et al. pacing lasted for >36 h.67 Unfortunately, both fetuses died, probably owing to arrhythmia and pericardial tamponade, respectively. Work is being done to develop leads and devices to improve fetal pacing.68–71
Fetal tachycardia
Re-entrant supraventricular tachycardia
The most common fetal tachycardia is re-entrant supraventricular tachycardia (Supplementary Figure 2).72–75 Hahurij and colleagues demonstrated that transient atrioventricular connections are normal during early human fetal development, but noted that persistence of such connections into late gestation could facilitate reentrant tachycardia.76 The presence of fetal ectopic atrial contractions (which are seen in 1–2% of pregnancies) may trigger supraventricular tachycardia, which is generally seen between 24 and 32 weeks of gestation. When supraventricular tachycardia breaks to sinus rhythm, the rate should return to normal for gestational age (in the absence of drug therapy). When fetal bradycardia occurs in conjunction with fetal tachycardia, long QT syndrome or inflammatory conditions, such as SSA/ Ro autoantibody exposure, should be suspected. In this circumstance, the choice of antiarrhythmic therapy can be complicated, for example, by the need to avoid medications that prolong the QT interval. In general, the best indication of alternative tachycardia mechanisms is intermittently or consistently altered atrioventricular or ventriculoatrial conduction observed by echocardiography. When atrioventricular or ventriculoatrial block is present during tachycardia (Figure 4), atrioventricular re-entrant supraventricular tachycardia is excluded and other forms of tachycardia should be suspected.
Figure 4.
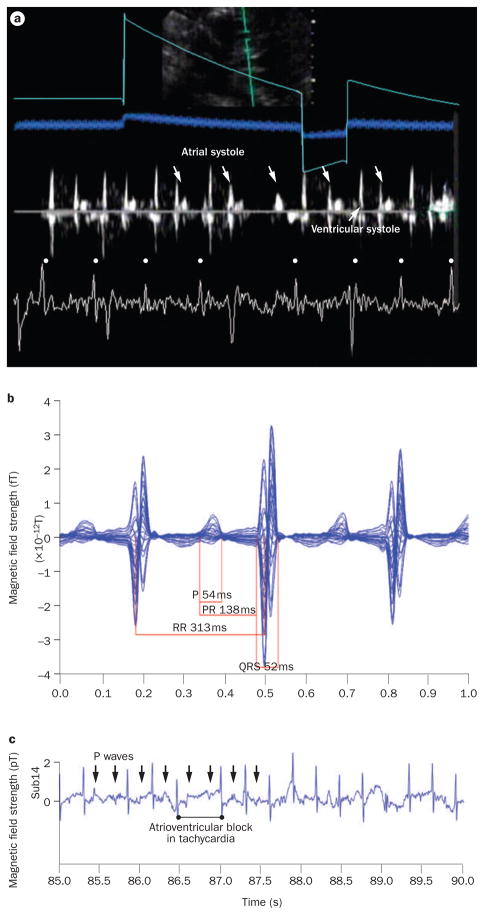
Combined fetal magnetocardiography and Doppler echocardiography tracing from a fetus with atrial ectopic tachycardia and intermittent atrioventricular block (during tachycardia) at 25 weeks and 5 days of gestation. a | A simultaneous echocardiography and fetal magnetocardiography tracing from this fetus showed atrial systole wall motion events (down-facing arrows) that documented the persistence of atrial activity during atrioventricular block of tachycardia. QRS complexes in the fetal magnetocardiography tracing are indicated by dots. b | Fetal magnetocardiography 20 s signal-averaged recording (1 s display). The atrial cycle length was ~313 ms (heart rate 192 bpm). c | A fetal magnetocardiography rhythm tracing from the same fetus, showing atrioventricular conduction block during tachycardia with perpetuation of atrial activity (arrows indicate P waves).
Atrial flutter
Atrial flutter is seen in about 30% of fetuses with tachyarrhythmia. Most fetuses with prenatal atrial flutter have an associated accessory atrioventricular connection,73,77 and can develop atrioventricular re-entrant supraventricular tachycardia in utero or postnatally. The atrial rate for atrial flutter is usually >400 bpm, and is usually sustained with variable atrioventricular block, whereas the atrial rate for other atrial tachycardias are either nonsustained (chaotic atrial tachycardia) or sustained at atrial rates of 180–240 bpm (atrial ectopic tachycardia).59,65,78
Other forms of tachycardia
Atrial ectopic tachycardia is a form of primary atrial tachycardia that arises in the atrium. In this condition, the atrioventricular node is passively activated (Figure 4). Other forms of tachycardia in which the atrioventricular node is a passive bystander include chaotic atrial tachycardia, junctional ectopic tachycardia, and ventricular tachycardia. Junctional and ventricular tachycardias are rare, but are becoming increasingly detected in utero.10,22,42,79,80 These forms of tachycardia are usually manifestations of SSA/Ro or SSB/La autoantibody-related myocarditis,10 active viral myocarditis, or genetic ion channelopathies.10,81,82
Treatment regimens
Fetal tachycardia of any form that is intermittent, not accompanied by cardiac or valve dysfunction, and present <50% of the time is best not treated, but needs to be monitored closely.83 Fetal heart rates should generally be measured in hospital by a medical professional. After instruction, mothers can utilize hand-held Doppler monitors to take measurements twice-daily at home; they should be asked to contact their obstetrician if the amount of time spent in fetal tachycardia substantially increases.84 Echocardiographic follow-up should initially happen at least twice weekly to look for any changes in fetal status.
Maternally administered antiarrhythmic agents are used to treat fetal tachycardia (Table 2). Treatment of fetal ventricular tachycardia depends on its etiology; fetal ventricular tachycardia related to torsades de pointes has been treated with maternally administered intravenous magnesium, lidocaine, and oral propranolol.1,81 If the ventricular tachycardia is thought to be associated with long QT syndrome, avoidance of drugs that prolong the QT interval further is critical, since such drugs can promote arrhythmia.10,22,42,79 Other forms of fetal monomorphic ventricular tachycardias behave differently. If the rate of the ventricular tachyarrhythmia is little more than the sinus rate, observation alone might be the best option. If the rate is >220 bpm, use of sotalol or amiodarone may be effective.78,85–87 Flecainide may also be effective, but should be used with caution in the presence of ventricular dysfunction, owing to an increased risk of adverse events (conduction disturbances, cardiac arrest, and death) associated with this agent.59,88–90 A short (1–2 week) course of dexamethasone 4–8 mg daily has been beneficial in cases where viral or isoimmune myocarditis is suspected.81,82
Table 2.
Therapies for fetal tachycardia
| Condition and drug | Dose range | Therapeutic level and effect | Adverse effects |
|---|---|---|---|
| Atrial flutter | |||
| Digoxin | LD 1,200 μg per 24 h IV in 3 divided doses; MD 375–750 μg daily PO in two equal daily doses | 0.7–2.0 ng/ml Mild nausea, fatigue and sinus bradycardia, first-degree atrioventricular block, rare nocturnal Wenchebach atrioventricular block |
Severe nausea and/or vomiting, severe sinus bradycardia or atrioventricular block, maternal and fetal proarrhythmia |
| Sotalol | 160–480 mg daily in two equal daily doses or every 8 h PO | NA Improved survival, bradycardia, first-degree atrioventricular block, P and QRS widening, QT prolongation |
Nausea and/or vomiting, dizziness, fatigue, bundle branch block, maternal and/or fetal proarrhythmia |
| Supraventicular tachycardia* | |||
| Flecainide‡ | 100–300 mg daily in equal doses given every 8 h PO | 0.2–1.0 μg/ml Mild IVCD, headache, QT prolongation |
Visual and central nervous system symptoms, bundle branch block, QT prolongation, fetal or neonatal proarrhythmia |
| Amiodarone‡ | LD 1,800–2,400 mg daily in equal doses given every 6 h for 48 h; MD 200–600 mg daily PO | 0.7–2.8 μg/ml Maternal and/or fetal sinus bradycardia, first-degree atrioventricular block, P and QRS widening, QT prolongation |
Nausea and/or vomiting, maternal and/or fetal thyroid dysfunction, photosensitivity rash, thrombocytopenia, bundle branch block, proarrhythmia, torsades de pointes in fetuses with long QT syndrome |
| Procainamide‡ | LD 500–600 mg over20 min IV; MD 2–6 mg/min IV Initially 1,250 mg, followed in 1 h by 750 mg, then 250–1,000 mg every 3–6 h PO | 4–10 μg/ml Uterine irritability, QT prolongation |
Nausea and/or vomiting, hypotension, proarrhythmia, blood dyscrasias |
| Ventricular tachycardia‡ | |||
| Magnesium sulfate81 | LD 2–4 g IV followed by 1–2 g/h IV | 1.5–3.0 mmol/l | Fatigue, central nervous system symptoms, stop for loss of patellar reflex at levels of 3.5–5.0 mmol/l, cardiac arrhythmias at high levels |
| Lidocaine | LD 1.0–1.5 mg/kg IV followed by 1–3 mg/min IV | 1.5–5.0 μg/ml | Central nervous system symptoms |
| Propranolol | 40–80 mg every 8 h PO | NA Increased uterine tone, bronchoconstriction |
Fatigue, bradycardia, hypotension |
| Dexamethasone | LD 4–8 mg PO; MD 4 mg daily | NA Fluid retention |
Similar to other corticosteroids. Short-term use preferred, fetal growth restriction |
| Sotalol | 160–480 mg daily in two equal daily doses or every 8 h PO | NA Bradycardia, first-degree atrioventricular block, P and QRS widening, QT prolongation |
Nausea and/or vomiting, dizziness, fatigue, bundle branch block, maternal and/or fetal proarrhythmia |
Sotalol or digoxin dosed as for atrial flutter are also commonly used to treat supraventricular tachycardia.
These doses of amiodarone, flecaininde and procainamide can be used to treat either ventricular or supraventricular tachycardias.
Abbreviations: IV, intravenous; IVCD, intraventricular conduction delay; LD, loading dose; MD, maintenance dose; PO, oral.
Fetal supraventricular tachycardia is clearly one of the success stories of fetal drug intervention. Survival in excess of 90% is now possible when existing guidelines are implemented.65,79,91 No studies have, however, systematically compared long-term neurodevelopmental outcomes associated with the various regimens in children. Furthermore, some controversy exists in relation to first-line and second-line treatment regimens for fetal supraventricular tachycardia. In general, digoxin is used as first-line treatment in the US, whereas in Europe flecainide is used for this purpose. For second-line treatment of drug-refractory supraventricular tachycardia with hydrops fetalis, Parilla and colleagues advocate early use of fetal intramuscular digoxin.91 This treatment substantially reduced the time to conversion91 compared with that achieved with maternal intravenous or oral digoxin, alone79 or in combination with amiodarone,67 without increasing mortality. For fetuses without ventricular dysfunction, flecainide or sotalol have been used as second-line agents, and both have efficacies of 60–80%; however, for fetuses with ventricular dysfunction or severe atrioventricular valve regurgitation, amiodarone is the most effective drug (>93% efficacy alone or in combination), and is associated with a low risk of proarrhythmia.79,87
A number of reports describe fetal umbilical cord or intramuscular injection of various antiarrhythmic agents.91–93 The risks associated with umbilical cord injection have outweighed the advantages in most cases, and the monitoring of drug levels in the umbilical cord has not improved fetal survival. Fetal intramuscular injection of digoxin allows rapid attainment of therapeutic concentrations of the drug in fetuses with hydrops fetalis, and can be used before or as an adjunct to more-potent medications in fetuses with hydrops fetalis due to tachycardia.91
Maternal or fetal adverse effects are common with fetal drug treatments but, in most healthy young women, these are generally tolerable and reversible. Amiodarone is associated with maternal and neonatal thyroid dysfunction.94 Sotalol and flecainide may be more pro-arrhythmic than amiodarone,85,95–97 and fetal sudden deaths have been reported with all these drugs, except (to our knowledge) amiodarone. In addition, neonatal electrocardiographic changes have been associated with flecainide treatment.95–97 Maternal adverse effects are usually mild with sotalol and flecainide, but electrocardiographic changes should be monitored with all antiarrhythmic agents. The use of β-blockers would seem to be the ideal drug treatment for tachycardia, but poor placental transfer hinders the effectiveness of these agents. Propranolol has been associated with increased uterine tone, whereas pro-cainamide, a Class IA antiarrhythmia agent, can increase uterine contractions.59,78 Finally, digoxin, which seems to be associated with the least risk to the mother and fetus, causes nausea in some patients when used in the doses needed to achieve fetal conversion.91 The most effective fetal treatment for atrial flutter is sotalol; however, in mild cases of atrial flutter, digoxin alone may be adequate.73,85,86,98,99 Amiodarone converts only 30% of fetal atrial flutter cases, but can slow the ventricular response.79
Fetal ectopy
Once considered an entirely benign arrhythmia, fetal ectopy is now thought to be a manifestation of a number of diseases. Cuneo and colleagues reported a 2.6% incidence of first-degree atrioventricular block in fetuses with isolated ectopy.56 Fetuses with suspected ectopy were diagnosed as having other serious diseases, such as second-degree atrioventricular block and long QT syndrome. Ectopy should not, therefore, be dismissed as benign without fetal assessment, especially if risk factors such as a family history of sudden death, prior fetal loss, or maternal pregnancy complications exist. Whether ectopy represents spontaneous automaticity of the atrium or re-entry is unclear. When coupling of the ectopic beat to the prior QRS is fixed, as opposed to variable, it is likely to be related to a re-entrant atrioventricular pathway; in this setting, the risk of supraventricular tachycardia is about 0.5% for simple ectopy (isolated, bigeminy, or trigeminy)78,100 (Figure 5) and up to 6% for complex ectopy (atrial couplets or triplets). This increased risk of supraventricular tachycardia extends into the neonatal period, although most ectopy resolves by 1 month of age. While atrial ectopy is about 10 times more common than ventricular ectopy, Doppler echocardiography alone is often insufficient to distinguish these two conditions, and M-mode echocardiography is useful in such cases.101 Ventricular ectopy is more likely than atrial ectopy to be noncoupled, late, or associated with atrioventricular valve regurgitation, echogenic myocardium, or ventricular dysfunction.
Figure 5.
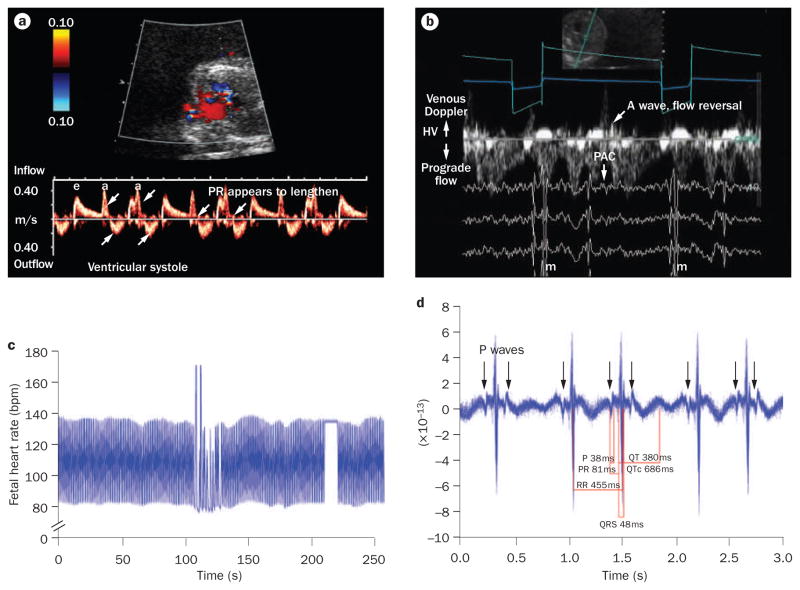
Fetal magnetocardiography and Doppler echocardiography tracings from a fetus with a rhythm pattern that simulated Mobitz type I second-degree atrioventricular block at 18 weeks and 1 day of gestation. This fetus presented with tachyarrhythmia (not shown) of 260 bpm. a | Doppler echocardiography of the left ventricular inflow and outflow shows that atrioventricular conduction appears prolonged and followed by dropped ventricular events (upward arrows) and hidden atrial events (downward arrows). b | Simultaneous fetal magnetocardiography and Doppler echocardiography demonstrates venous flow reversal (diagonal arrow) consistent with cannon A waves after alternate QRS complexes that are caused by blocked PACs (down-facing arrows). c | The fetal heart rate trends show persistence of this alternating rhythm over most of the 5 min recording. d | A 20 s signal-averaged trace of the QRS interval before the pause led to a final diagnosis of blocked atrial trigeminy. Abbreviations: a, atrial; e, passive inflow; HV, hepatic vein; PAC, premature atrial contraction.
Blocked atrial bigeminy can accompany isolated ectopy, and can be present for prolonged periods in the fetus. Blocked atrial bigeminy can usually be easily distinguished from sinus bradycardia or atrioventricular block by examination of the Doppler flow pattern in the inferior vena cava or hepatic veins, which shows flow reversal. Neither isolated ectopy nor blocked atrial bigeminy require medical treatment, and are not usually associated with cardiac failure.78
Fetal genetic ion channelopathies
Stillbirth and intrauterine fetal demise after 20 weeks’ gestation are linked to known or suspected ion channelopathy syndromes in ~10% of cases, on the basis of genetic findings at autopsy.5,102,103 Among infants who presented with such syndromes prenatally, however, the incidence of mutations at unidentified genetic loci is increased to about 25%, which suggests that such early presentation indicates a severe form of disease that results in under-representation of these mutations in the postnatal human population. Greater application of recording tools, such as fMCG or fECG, may improve prenatal detection and allow identification and prenatal treatment of highly lethal variants. In addition, the diligent application of gestational-age-dependent criteria16,17 for bradycardia will help to identify the subset of fetuses with inappropriately low heart rates. Short QT syndrome in utero has not been reported. In their examination of data from the International Long QT Syndrome Registry,103 which is dominated by patients with long QT syndrome type 1, Rashba et al. suggested that intra-uterine fetal demise is not more common than in the general population, but noted that a number of rare variants of ion channelopathy syndromes associated with fetal or neonatal demise have been reported.103 SCN5A defects and mutations in SCN5 beta subunits (associated with long QT syndrome type 3 or Brugada syndrome), have been reported most in the fetus and neonate, along with defects in KCNE1, KCNE2, KCNH1, KCNQ1 and KCNH2 (formerly HERG). Spontaneous and complex heterozygous mutations predominate.5,23,25,102–107
As mentioned above, the presence of fetal bradycardia should raise suspicion of fetal long QT syndrome. Beinder et al. showed that about 70% of individuals who were later diagnosed as having long QT syndrome presented initially with fetal bradycardia or the combination of tachycardia and bradycardia on prenatal records or during labor.18 At present, the frequency of long QT intervals occurring in conjunction with fetal sinus bradycardia is unknown, and investigations with fMCG, fECG, or postnatal electrocardiography are warranted.
Future technologies
Superconducting quantum interference device (SQUID)-based magnetometers are currently used to make fMCG recordings. SQUIDs are extremely sensitive flux-to-voltage converters based on superconductor physics principles. Unfortunately, these devices have several major disadvantages that restrict their widespread clinical application: they are bulky, expensive, and require regular maintenance, which involves replenishing a reservoir of liquid helium. In addition, they must be operated in a magnetically shielded room.
In the past few years, atomic magnetometry (a cheaper and more practical technology than SQUIDs) has been developed.108 Atomic magnetometers use a laser to spin-polarize a gas of alkali atoms. These atoms precess when placed in a magnetic field, and the strength of the magnetic field (which is proportional to the steady-state precession angle) can be measured from the change in polarization of a second laser beam passing through the gas. Atomic magnetometers have the disadvantages that they must be operated in a near-zero magnetic field and the alkali atoms must be heated to around 200 °C. Magnetic shielding is also required, but the compactness of atomic magnetometers may permit local shielding of the patient, rather than shielding of the entire room. Since magnetic field strength is proportional to the inverse square of the distance from the field source, placement of the recording system close to the source of the magnetic signal could have a dramatic impact on the signal-to-noise ratio. A transvaginal recording SQUID is currently being developed for study in pregnant sheep, and use of such a transvaginal device or atomic magnetometry could serve to establish fetal cardiac electrophysiology at the gestation stages when most fetal losses occur, as well as to improve fetal cardiac monitoring in obese patients.
Another new approach is mobile truck-based fMCG. This technique is a reasonably straightforward extension of other mobile medical imaging systems, such as MRI and nuclear medicine devices, although some issues remain to be addressed with regard to magnetic shielding and robustness of the instrumentation. Our research group is currently collaborating with a small consortium of companies and universities to develop the first mobile system for fMCG. Mobile technology may enable the provision of preintervention evaluations and same-day or next-day follow-up evaluations for pregnant patients who are undergoing fetal cardiac and noncardiac surgical interventions.
Two technologies once thought to be completely incompatible, fMCG and Doppler echocardiography, can now be simultaneously recorded and their signals electronically superimposed during postprocessing (by use of a timing signal in both recordings to allow their alignment.) The combined images provide a comprehensive electromechanical assessment of the human fetal heart (Figures 2, 4 and 5).
Chan and colleagues have published promising preliminary work in the field of fetal stem-cell therapy that could ultimately lead to in utero fetal treatments for potentially fatal ion channelopathies and other genetic diseases.109 Stem-cell transplantation must be done late in the first trimester, while the fetus is still immunologically immature, to be most effective. Fetal bradycardia associated with stem-cell harvesting has, however, been reported in up to 33% of fetuses, which again emphasizes the need for and importance of fetal periprocedural electrophysiological monitoring. Such monitoring could provide clues to the etiology of fetal bradycardia. Transvaginal fMCG recording has the potential to facilitate improved monitoring for bradycardia, ischemia, or arrhythmias in the future.
Conclusions
While mortality in the neonatal, infant, and child populations has declined substantially over the past three decades, fetal mortality over the same period shows only a modest decrease. This disparity is largely due to the lack of advanced medical technologies to improve diagnosis and therapy for the fetus. New models of subspecialty consultation in the care continuum of the fetus are now being implemented, whereby pediatric subspecialists take the lead in directing the care of the fetus when the disease state is primarily confined to the fetus. Pediatric-focused or pediatric-based perinatal centers enable hospitalization of patients with high-risk pregnancies in a setting where the fetal to neonatal transition can be optimized. Fetal cardiac, neuroscience, and surgical research centers, where interdisciplinary teams of scientists and clinicians work side by side using advanced methods of fetal diagnosis and management, are seen in only a few centers and could be expanded. Research emphasis should be placed on the development of bioinstrumentation technology, advances in medical imaging, drug delivery tools, and altered medical care models for the fetus. Determining how best to treat serious fetal arrhythmias and other life-threatening fetal diseases—and to identify when such treatments would be most effective—should be a priority, because the medical consequences of unrecognized fetal diseases are costly to families and to our health-care systems.
Medscape CME Continuing Medical Education online.
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Nature Publishing Group. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this educational activity for a maximum of 1.0 AMA PRA Category 1 Credits™. Physicians should only claim credit commensurate with the extent of their participation in the activity. All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at http://www.medscapecme.com/journal/nrcardio; and (4) view/print certificate.
Learning Objectives
Upon completion of this activity, participants should be able to:
Describe categories of fetal arrhythmias.
Identify the most widely used methods of assessing fetal arrhythmias.
Describe the types of fetal bradyarrhythmias.
Identify triggers for fetal arrhythmias during pregnancy.
Describe treatment approaches for fetal tachycardia.
Key points.
Fetal demise related to cardiac depolarization or repolarization abnormalities may be preventable
Genetic ion channel diseases often present with fetal bradycardia
The prognosis of patients with complete atrioventricular block depends on the etiology of this condition
Fetal magnetocardiography enhances diagnosis and treatment
Review criteria.
Articles in the English language or with abstracts in English published from January 1994 to November 2009 were selected from MEDLINE and PubMed databases on the basis of their citation frequency. Older references and publications (1966–1994) that contributed to the present understanding of disease processes were included on the basis of the authors’ personal experience. Search terms included (alone and in combination) “fetus”, “fetal”, “arrhythmia”, “tachycardia”, “atrial flutter”, “congenital heart block”, “bradycardia”, “junctional ectopic tachycardia”, “ventricular tachycardia”, “long QT syndrome”, “genetic ion channelopathy”, “drugs”, “antiarrhythmic medications”, “magnetocardiography”, “electrocardiography”, “echocardiography”, “stillbirth”, “fetal demise”, “hydrops fetalis”, and “prematurity”.
Supplementary Material
Acknowledgments
Ours is team science. We acknowledge Nana Aba Mensah Brown, Suhong Yu, Hui Zhao, Zhimin Li, Zhen Ji, Medical Physics graduate students U. W. Madison who processed the data, William Lutter, Medical Physicist who supported the technology infrastructure, Gretchen Eckstein, research obstetrics nurse, Bageshree Cheulkar, research assistant to Dr Strasburger, Bettina Cuneo, interpretation and patient management, and the pediatric cardiologists and maternal fetal medicine specialists who supported this research through their referrals. Grant support includes NIH R01HL63174, R21HD049022, R42HL092755, R44HL082017, R43HD055091, and the Advancing a Healthier Wisconsin Fund. Désirée Lie, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the MedscapeCME-accredited continuing medical education activity associated with this article.
Footnotes
Supplementary information is linked to the online version of the paper at www.nature.com/nrcardio
Competing interests
The authors and the Journal Editor B. Mearns declare no competing interests. The CME questions author D. Lie has served as a nonproduct speaker for “Topics in Health” for Merck Speaker Services.
Contributor Information
Janette F. Strasburger, Division of Pediatric Cardiology, Department of Pediatrics, Children’s Hospital of Wisconsin, 9000 W. Wisconsin Avenue, Milwaukee, WI 53226, USA
Ronald T. Wakai, Department of Medical Physics, 1163 Wisconsin Institutes for Medical Research, University of Wisconsin–Madison, 1111 Highland Avenue, Madison, WI 53705, USA
References
- 1.Cuneo BF, et al. Prenatal diagnosis and in utero treatment of torsades de pointes associated with congenital long QT syndrome. Am J Cardiol. 2003;91:1395–1398. doi: 10.1016/s0002-9149(03)00343-6. [DOI] [PubMed] [Google Scholar]
- 2.Hamada H, et al. Prenatal diagnosis of long QT syndrome using fetal magnetocardiography. Prenat Diagn. 1999;19:677–680. doi: 10.1002/(sici)1097-0223(199907)19:7<677::aid-pd597>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Horigome H, Iwashita H, Yoshinaga M, Shimizu W. Magnetocardiographic demonstration of torsade de pointes in a fetus with congenital long QT syndrome. J Cardiovasc Electrophysiol. 2008;19:334–335. doi: 10.1111/j.1540-8167.2007.01026.x. [DOI] [PubMed] [Google Scholar]
- 4.Hosono T, Shinto M, Chiba Y, Kandori A, Tsukada K. Prenatal diagnosis of fetal complete atrioventricular block with QT prolongation and alternating ventricular pacemakers using multi-channel magnetocardiography and current-arrow maps. Fetal Diagn Ther. 2002;17:173–176. doi: 10.1159/000048033. [DOI] [PubMed] [Google Scholar]
- 5.Miller TE, et al. Recurrent third-trimester fetal loss and maternal mosaicism for long-QT syndrome. Circulation. 2004;109:3029–3034. doi: 10.1161/01.CIR.0000130666.81539.9E. [DOI] [PubMed] [Google Scholar]
- 6.Beery TA, Shooner KA, Benson DW. Neonatal long QT syndrome due to a de novo dominant negative hERG mutation. Am J Crit Care. 2007;16:412–416. [PubMed] [Google Scholar]
- 7.Baruteau A-E, Study AFM. Non-immune isolated atrioventricular block in childhood: a French multicentric study [Abstract] Heart Rhythm. 2009;39(Suppl):AB19–AB31. [Google Scholar]
- 8.Tester DJ, McCormack J, Ackerman MJ. Prenatal molecular genetic diagnosis of congenital long QT syndrome by strategic genotyping. Am J Cardiol. 2004;93:788–791. doi: 10.1016/j.amjcard.2003.11.061. [DOI] [PubMed] [Google Scholar]
- 9.Zhao H, Strasburger JF, Cuneo BF, Wakai RT. Fetal cardiac repolarization abnormalities. Am J Cardiol. 2006;98:491–496. doi: 10.1016/j.amjcard.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 10.Dubin AM, et al. Congenital junctional ectopic tachycardia and congenital complete atrioventricular block: a shared etiology? Heart Rhythm. 2005;2:313–315. doi: 10.1016/j.hrthm.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Rein AJ, et al. Early diagnosis and treatment of atrioventricular block in the fetus exposed to maternal anti-SSA/Ro–SSB/La antibodies: a prospective, observational, fetal kinetocardiogram-based study. Circulation. 2009;119:1867–1872. doi: 10.1161/CIRCULATIONAHA.108.773143. [DOI] [PubMed] [Google Scholar]
- 12.Rein AJ, et al. Use of tissue velocity imaging in the diagnosis of fetal cardiac arrhythmias. Circulation. 2002;106:1827–1833. doi: 10.1161/01.cir.0000031571.92807.cc. [DOI] [PubMed] [Google Scholar]
- 13.Wakai RT, Lengle JM, Leuthold AC. Transmission of electric and magnetic fetal cardiac signals in a case of ectopia cordis: the dominant role of the vernix caseosa. Phys Med Biol. 2000;45:1989–1995. doi: 10.1088/0031-9155/45/7/320. [DOI] [PubMed] [Google Scholar]
- 14.Acherman RJ, et al. Fetal bradycardia. A practical approach. Fetal Matern Med Rev. 2007;18:225–255. [Google Scholar]
- 15.ACOG Practice Bulletin No. 106: Intrapartum fetal heart rate monitoring: nomenclature, interpretation, and general management guidelines. Obstet Gynecol. 2009;114:192–202. doi: 10.1097/AOG.0b013e3181aef106. [No authors listed] [DOI] [PubMed] [Google Scholar]
- 16.Serra V, Bellver J, Moulden M, Redman CW. Computerized analysis of normal fetal heart rate pattern throughout gestation. Ultrasound Obstet Gynecol. 2009;34:74–79. doi: 10.1002/uog.6365. [DOI] [PubMed] [Google Scholar]
- 17.Robinson SM, Wheeler T, Hayes MC, Barker DJ, Osmond C. Fetal heart rate and intrauterine growth. Br J Obstet Gynaecol. 1991;98:1223–1227. doi: 10.1111/j.1471-0528.1991.tb15393.x. [DOI] [PubMed] [Google Scholar]
- 18.Beinder E, Grancay T, Menéndez T, Singer H, Hofbeck M. Fetal sinus bradycardia and the long QT syndrome. Am J Obstet Gynecol. 2001;185:743–747. doi: 10.1067/mob.2001.117973. [DOI] [PubMed] [Google Scholar]
- 19.Cuneo BF, Strasburger JF, Niksch A, Ovadia M, Wakai RT. An expanded phenotype of maternal SSA/SSB antibody-associated fetal cardiac disease. J Matern Fetal Neonatal Med. 2009;22:233–238. doi: 10.1080/14767050802488220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozkutlu S, Onderoglu L, Karagoz T, Celiker A, Sahiner UM. Isolated noncompaction of left ventricular myocardium with fetal sustained bradycardia due to sick sinus syndrome. Turk J Pediatr. 2006;48:383–386. [PubMed] [Google Scholar]
- 21.Maeno Y, et al. Prenatal diagnosis of sustained bradycardia with 1:1 atrioventricular conduction. Ultrasound Obstet Gynecol. 2003;21:234–238. doi: 10.1002/uog.71. [DOI] [PubMed] [Google Scholar]
- 22.Cuneo BF, Strasburger JF, Wakai RT. Magnetocardiography in the evaluation of fetuses at risk for sudden cardiac death before birth. J Electrocardiol. 2008;41:116.e1–116.e6. doi: 10.1016/j.jelectrocard.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baruteau AE, Schleich JM. Antenatal presentation of congenital long QT syndrome: a prenatal diagnosis not to be missed. Pediatr Cardiol. 2008;29:1131–1132. doi: 10.1007/s00246-008-9271-7. [DOI] [PubMed] [Google Scholar]
- 24.Beinder E, Buheitel G, Hofbeck M. Are some cases of sudden intrauterine unexplained death due to the long QT syndrome? Prenat Diagn. 2003;23:1097–1098. doi: 10.1002/pd.702. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz PJ. Stillbirths, sudden infant deaths, and long-QT syndrome: puzzle or mosaic, the pieces of the jigsaw are being fitted together. Circulation. 2004;109:2930–2932. doi: 10.1161/01.CIR.0000133180.77213.43. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz PJ, et al. Prolongation of the QT interval and the sudden infant death syndrome. N Engl J Med. 1998;338:1709–1714. doi: 10.1056/NEJM199806113382401. [DOI] [PubMed] [Google Scholar]
- 27.Berg C, et al. Atrioventricular block detected in fetal life: associated anomalies and potential prognostic markers. Ultrasound Obstet Gynecol. 2005;26:4–15. doi: 10.1002/uog.1918. [DOI] [PubMed] [Google Scholar]
- 28.Glatz AC, et al. Outcome of high-risk neonates with congenital complete heart block paced in the first 24 hours after birth. J Thorac Cardiovasc Surg. 2008;136:767–773. doi: 10.1016/j.jtcvs.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 29.Cuneo BF. Outcome of fetal cardiac defects. Curr Opin Pediatr. 2006;18:490–496. doi: 10.1097/01.mop.0000245348.52960.11. [DOI] [PubMed] [Google Scholar]
- 30.Villain E, Marijon E, Georgin S. Is isolated congenital heart block with maternal antibodies a distinct and more severe form of the disease in childhood? Heart Rhythm. 2005;2 (Suppl 1):S45. [Google Scholar]
- 31.Lopes LM, et al. Perinatal outcome of fetal atrioventricular block: one-hundred-sixteen cases from a single institution. Circulation. 2008;118:1268–1275. doi: 10.1161/CIRCULATIONAHA.107.735118. [DOI] [PubMed] [Google Scholar]
- 32.Buyon JP, Winchester R. Congenital complete heart block. A human model of passively acquired autoimmune injury. Arthritis Rheum. 1990;33:609–614. doi: 10.1002/art.1780330502. [DOI] [PubMed] [Google Scholar]
- 33.Boutjdir M. Molecular and ionic basis of congenital complete heart block. Trends Cardiovasc Med. 2000;10:114–122. doi: 10.1016/s1050-1738(00)00059-1. [DOI] [PubMed] [Google Scholar]
- 34.Buyon JP, Clancy RM. Dying right to live longer: positing apoptosis as a link between maternal autoantibodies and congenital heart block. Lupus. 2008;17:86–90. doi: 10.1177/0961203307085115. [DOI] [PubMed] [Google Scholar]
- 35.Buyon JP. Neonatal lupus syndrome. In: Lahita RG, editor. Systemic Lupus Erythematosus. 4. Vol. 473. Elsevier Academic; 1998. [Google Scholar]
- 36.Friedman DM, et al. Prospective evaluation of fetuses with autoimmune-associated congenital heart block followed in the PR Interval and Dexamethasone Evaluation (PRIDE) study. Am J Cardiol. 2009;103:1102–1106. doi: 10.1016/j.amjcard.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buyon JP, Clancy RM, Friedman DM. Cardiac manifestations of neonatal lupus erythematosus: guidelines to management, integrating clues from the bench and bedside. Nat Clin Pract Rheumatol. 2009;5:139–148. doi: 10.1038/ncprheum1018. [DOI] [PubMed] [Google Scholar]
- 38.Buyon JP, Clancy RM, Friedman DM. Autoimmune associated congenital heart block: integration of clinical and research clues in the management of the maternal/fetal dyad at risk. J Intern Med. 2009;265:653–662. doi: 10.1111/j.1365-2796.2009.02100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Motta M, Rodriguez-Perez C, Tincani A, Lojacono A, Chirico G. Outcome of infants from mothers with anti-SSA/Ro antibodies. J Perinatol. 2007;27:278–283. doi: 10.1038/sj.jp.7211688. [DOI] [PubMed] [Google Scholar]
- 40.Friedman DM, et al. Utility of cardiac monitoring in fetuses at risk for congenital heart block: the PR Interval and Dexamethasone Evaluation (PRIDE) prospective study. Circulation. 2008;117:485–493. doi: 10.1161/CIRCULATIONAHA.107.707661. [DOI] [PubMed] [Google Scholar]
- 41.Sonesson SE, Salomonsson S, Jacobsson LA, Bremme K, Wahren-Herlenius M. Signs of first-degree heart block occur in one-third of fetuses of pregnant women with anti-SSA/Ro 52 kD antibodies. Arthritis Rheum. 2004;50:1253–1261. doi: 10.1002/art.20126. [DOI] [PubMed] [Google Scholar]
- 42.Zhao H, et al. Electrophysiological characteristics of fetal atrioventricular block. J Am Coll Cardiol. 2008;51:77–84. doi: 10.1016/j.jacc.2007.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gardiner HM, et al. Fetal ECG: a novel predictor of atrioventricular block in anti-Ro positive pregnancies. Heart. 2007;93:1454–1460. doi: 10.1136/hrt.2006.099242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Z, Strasburger JF, Cuneo BF, Gotteiner NL, Wakai RT. Giant fetal magnetocardiogram P waves in congenital atrioventricular block: a marker of cardiovascular compensation? Circulation. 2004;110:2097–2101. doi: 10.1161/01.CIR.0000144302.30928.AA. [DOI] [PubMed] [Google Scholar]
- 45.Costedoat-Chalumeau N, Amoura Z, Villain E, Cohen L, Piette JC. Anti-SSA/Ro antibodies and the heart: more than complete congenital heart block? A review of electrocardiographic and myocardial abnormalities and of treatment options. Arthritis Res Ther. 2005;7:69–73. doi: 10.1186/ar1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nield LE, et al. Endocardial fibroelastosis associated with maternal anti-Ro and anti-La antibodies in the absence of atrioventricular block. J Am Coll Cardiol. 2002;40:796–802. doi: 10.1016/s0735-1097(02)02004-1. [DOI] [PubMed] [Google Scholar]
- 47.Pises N, et al. Positive maternal anti-SSA/SSB antibody-related fetal right ventricular endocardial fibroelastosis without atrioventricular block, reversal of endocardial fibroelastosis. Prenat Diagn. 2009;29:177–178. doi: 10.1002/pd.2073. [DOI] [PubMed] [Google Scholar]
- 48.Nield LE, et al. Maternal anti-Ro and anti-La antibody-associated endocardial fibroelastosis. Circulation. 2002;105:843–848. doi: 10.1161/hc0702.104182. [DOI] [PubMed] [Google Scholar]
- 49.Bazett HC. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–370. [Google Scholar]
- 50.Kussman BD, Madril DR, Thiagarajan RR, Walsh EP, Laussen PC. Anesthetic management of the neonate with congenital complete heart block: a 16-year review. Paediatr Anaesth. 2005;15:1059–1066. doi: 10.1111/j.1460-9592.2005.01634.x. [DOI] [PubMed] [Google Scholar]
- 51.Buyon JP. Dispelling the preconceived notion that lupus pregnancies result in poor outcomes. J Rheumatol. 2005;32:1641–1642. [PubMed] [Google Scholar]
- 52.Stinstra J, et al. Multicenter study of fetal cardiac time intervals using magnetocardiography. BJOG. 2002;109:1235–1243. doi: 10.1046/j.1471-0528.2002.01057.x. [DOI] [PubMed] [Google Scholar]
- 53.Nakai K, et al. Development of 64-channel magnetocardiography and clinical application [Japanese] Rinsho Byori. 2006;54:844–849. [PubMed] [Google Scholar]
- 54.Horigome H, et al. Standardization of the PQRST waveform and analysis of arrhythmias in the fetus using vector magnetocardiography. Pediatr Res. 2006;59:121–125. doi: 10.1203/01.pdr.0000190578.81426.fc. [DOI] [PubMed] [Google Scholar]
- 55.Cuneo BF, et al. Atrial and ventricular rate response and patterns of heart rate acceleration during maternal-fetal terbutaline treatment of fetal complete heart block. Am J Cardiol. 2007;100:661–665. doi: 10.1016/j.amjcard.2007.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cuneo BF, Strasburger JF, Wakai RT, Ovadia M. Conduction system disease in fetuses evaluated for irregular cardiac rhythm. Fetal Diagn Ther. 2006;21:307–313. doi: 10.1159/000091362. [DOI] [PubMed] [Google Scholar]
- 57.Comani S, Van Leeuwen P, Lange S, Geue D, Gronemeyer D. Influence of gestational age on the effectiveness of spatial and temporal methods for the reconstruction of the fetal magnetocardiogram. Biomed Tech (Berl) 2009;54:29–37. doi: 10.1515/BMT.2009.005. [DOI] [PubMed] [Google Scholar]
- 58.Menéndez T, et al. Usefulness of magnetocardiography for the investigation of fetal arrhythmias. Am J Cardiol. 2001;88:334–336. doi: 10.1016/s0002-9149(01)01658-7. [DOI] [PubMed] [Google Scholar]
- 59.Strasburger JF. Prenatal diagnosis of fetal arrhythmias. Clin Perinatol. 2005;32:891–912. doi: 10.1016/j.clp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 60.Skog A, Wahren-Herlenius M, Sundstrom B, Bremme K, Sonesson SE. Outcome and growth of infants fetally exposed to heart block-associated maternal anti-Ro52/SSA autoantibodies. Pediatrics. 2008;121:e803–e809. doi: 10.1542/peds.2007-1659. [DOI] [PubMed] [Google Scholar]
- 61.Eronen M, et al. Short- and long-term outcome of children with congenital complete heart block diagnosed in utero or as a newborn. Pediatrics. 2000;106 (Pt 1):86–91. doi: 10.1542/peds.106.1.86. [DOI] [PubMed] [Google Scholar]
- 62.Moak JP, et al. Congenital heart block: development of late-onset cardiomyopathy, a previously underappreciated sequela. J Am Coll Cardiol. 2001;37:238–242. doi: 10.1016/s0735-1097(00)01048-2. [DOI] [PubMed] [Google Scholar]
- 63.Fesslova V, et al. The impact of treatment of the fetus by maternal therapy on the fetal and postnatal outcomes for fetuses diagnosed with isolated complete atrioventricular block. Cardiol Young. 2009;19:282–290. doi: 10.1017/S1047951109004053. [DOI] [PubMed] [Google Scholar]
- 64.Gregoratos G, et al. ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines) J Am Coll Cardiol. 2002;40:1703–1719. doi: 10.1016/s0735-1097(02)02528-7. [DOI] [PubMed] [Google Scholar]
- 65.Srinivasan S, Strasburger J. Overview of fetal arrhythmias. Curr Opin Pediatr. 2008;20:522–531. doi: 10.1097/MOP.0b013e32830f93ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carpenter RJ, Jr, et al. Fetal ventricular pacing for hydrops secondary to complete atrioventricular block. J Am Coll Cardiol. 1986;8:1434–1436. doi: 10.1016/s0735-1097(86)80319-9. [DOI] [PubMed] [Google Scholar]
- 67.Walkinshaw SA, Welch CR, McCormack J, Walsh K. In utero pacing for fetal congenital heart block. Fetal Diagn Ther. 1994;9:183–185. doi: 10.1159/000263929. [DOI] [PubMed] [Google Scholar]
- 68.Kohl T, et al. Fetoscopic direct fetal cardiac access in sheep: an important experimental milestone along the route to human fetal cardiac intervention. Circulation. 2000;102:1602–1604. doi: 10.1161/01.cir.102.14.1602. [DOI] [PubMed] [Google Scholar]
- 69.Kikuchi Y, Shiraishi H, Igarashi H, Chunfeng L, Yanagisawa M. Cardiac pacing in fetal lambs: intrauterine transvenous cardiac pacing for fetal complete heart block. Pacing Clin Electrophysiol. 1995;18 (Pt 1):417–423. doi: 10.1111/j.1540-8159.1995.tb02540.x. [DOI] [PubMed] [Google Scholar]
- 70.Dell’Orfano J, et al. The monolithic fetal pacemaker: prototype lead design for closed thorax deployment. Pacing Clin Electrophysiol. 2003;26 (Pt 1):805–811. doi: 10.1046/j.1460-9592.2003.t01-1-00143.x. [DOI] [PubMed] [Google Scholar]
- 71.Assad RS, et al. New lead for in utero pacing for fetal congenital heart block. J Thorac Cardiovasc Surg. 2003;126:300–302. doi: 10.1016/s0022-5223(03)00220-4. [DOI] [PubMed] [Google Scholar]
- 72.Wakai RT, Strasburger JF, Li Z, Deal BJ, Gotteiner NL. Magnetocardiographic rhythm patterns at initiation and termination of fetal supraventricular tachycardia. Circulation. 2003;107:307–312. doi: 10.1161/01.cir.0000043801.92580.79. [DOI] [PubMed] [Google Scholar]
- 73.Naheed ZJ, Strasburger JF, Deal BJ, Benson DW, Jr, Gidding SS. Fetal tachycardia: mechanisms and predictors of hydrops fetalis. J Am Coll Cardiol. 1996;27:1736–1740. doi: 10.1016/0735-1097(96)00054-x. [DOI] [PubMed] [Google Scholar]
- 74.Ko JK, Deal BJ, Strasburger JF, Benson DW., Jr Supraventricular tachycardia mechanisms and their age distribution in pediatric patients. Am J Cardiol. 1992;69:1028–1032. doi: 10.1016/0002-9149(92)90858-v. [DOI] [PubMed] [Google Scholar]
- 75.Kannankeril PJ, Gotteiner NL, Deal BJ, Johnsrude CL, Strasburger JF. Location of accessory connection in infants presenting with supraventricular tachycardia in utero: clinical correlations. Am J Perinatol. 2003;20:115–119. doi: 10.1055/s-2003-40014. [DOI] [PubMed] [Google Scholar]
- 76.Hahurij ND, et al. Accessory atrioventricular myocardial connections in the developing human heart: relevance for perinatal supraventricular tachycardias. Circulation. 2008;117:2850–2858. doi: 10.1161/CIRCULATIONAHA.107.756288. [DOI] [PubMed] [Google Scholar]
- 77.Johnson WH, Jr, Dunnigan A, Fehr P, Benson DW., Jr Association of atrial flutter with orthodromic reciprocating fetal tachycardia. Am J Cardiol. 1987;59:374–375. doi: 10.1016/0002-9149(87)90823-x. [DOI] [PubMed] [Google Scholar]
- 78.Strasburger JF, Cheulkar B, Wichman HJ. Perinatal arrhythmias: diagnosis and management. Clin Perinatol. 2007;34:627–652. doi: 10.1016/j.clp.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Strasburger JF, et al. Amiodarone therapy for drug-refractory fetal tachycardia. Circulation. 2004;109:375–379. doi: 10.1161/01.CIR.0000109494.05317.58. [DOI] [PubMed] [Google Scholar]
- 80.Simpson JM. Fetal arrhythmias. Ultrasound Obstet Gynecol. 2006;27:599–606. doi: 10.1002/uog.2819. [DOI] [PubMed] [Google Scholar]
- 81.Simpson JM, Maxwell D, Rosenthal E, Gill H. Fetal ventricular tachycardia secondary to long QT syndrome treated with maternal intravenous magnesium: case report and review of the literature. Ultrasound Obstet Gynecol. 2009;34:475–480. doi: 10.1002/uog.6433. [DOI] [PubMed] [Google Scholar]
- 82.Maeno Y, Hirose A, Kanbe T, Hori D. Fetal arrhythmia: prenatal diagnosis and perinatal management. J Obstet Gynaecol Res. 2009;35:623–629. doi: 10.1111/j.1447-0756.2009.01080.x. [DOI] [PubMed] [Google Scholar]
- 83.Cuneo BF, Strasburger JF. Management strategy for fetal tachycardia. Obstet Gynecol. 2000;96:575–581. doi: 10.1016/s0029-7844(00)00996-0. [DOI] [PubMed] [Google Scholar]
- 84.Cuneo BF. Treatment of fetal tachycardia. Heart Rhythm. 2008;5:1216–1218. doi: 10.1016/j.hrthm.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 85.Oudijk MA, et al. Sotalol in the treatment of fetal dysrhythmias. Circulation. 2000;101:2721–2726. doi: 10.1161/01.cir.101.23.2721. [DOI] [PubMed] [Google Scholar]
- 86.D’Alto M, et al. The challenge of fetal dysrhythmias: echocardiographic diagnosis and clinical management. J Cardiovasc Med (Hagerstown) 2008;9:153–160. doi: 10.2459/JCM.0b013e3281053bf1. [DOI] [PubMed] [Google Scholar]
- 87.Jouannic JM, et al. Fetal supraventricular tachycardia: a role for amiodarone as second-line therapy? Prenat Diagn. 2003;23:152–156. doi: 10.1002/pd.542. [DOI] [PubMed] [Google Scholar]
- 88.Van Leeuwen P, et al. Effect of prenatal antiarrhythmic treatment on cardiac function in a twin pregnancy. Pacing Clin Electrophysiol. 2008;31:1213–1217. doi: 10.1111/j.1540-8159.2008.01165.x. [DOI] [PubMed] [Google Scholar]
- 89.Fish FA, Gillette PC, Benson DW., Jr Proarrhythmia, cardiac arrest and death in young patients receiving encainide and flecainide The Pediatric Electrophysiology Group. J Am Coll Cardiol. 1991;18:356–365. doi: 10.1016/0735-1097(91)90586-x. [DOI] [PubMed] [Google Scholar]
- 90.Fenrich AL, Jr, Perry JC, Friedman RA. Flecainide and amiodarone: combined therapy for refractory tachyarrhythmias in infancy. J Am Coll Cardiol. 1995;25:1195–1198. doi: 10.1016/0735-1097(94)00513-p. [DOI] [PubMed] [Google Scholar]
- 91.Parilla BV, Strasburger JF, Socol ML. Fetal supraventricular tachycardia complicated by hydrops fetalis: a role for direct fetal intramuscular therapy. Am J Perinatol. 1996;13:483–486. doi: 10.1055/s-2007-994432. [DOI] [PubMed] [Google Scholar]
- 92.Weiner CP, Thompson MI. Direct treatment of fetal supraventricular tachycardia after failed transplacental therapy. Am J Obstet Gynecol. 1988;158 (Pt 1):570–573. doi: 10.1016/0002-9378(88)90027-0. [DOI] [PubMed] [Google Scholar]
- 93.Hallak M, Neerhof MG, Perry R, Nazir M, Huhta JC. Fetal supraventricular tachycardia and hydrops fetalis: combined intensive, direct, and transplacental therapy. Obstet Gynecol. 1991;78 (Pt 2):523–525. [PubMed] [Google Scholar]
- 94.Lomenick JP, Jackson WA, Backeljauw PF. Amiodarone-induced neonatal hypothyroidism: a unique form of transient early-onset hypothyroidism. J Perinatol. 2004;24:397–399. doi: 10.1038/sj.jp.7211104. [DOI] [PubMed] [Google Scholar]
- 95.Trotter A, Kaestner M, Pohlandt F, Lang D. Unusual electrocardiogram findings in a preterm infant after fetal tachycardia with hydrops fetalis treated with flecainide. Pediatr Cardiol. 2000;21:259–262. doi: 10.1007/s002460010053. [DOI] [PubMed] [Google Scholar]
- 96.Rasheed A, Simpson J, Rosenthal E. Neonatal ECG changes caused by supratherapeutic flecainide following treatment for fetal supraventricular tachycardia. Heart. 2003;89:470. doi: 10.1136/heart.89.4.470-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hall CM, Ward Platt MP. Neonatal flecainide toxicity following supraventricular tachycardia treatment. Ann Pharmacother. 2003;37:1343–1344. doi: 10.1345/aph.1C487. [DOI] [PubMed] [Google Scholar]
- 98.Jaeggi ET, Nii M. Fetal brady- and tachyarrhythmias: new and accepted diagnostic and treatment methods. Semin Fetal Neonatal Med. 2005;10:504–514. doi: 10.1016/j.siny.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 99.Casey FA, McCrindle BW, Hamilton RM, Gow RM. Neonatal atrial flutter: significant early morbidity and excellent long-term prognosis. Am Heart J. 1997;133:302–306. doi: 10.1016/s0002-8703(97)70224-2. [DOI] [PubMed] [Google Scholar]
- 100.Copel JA, Liang RI, Demasio K, Ozeren S, Kleinman CS. The clinical significance of the irregular fetal heart rhythm. Am J Obstet Gynecol. 2000;182:813–819. doi: 10.1016/s0002-9378(00)70330-9. [DOI] [PubMed] [Google Scholar]
- 101.Fyfe DA, Meyer KB, Case CL. Sonographic assessment of fetal cardiac arrhythmias. Semin Ultrasound CT MR. 1993;14:286–297. doi: 10.1016/s0887-2171(05)80103-9. [DOI] [PubMed] [Google Scholar]
- 102.Tan B-H, et al. Sudden infant death syndrome-associated mutations in the sodium channel beta subunits. Heart Rhythm. doi: 10.1016/ j.hrthm.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rashba EJ, et al. Influence of pregnancy on the risk for cardiac events in patients with hereditary long QT syndrome. LQTS Investigators. Circulation. 1998;97:451–456. doi: 10.1161/01.cir.97.5.451. [DOI] [PubMed] [Google Scholar]
- 104.Tester DJ, Ackerman MJ. Sudden infant death syndrome: how significant are the cardiac channelopathies? Cardiovasc Res. 2005;67:388–396. doi: 10.1016/j.cardiores.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 105.Shim SH, Ito M, Maher T, Milunsky A. Gene sequencing in neonates and infants with the long QT syndrome. Genet Test. 2005;9:281–284. doi: 10.1089/gte.2005.9.281. [DOI] [PubMed] [Google Scholar]
- 106.Hofbeck M, Ulmer H, Beinder E, Sieber E, Singer H. Prenatal findings in patients with prolonged QT interval in the neonatal period. Heart. 1997;77:198–204. doi: 10.1136/hrt.77.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ackerman MJ, et al. Postmortem molecular analysis of SCN5A defects in sudden infant death syndrome. JAMA. 2001;286:2264–2269. doi: 10.1001/jama.286.18.2264. [DOI] [PubMed] [Google Scholar]
- 108.Kominis IK, Kornack TW, Allred JC, Romalis MV. A subfemtotesla multichannel atomic magnetometer. Nature. 2003;422:596–599. doi: 10.1038/nature01484. [DOI] [PubMed] [Google Scholar]
- 109.Chan J, Kumar S, Fisk NM. First trimester embryo-fetoscopic and ultrasound-guided fetal blood sampling for ex vivo viral transduction of cultured human fetal mesenchymal stem cells. Hum Reprod. 2008;23:2427–2437. doi: 10.1093/humrep/den302. [DOI] [PubMed] [Google Scholar]
- 110.Zhao H, Chen M, Van Veen BD, Strasburger JF, Wakai RT. Simultaneous fetal magnetocardiography and ultrasound/ Doppler imaging. IEEE Trans Biomed Eng. 2007;54 (Pt 2):1167–1171. doi: 10.1109/TBME.2006.889198. [DOI] [PubMed] [Google Scholar]
- 111.Schmidt KG, Ulmer HE, Silverman NH, Kleinman CS, Copel JA. Perinatal outcome of fetal complete atrioventricular block: a multicenter experience. J Am Coll Cardiol. 1991;17:1360–1366. doi: 10.1016/s0735-1097(10)80148-2. [DOI] [PubMed] [Google Scholar]
- 112.Jaeggi ET, Hornberger LK, Smallhorn JF, Fouron JC. Prenatal diagnosis of complete atrioventricular block associated with structural heart disease: combined experience of two tertiary care centers and review of the literature. Ultrasound Obstet Gynecol. 2005;26:16–21. doi: 10.1002/uog.1919. [DOI] [PubMed] [Google Scholar]
- 113.Eronen M. Outcome of fetuses with heart disease diagnosed in utero. Arch Dis Child Fetal Neonatal Ed. 1997;77:F41–F46. doi: 10.1136/fn.77.1.f41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Machado MV, Tynan MJ, Curry PV, Allan LD. Fetal complete heart block. Br Heart J. 1988;60:512–515. doi: 10.1136/hrt.60.6.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gembruch U, Hansmann M, Redel DA, Bald R, Knöpfle G. Fetal complete heart block: antenatal diagnosis, significance and management. Eur J Obstet Gynecol Reprod Biol. 1989;31:9–22. doi: 10.1016/0028-2243(89)90022-1. [DOI] [PubMed] [Google Scholar]
- 116.Maeno Y, et al. Clinical course of fetal congenital atrioventricular block in the Japanese population: a multicenter experience. Heart. 2005;91:1075–1079. doi: 10.1136/hrt.2003.033407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.