Abstract
The oculomotor vermis (OMV) of the cerebellum is necessary for the generation of the accurate rapid eye movements called saccades. Large lesions of the midline cerebellar cortex involving the OMV cause saccades to become hypometric and more variable. However, saccades were not examined immediately after these lesions so the interpretation of the resulting deficits might have been contaminated by some adaptation to the saccade dysmetria. Therefore, to better understand the contribution of the OMV to normal saccades, we impaired its operation locally by injecting small amounts of either an agonist or antagonist of γ-aminobutyric acid (GABA), which is a ubiquitous neurotransmitter throughout the cerebellar cortex. Muscimol, a GABA agonist, inactivated part of the OMV, whereas bicuculline, an antagonist, disinhibited it. Muscimol caused all ipsiversive horizontal saccades from 5 to 30° to become hypometric. In contrast, bicuculline produced an amplitude-dependent dysmetria: ipsiversive horizontal saccades elicited by target steps <10° became hypometric, whereas those in response to larger steps became hypermetric. At the transition target amplitude, saccade amplitudes were quite variable with some being hypo- and others hypermetric. After most injections of either agent, saccades had lower peak velocities and longer durations than pre-injection saccades of the same amplitude. The longer durations were associated with a prolongation of the deceleration phase. Both agents produced inconsistent effects on contraversive saccades. These results establish that the oculomotor vermis helps control the characteristics of normal ipsiversive saccades and that GABAergic inhibitory processes are a crucial part of this process.
Keywords: monkey, cerebellum, muscimol, bicuculline, saccades, GABA
INTRODUCTION
Damage to the cerebellar oculomotor vermis (OMV; lobules VI and VIIa) in both humans (Straube et al., 2001; Waespe and Baumgartner, 1992; Waespe and Müller-Meisser, 1996) and monkeys (Barash et al., 1999; Takagi et al., 1998) impairs the accuracy of saccades. In those studies, however, large areas of the OMV were compromised and saccades were evaluated after some recovery could have occurred, possibly obscuring the role of the OMV in the generation of normal saccades. Therefore, we examined saccades after small, reversible inactivations of the OMV produced by injections of muscimol, an agonist of γ-aminobutyric acid (GABA), which is a ubiquitous inhibitory neurotransmitter of the vermis. Furthermore, we also examined the effect of blocking the GABA inhibitory networks of the OMV by injecting bicuculline. We expected that this disinhibition would produce deficits opposite to those of muscimol.
The expected deficits produced by our injections can be revealed by considering a simplified subcortical circuit for the generation of ipsiversive saccades (Scudder et al., 2002) (Fig. 1). The superior colliculus (SC) generates a saccade command that drives contralateral pre-motor excitatory burst neurons (EBNs), which deliver a burst of spikes to motoneurons (MNs). The SC, via the nucleus reticularis tegmenti pontis (NRTP), also influences the OMV, which in turn inhibits neurons in the ipsilateral caudal fastigial nucleus (cFN) via its only output neuron, the Purkinje (P-) cell. The cFN projects to contralateral inhibitory burst neurons (IBNs) (Scudder et al., 2000; Noda et al., 1990), which also can influence the bursts of MNs. After inactivation of the OMV by muscimol, we expect the decrease in P-cell activity to cause a disinhibition of the cFN. This increase in cFN activity would increase IBN activity and provide more inhibition of the MNs, thereby causing ipsiversive saccades to become hypometric (filled arrow, Fig. 1). Disinhibition of the cFN by bicuculline also causes ipsiversive saccades to become hypometric (Sato and Noda, 1992).
Figure 1.
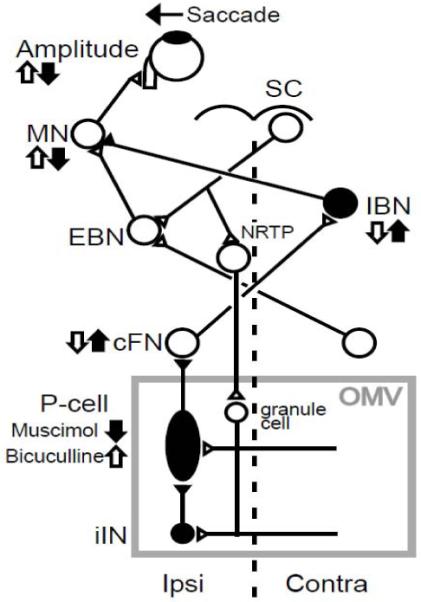
Schematic of the brain stem–cerebellar circuitry involved with leftward saccades. A saccade command from the superior colliculus (SC) reaches excitatory burst neurons (EBN), which drive abducens motoneurons (MN). The SC signal also reaches the oculomotor vermis (OMV) via the nucleus reticularis tegmenti pontis (NRTP). P-cells in OMV inhibit neurons in the caudal fastigial nucleus (cFN). cFN neurons drive inhibitory burst neurons (IBN), which inhibit MNs. Inhibitory interneurons in the OMV (iIN) help shape P-cell activity. Vertical arrows indicate the expected changes in activity that muscimol (filled) or bicuculline (open) injections should produce. Inhibitory neurons are filled; excitatory neurons are open.
P-cell activity is shaped by several GABAergic inhibitory interneurons (Golgi, basket, stellate and Lugaro cells, represented collectively as “iIN” in Fig. 1) either by direct inhibition or via inhibition of other cells, e.g., granule cells that synapse on P-cells (Geurts et al., 2003). Therefore, disinhibition of the OMV by bicuculline would effectively increase P-cell activity. This increase would lead to a reduction of both cFN and IBN activity, which would decrease the inhibition to MNs, thereby causing hypermetric ipsiversive saccades (open arrow, Fig. 1). Inactivation of the cFN by muscimol also causes ipsiversive saccades to become hypermetric (Robinson et al., 1993; Goffart et al., 2004).
As predicted from the circuit in Fig. 1, our muscimol injections caused the amplitudes of all ipsiversive saccades to become hypometric. On the other hand, the hypermetria expected to arise after bicuculline injections occurred only for saccades elicited by target steps greater than 10°, whereas smaller target steps elicited hypometric saccades. Moreover, both muscimol and bicuculline produced a similar slowing of saccades.
RESULTS
Effects on saccade amplitude
muscimol injections
A representative example of the effect of muscimol on the amplitude of ipsiversive horizontal saccades to 5, 10, 15 and 20° target steps is shown in Fig. 2A. At all target amplitudes, saccade gain was significantly smaller after the injection (unpaired t-test with Bonferroni correction, p<0.05) as reflected by a negative percentage gain change (Fig. 2B, dark line labeled A). Negative percent gain changes (hypometrias) occurred at all tested target amplitudes (some up to 25 and 30°) in all 7 experiments (Fig. 2B, Table 1). All gain changes were significant.
Figure 2.
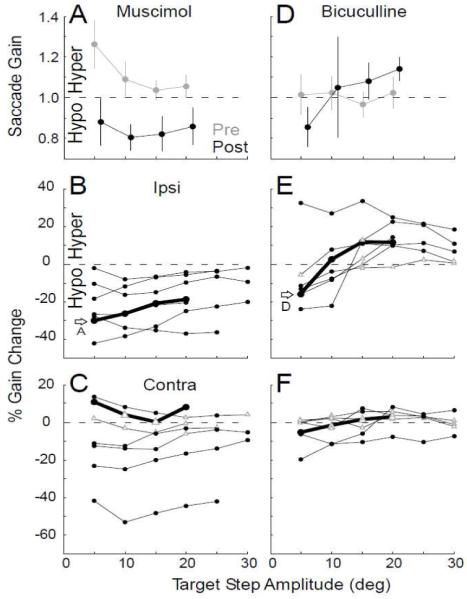
Effect of representative muscimol (A,B,C) and bicuculline (D,E,F) injections on saccades to different target step sizes. (A,D) Mean pre- (gray) and post-injection (black) ipsiversive saccade gain at different target step sizes for an exemplar experiment. Error bar: standard deviation. (B,C,E,F) Percent gain change of ipsiversive (B,E) and contraversive (C,F) saccades for all injections. The thick black line and open arrow identify the data in A, D, respectively. Filled circles: significant percent gain changes (p<0.05); open triangles: those that are not (p>0.05).
Table 1.
Summary of the results of all 14 experiments
| Injection # | Agent | Monkey | OMV side |
Volume (nl) |
Ipsi %GainΔ | Contra %GainΔ | Latency | Ipsi p-vel decrease |
Ipsi dur increase |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| at 5° | at 20° | at 5° | at 20° | Ipsi | Contra | |||||||
| 1 | Muscimol | B | Right | 200 | −42.2 | −25.0 | 13.8 | ns | ↑ | ↓ | * | * |
| 2 Fig[3]A-C | Muscimol | B | Left | 320 | −0.6 | −4.5 | −23.4 | −16.6 | ↑ | ↓ | * | * |
| 3 | Muscimol | W | Right | 1000 | −27.7 | −37.2 | −42.0 | −44.7 | ↑ | ↑ | * | * |
| 4 Fig[2,4]A | Muscimol | B | Right | 120 | −30.3 | −18.7 | 10.8 | 8.3 | ns | ns | * | * |
| 5 | Muscimol | B | Left | 120 | −26.7 | −20.4 | −11.1 | ns | ↑ | ↑ | ns | ns |
| 6 | Muscimol | B | Left | 200 | −18.5 | −5.7 | ns | −3.3 | ↑ | ↓ | * | ns |
| 7 | Muscimol | B | Left | 300 | −10.7 | −9.9 | −12.7 | ns | ↑ | ↓ | * | * |
| 8 | Bicuculline | B | Right | 160 | −11.5 | ns | ns | 3.7 | ↑ | ↑ | * | ns |
| 9 | Bicuculline | B | Left | 220 | −13.3 | 10.3 | ns | ns | ↑ | ↑ | * | * |
| 10 Fig[3]D-F | Bicuculline | W | Left | 300 | ns | 9.8 | ns | ns | ↑ | ↑ | * | * |
| 11 Fig[2,4]D | Bicuculline | B | Left | 140 | −15.7 | 11.5 | −5.4 | ns | ns | ns | ns | ns |
| 12 | Bicuculline | B | Left | 280 | 32.6 | 25.0 | −20.0 | −7.7 | ↓ | ↑ | * | * |
| 13 | Bicuculline | B | Left | 140 | −23.9 | 22.5 | −6.4 | 8.2 | ↑ | ns | * | * |
| 14 | Bicuculline | B | Right | 100 | −15.9 | 14.3 | ns | ns | ↑ | ↑ | * | ns |
First four columns indicate the agent, the monkey, the side of the vermis injected and the injection volume. The next columns show the percent gain change of ipsi- and contraversive saccades to target steps of 5 and 20°. Numbers indicate a significant gain change (negative is hypometria); “ns” indicates a non-significant change. In the latency column, arrows indicate the direction of significant increases (↑) or decreases (↓) [unpaired t-test, p<0.05];” ns” indicates a non-significant change. Last 2 columns show significant (*) or non-significant (ns) [unpaired t-test, p<0.05] decreases and increases, respectively, in ipsiversive peak velocity and duration.
The effect on contraversive saccades varied from experiment to experiment (Fig. 2C, Table 1). Saccades to almost all target step sizes exhibited significant changes (filled black circles). However, those changes were inconsistent and could result in either a hypo- or hypermetria.
Large lesions of the OMV increase the variability of saccade amplitudes (Barash et al., 1999; Takagi et al., 1998). However, our small muscimol injections had no consistent effect on the variability of either ipsi- or contraversive saccade amplitudes to any target step size in any experiment. For example, for the experiment illustrated in Fig. 2A, the SD of gain was ±0.12, 0.08, 0.05 and 0.05 before and ±0.11, 0.06, 0.08 and 0.09 after the injection for target steps of 5, 10, 15 and 20°, respectively. The variance was significantly different (here decreased) only for saccades to 5° target steps (F-test, p<0.05).
The effect on variance may depend on the amount of muscimol injected. In 5 of the remaining experiments in which the injection also was <320 nl, saccades to only one or two target amplitudes (never the same ones) showed significant changes in variability. However, in the one experiment with a 1000 nl injection, the variance in saccade amplitude was significantly increased for all target amplitudes. The same dependence of variability on injection size occurred for contraversive saccades.
Bicuculline injections
The effect of bicuculline on saccade amplitudes was quite different: ipsiversive saccades to small target steps usually became hypometric, whereas those to large target steps (≥15°) became hypermetric. In the representative example illustrated in Fig. 2D, post-injection saccades to 5° target steps were hypometric, but those to 15 and 20° steps were hypermetric (Fig. 2E, thick line labeled D). Pre- and post-injection gains were significantly different for saccades to 5, 15 and 20° target steps (p<0.05), but not for saccades to 10° target steps. This pattern of gain changes with amplitude also occurred for 5 of the remaining 6 experiments (Fig. 2E, Table 1). The effect on contraversive saccade gain again was very modest and inconsistent (Fig. 2F, Table 1).
Effects on saccade main sequence relations
Muscimol caused modest changes in the dynamics of ipsiversive saccades. As shown for another representative experiment, saccades after muscimol injections had lower peak velocities (Fig. 3A) and longer durations (Fig. 3B) than normal, especially for larger saccade amplitudes. Exponential curves fitted to the peak velocity data and linear regressions fitted to the duration data were significantly different (F-test, p<0.05) for pre- and post-injection saccades. Muscimol caused a significant decrease in peak velocity in 6 of 7 experiments. In 5 of those 6, linear regressions of duration with amplitude revealed a significant reciprocal increase in duration (Table 1).
Figure 3.
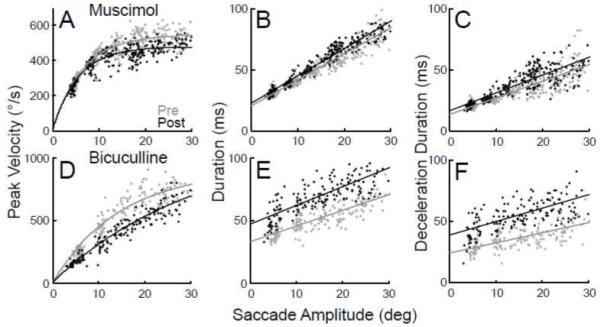
Effect of muscimol (A,B,C) and bicuculline (D,E,F) injections on ipsiversive saccade main sequence relations. (A,D) Peak velocity versus saccade amplitude for pre- (gray) and post-injection (black) data of an exemplar experiment fit with exponentials. Duration (B,E) and deceleration duration (C,F) versus saccade amplitude with linear fits.
Bicuculline also tended to slow saccades. In another representative bicuculline experiment illustrated in Fig. 3D and E, ipsiversive saccades had lower peak velocities and longer durations than normal. Comparison of the exponential fits of the peak velocity relations of pre- and post-injection data revealed a significant slowing in 6 of 7 experiments. In 4 of those 6, linear regression fits revealed that saccade duration also was significantly greater than normal (Table 1).
Both agents increased the duration of amplitude-matched saccades after 5 of 7 muscimol and 4 of 7 bicuculline injections, respectively. In all these experiments, the increase in duration was associated with a significant increase in the deceleration phase of the saccade, but not by in increase in the acceleration phase, as shown in Fig. 3C and F for the injection data illustrated in Fig. 3A, B and D, E, respectively. This lengthening of the deceleration phase accounted for most of the increase in overall duration. For example, at a saccade amplitude of 20°, the average increase in deceleration duration accounted for 90% and 107% of the increase in overall duration produced by the muscimol and bicuculline injections in Figs. 3C and F, respectively. Across the 5 muscimol injections that produced increased durations, the percentage ranged from 64 to l04% (mean=88.5%), whereas the percentage range across the 4 bicuculline injections that caused increased durations was 74 to 107% (mean=89.7%).
After those bicuculline injections where there was a transition from hypo- to hypermetria, the saccade gains at the transition target amplitude were more variable than for other target amplitudes (e.g., Fig. 2D at 10°). This was because some saccades were hypometric (gains <1.0), whereas others were hypermetric (gains >1.0). The hypermetric saccades at the transition had long deceleration phases that were even longer than those associated with the hypermetric saccades to the larger target amplitudes.
Effect on other saccade characteristics
Muscimol and bicuculline not only changed the amplitudes of saccades to horizontal target steps but also affected vertical and oblique saccades. Fig. 4A shows the effect of muscimol on the saccade trajectories to 10° target steps in 8 different directions for the experiment illustrated in Fig. 2A. Saccades with ipsiversive horizontal components were hypometric in all oblique directions and vertical saccades veered slightly in the contraversive direction. In contrast, after a bicuculline injection (Fig. 4B), saccades to 20° target steps in all oblique directions with ipsiversive horizontal components tended to overshoot and vertical saccades veered slightly in the ipsiversive direction. Similar dysmetrias occurred for saccades with ipsiversive horizontal components after all muscimol and bicuculline injections, respectively.
Figure 4.
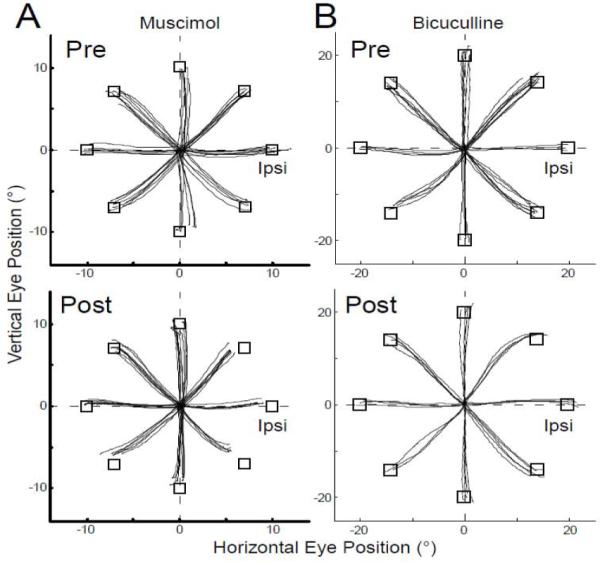
Trajectories of saccades from straight ahead to targets located at an eccentricity of 10° (A) or 20° (B) in 8 equally spaced directions (open squares). (A) Vertical vs. horizontal eye position before and after the muscimol injection illustrated in Fig. 2A. (B) Vertical vs. horizontal eye position before and after the bicuculline injection illustrated in Fig. 2D. All saccades start from their actual initial eye positions.
Neither agent produced a consistent offset of horizontal eye position when the monkey fixated targets located straight ahead. This can be appreciated from Fig. 4, where the centrifugal saccades from a straight-ahead target position are all plotted from their actual starting positions. For each experiment, we tested the fixation offset of all initial eye positions at saccade onset relative to each horizontal target step. The fixation offset produced by neither muscimol nor bicuculline depended on orbital eye position nor did it vary consistently from experiment to experiment. Therefore, we averaged all the offset data across all 7 experiments for each agent. The mean difference of absolute horizontal offset during fixation before and after the muscimol or bicuculline injections was only 0.26±0.47° or 0.22±0.40°, respectively. The mean difference of absolute offset of vertical eye position was slightly greater and averaged 0.75±0.44° and 0.41±0.18° after the muscimol and bicuculline injections, respectively. Moreover, the horizontal and vertical directions of the offset (ipsiversive, contraversive, up and down) were not consistent from experiment to experiment.
Finally, the latency of saccades to ipsiversive target steps was significantly increased in most experiments after both muscimol (6/7 experiments, mean increase across 6 experiments=50.5±74.2ms) and bicuculline (5/7 experiments, mean increase across 5 experiments=31.9±39.7ms) (unpaired t test, p<0.05, Table. 1). For contraversive saccades, there was no consistent change in latency after muscimol injections and only a modest increase after bicuculline (5/7 experiments, mean across 5 experiments=17.9±8.3ms).
DISCUSSION
We used pharmacological manipulations to explore the role of the oculomotor vermis in producing accurate targeting saccades. Our rather small injections of muscimol and bicuculline produced robust and consistent deficits in ipsiversive saccades and no consistent dysmetria of contraversive saccades. Muscimol caused ipsiversive hypometria, whereas bicuculline caused larger ipsiversive saccades to become hypermetric but smaller ones to become hypometric. The dysmetrias produced by these agents substantiates the idea that the OMV helps control the size and hence accuracy of ipsiversive saccades. Moreover, our data show that inhibitory processes mediated by GABA are likely to be an important element in this control.
muscimol injections
Consistent with the circuitry in Fig. 1, hypometric ipsiversive saccades occurred when muscimol turned off the OMV, thereby suppressing P-cell activity and increasing both cFN and IBN activity.
Large excisions in the OMV cause both leftward and rightward saccades to become hypometric (Barash et al., 1999; Takagi et al., 1998). Therefore, our injections, which produced consistent deficits only of ipsiversive saccades, likely affected only one side of the OMV. Both our study and that of Takagi et al. (1998) produced hypometrias with similar ranges: ~5 to 30% and ~15% to ~50%, respectively. In their study and ours, saccades also had slower peak velocities and longer durations after impairments of the OMV.
Our small injections into the OMV produced only very small, inconsistent offsets in fixation. The offsets produced in other studies by much larger surgical lesions were either, like ours, inconsistent (Takagi et al., 1998). We can conclude, however, that whereas inactivation of a small part of the OMV is sufficient to cause a significant impairment of saccade amplitudes it causes very little, if any, offset in fixation.
bicuculline injections
Unexpectedly, bicuculline induced a size-dependent dysmetria after all but one injection. Only ipsiversive saccades greater than ~10° displayed the hypermetria that would be expected if bicuculline produced the opposite effect of muscimol. In contrast, smaller saccades became hypometric. It is unclear how GABA inhibition would operate to have opposite effects on large and small saccades, but the results of the bicuculline injections appear to suggest that GABA-mediated inhibitory processes play a different role in the control of large and small saccades.
After both the muscimol and bicuculline injections, the ipsiversive dysmetria was not correlated with the amount or location of the injection. However, all but 1 of the 14 injections ranged between ~100 and ~320nl. Perhaps the agents had to have a certain concentration near a certain number of cortical elements to produce a consistent dysmetria, and our effective small injections were made closer to those elements than the injections requiring larger volumes. Similarly, injections closer to the midline were not more effective. The more modest and inconsistent dysmetrias of contraversive saccades also were independent of the amount or location of the injection. For example, injections #2, 5 and 7 were all at the same location relative to the midline, but they produced different effects (Table 1). Also injection #6 was ~0.5mm from the estimated midline but produced deficits that were relatively similar to those caused by injection #7, which was ~1.5mm from the midline.
Saccade main sequence relations after injections
In most experiments, all saccades, especially the larger ones, became slower after either muscimol or bicuculline injections. In most experiments, the lower peak velocities were accompanied by an increase in saccade duration. Because the two agents had opposite effects on saccade gain at all but the smaller target amplitudes, we were surprised that both agents produced a similar slowing. The slowing was not simply due to a possible common fatigue caused by many targeting saccades (Golla et al., 2008), because after bicuculline, the post-injection data were collected within the first 20 min after the injection, whereas the post-injection muscimol data were taken more than 40 min after the injection. Instead, our findings imply that the OMV, both its P-cells and inhibitory interneurons, must be functioning normally in order to maintain normal saccade dynamics.
After most injections, saccade durations become longer and peak velocities become slower than normal (Fig. 3). Moreover, ~89% of this increase in duration, on average, can be accounted for by an increase in the duration of deceleration. Consequently, after either GABA suppression or facilitation, the cerebellum does not provide a signal that is appropriate to help produce the correct saccade duration. Therefore, our data suggest that the role of the normal processes involving GABA in the vermis is to assure that the deceleration phase of a saccade is correct. A similar observation was made in a study of saccade adaptation on human patients with cerebellar degeneration (Xu-Wilson et al., 2009).
Comparison to the effects of injections into the cFN
Our injections into the OMV produced deficits of ipsiversive saccades that are consistent with previous pharmacological injections into the cFN. Our bicuculline disinhibition of OMV P-cells increases inhibition of the cFN, which also is produced by injection of muscimol directly into the cFN. Like our bicuculline injections into the OMV, muscimol injected into the cFN also causes ipsiversive hypermetria (Goffart et al. 2004; Robinson et al., 1993). Similarly, our muscimol injections into the OMV suppress its activity, thereby decreasing cFN inhibition, which also occurs after injection of bicuculline directly into the cFN. As expected, saccades become hypometric after either injection (Sato and Noda, 1992).
Muscimol injections into the cFN also cause contraversive saccades to become hypometric (Goffart et al. 2004; Robinson et al., 1993), whereas our injections of muscimol into the OMV produced hypermetria in some experiments and hypometria in others (Fig. 2C). We suggest that these inconsistent effects on contraversive saccades might have been caused by spillover of some of our injections across the midline to the contralateral OMV. Because inactivation of both cFNs induces a bidirectional hypermetria (Robinson et al., 1993), we would expect both ipsi- and contraversive saccades would become hypometric if muscimol had spread beyond the midline of the vermis. Therefore, we feel it is impossible to conclude anything about the OMV influence on contraversive saccades from our experiments.
Finally, the injection of neither agent into the OMV caused an offset in fixation eye position like that produced by injections of either muscimol (Guerrasio et al. 2010; Goffart et al. 2004; Robinson et al., 1993) or bicuculline (Sato and Noda, 1992) into the cFN. Because the output of all the ipsilateral OMV converges on the ipsilateral cFN (Yamada and Noda, 1987), an injection into one cFN could be equivalent to affecting one entire side of the OMV. Perhaps our small injections simply did not compromise a sufficient amount of the OMV to produce an eye fixation offset as was produced after large excisions of the OMV (Takagi et al., 1998).
EXPERIMENTAL PROCEDURE
We performed pharmacological injections into the OMV of two rhesus monkeys (Macaca mulatta, male, Monkey B: 7.4 and Monkey W: 5.0 kg). They also provided data for a previous study (Kojima et al., 2010) in which the surgical and recording procedures have been described in detail. Briefly, we implanted each monkey with head stabilization lugs, an eye coil and a recording chamber aimed straight down at 14.5 mm posterior to the inter-aural line on the midline (Kojima et al. 2010). Once a monkey was trained to track a spot target, we recorded from the OMV with a tungsten micro-electrode. We determined the limits of the OMV on the basis of its characteristic bursting activity for saccades in all directions. We estimated the location of the OMV midline functionally by determining the horizontal error direction selectivity of the complex spike (CS) discharge of P-cells (Soetedjo et al. 2008). We drew the midline between medio-lateral cell clusters that had opposite preferred CS directions.
Injection of pharmacological agents
We injected either muscimol (2μg/μL, MP Biomedicals) or bicuculline (5μg/μL, Bicuculline Methochloride, MP Biomedicals) dissolved in saline solution through a 35-gauge stainless steel tube that was insulated by epoxylite except for its beveled tip to allow the recording of background activity. We advanced the tube until we heard the strongest saccade-related bursts of activity. We then waited ~ 5 min to allow the tissue to stabilize. We began by injecting a small volume (100 nl) and monitored its effect on the amplitude of saccades made to between 4 to 6 different horizontal target steps from 5° to either 20, 25 or 30° in size, in 5° steps. The target moved to the various horizontal locations between ±20° pseudo randomly. We delivered more of the pharmacological agent every 10 min until a horizontal dysmetria occurred. In different experiments, we injected a total of 120 to 320 nl of muscimol in monkey B and 1000 nl in monkey W and 100 to 280 nl of bicuculline in monkey B and 300 nl in monkey W (Table 1). The bursting activity decreased and increased following muscimol and bicuculline injections, respectively. Although some of the larger injections may have spread across the midline (Arikan et al., 2002), it is unlikely that any spread to the cFN because we never observed the deficits produced when either muscimol (Robinson et al., 2002) or bicuculline (Sato and Noda, 1992) are injected there.
Experimental protocols
During each experiment, monkeys made saccades to horizontal target steps for up to 100 min. Each time the monkey made a saccade, we turned off the target at the end of saccade (triggered online when the falling phase of eye velocity reached 20°/s) for 500 ms to prevent adaptation of the dysmetric saccades caused by the injection. We compared 20 pre-injection saccades with 20 saccades collected after the injection-induced dysmetria had stabilized. For each drug, the first 2 injections on monkey B and the first on monkey W (Table 1 #1-3, #8-10) were used to test the effect of the drug on the time course of saccade gain [saccade amplitude/(|saccade initial position - target end position|)].
In both monkeys, muscimol caused a gradual decrease in ipsiversive saccade gain, which stabilized after 40-50 min and remained so for almost another hour. Fig. 5A shows data from a representative example. Similar time courses of decreases in saccade amplitude occurred for all target sizes. A much smaller and inconsistent contraversive hypometria also stabilized after 40-50 min. For the last 4 experiments, therefore, the animals were placed in the dark for 40 min after the injection before the data were collected.
Figure 5.
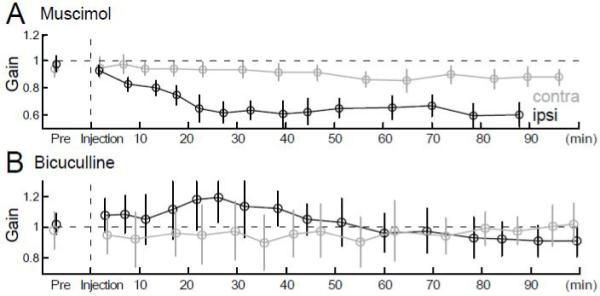
Time course of the effects of representative muscimol (A) and bicuculline (B) injections on ipsiversive (black) and contraversive (gray) saccade gains. Target steps were 15° in A and 10° in B. Vertical dashed lines indicate the end of the injection. Each point in A and B is the average of 15 and 21 saccades, respectively; bars indicate standard deviations.
In contrast, bicuculline produced an immediate ipsiversive saccade dysmetria, which remained stable for ~40 min in both monkeys. In the representative example shown in Fig. 5B, the deficit was a hypermetria. Contraversive saccades either were not affected or became only slightly dysmetric immediately after the injection. Therefore, we compared pre-injection saccades with those that occurred within the first 20 min after the injection.
In experiments #4-7 after muscimol and #11-14 after bicuculline injections, we also tested saccades made to 10° target steps in 8 different directions (every 45°) from straight ahead before the injection and when the dysmetria had become stable after the injection.
Data analysis
Our data analyses have been described in detail in our previous paper (Kojima et al., 2010). Briefly, eye and target position were analyzed off-line to identify each primary target step and the saccade it evoked. Custom computer programs that ran on the Spike-2 (CED) system then determined saccade onset and offset by a velocity criterion (20°/s). From these markings, the program calculated target amplitude and saccade amplitude, duration and peak velocity, as well as saccade gain. These data were exported to Matlab (Mathworks) for further analysis. To assess the effect of the drugs on saccade amplitude across experiments, we calculated the gain and the percent gain change as (saccade gain after injection - gain before injection)/(gain before injection) X100.
To evaluate possible effects on the main sequence relations for saccades (Bahill et al., 1981), we plotted peak saccade velocity and duration against amplitude and fitted the velocity relation with an exponential function (eq1) and the duration and the deceleration duration relation with a linear regression (eq2). We tested the statistical difference of the residual errors between the pre- and post-injection fits with an F-test (Motulsky and Christopoulos, 2004).
| (eq1) |
| (eq2) |
All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (1997) and exceeded the minimal requirements recommended by the Institute of Laboratory Animal Resources and the Association for Assessment and Accreditation of Laboratory Animal Care International. All the procedures were evaluated and approved by the local Animal Care and Use Committee of the University of Washington.
ACKNOWLEDGEMENTS
We are grateful for the valuable comments of S. Bierer, E. Buzunov, C. Kaneko, L. Ling, M. Mustari, and A. Weiss on an early version of the manuscript. This study was supported by National Institute of Health (NIH) grants EY00745 and EY019258 and RR00166 from the National Center for Research Resources (NCRR), a component of the NIH. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arikan R, Blake NM, Erinjeri JP, Woolsey TA, Giraud L, Highstein SM. A method to measure the effective spread of focally injected muscimol into the central nervous system with electrophysiology and light microscopy. J Neurosci Methods. 2002;118:51–7. doi: 10.1016/s0165-0270(02)00143-7. [DOI] [PubMed] [Google Scholar]
- Barash S, Melikyan A, Sivakov A, Zhang M, Glickstein M, Thier P. Saccadic dysmetria and adaptation after lesions of the cerebellar cortex. J Neurosci. 1999;19:10931–10939. doi: 10.1523/JNEUROSCI.19-24-10931.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahill AT, Brockenbrough A, Troost BT. Variability and development of a normative data base for saccadic eye movements. Invest Ophthalmol Vis Sci. 1981;21:116–25. [PubMed] [Google Scholar]
- Geurts FJ, De Schutter E, Dieudonné S. Unraveling the cerebellar cortex: cytology and cellular physiology of large-sized interneurons in the granular layer. Cerebellum. 2003;2:290–9. doi: 10.1080/14734220310011948. [DOI] [PubMed] [Google Scholar]
- Goffart L, Chen LL, Sparks DL. Deficits in saccades and fixation during muscimol inactivation of the caudal fastigial nucleus in the rhesus monkey. J Neurophysiol. 2004;92:3351–67. doi: 10.1152/jn.01199.2003. [DOI] [PubMed] [Google Scholar]
- Golla H, Tziridis K, Haarmeier T, Catz N, Barash S, Thier P. Reduced saccadic resilience and impaired saccadic adaptation due to cerebellar disease. Eur J Neurosci. 2008;27:132–44. doi: 10.1111/j.1460-9568.2007.05996.x. [DOI] [PubMed] [Google Scholar]
- Guerrasio L, Quinet J, Büttner U, Goffart L. The fastigial oculomotor region and the control of foveation during fixation. J Neurophysiol. 2010;103:1988–2001. doi: 10.1152/jn.00771.2009. [DOI] [PubMed] [Google Scholar]
- Kojima Y, Soetedjo R, Fuchs AF. Changes in simple spike activity of some Purkinje cells in the oculomotor vermis during saccade adaptation are appropriate to participate in motor learning. J Neurosci. 2010;30:3715–27. doi: 10.1523/JNEUROSCI.4953-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky H, Christopoulos A. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting. 1 edition Oxford University Press; USA: 2004. Using global fitting to test a treatment effect in one experiment; pp. 163–165. [Google Scholar]
- Noda H, Sugita S, Ikeda Y. Afferent and efferent connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J Comp Neurol. 1990;302:330–48. doi: 10.1002/cne.903020211. [DOI] [PubMed] [Google Scholar]
- Ritchie L. Effects of cerebellar lesions on saccadic eye movements. J Neurophysiol. 1976;39:1246–56. doi: 10.1152/jn.1976.39.6.1246. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Fuchs AF, Noto CT. Cerebellar influences on saccade plasticity. Ann N Y Acad Sci. 2002;956:155–63. doi: 10.1111/j.1749-6632.2002.tb02816.x. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Straube A, Fuchs AF. Role of the caudal fastigial nucleus in saccade generation. II. Effects of muscimol inactivation. J Neurophysiol. 1993;70:1741–58. doi: 10.1152/jn.1993.70.5.1741. [DOI] [PubMed] [Google Scholar]
- Sato H, Noda H. Saccadic dysmetria induced by transient functional decortication of the cerebellar vermis. Exp Brain Res. 1992;88:455–8. doi: 10.1007/BF02259122. [DOI] [PubMed] [Google Scholar]
- Scudder CA, Kaneko CRS, Fuchs AF. The brainstem burst generator for saccadic eye movements. A modern synthesis. Exp Brain Res. 2002;142:439–462. doi: 10.1007/s00221-001-0912-9. [DOI] [PubMed] [Google Scholar]
- Scudder CA, McGee DM, Balaban CD. Connections of monkey saccade-related fastigial nucleus neurons revealed by anatomical and physiological methods. Soc Neurosci Abstr. 2000;26:363.18. [Google Scholar]
- Soetedjo R, Kojima Y, Fuchs AF. Complex spike activity in the oculomotor vermis of the cerebellum: a vectorial error signal for saccade motor learning? J Neurophysiol. 2008;100:1949–1966. doi: 10.1152/jn.90526.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube A, Deubel H, Ditterich J, Eggert T. Cerebellar lesions impair rapid saccade adaptation. Neurology. 2001;57:2105–2108. doi: 10.1212/wnl.57.11.2105. [DOI] [PubMed] [Google Scholar]
- Takagi J, Zee D, Tamargo R. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol. 1998;80:1911–1931. doi: 10.1152/jn.1998.80.4.1911. [DOI] [PubMed] [Google Scholar]
- Waespe W, Baumgartner R. Enduring dysmetria and impaired gain adaptivity of saccadic eye movements in Wallenberg’s lateral medullary syndrome. Brain. 1992;115:1125–1146. [PubMed] [Google Scholar]
- Waespe W, Müller-Meisser E. Directional reversal of saccadic dysmetria and gain adaptivity in a patient with a superior cerebellar artery infarction. Neuro-ophthalmology. 1996;16:65–74. [Google Scholar]
- Xu-Wilson M, Chen-Harris H, Zee DS, Shadmehr R. Cerebellar contributions to adaptive control of saccades in humans. J Neurosci. 2009;29:12930–9. doi: 10.1523/JNEUROSCI.3115-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J, Noda H. Afferent and efferent connections of the oculomotor cerebellar vermis in the macaque monkey. J Comp Neurol. 1987;265:224–41. doi: 10.1002/cne.902650207. [DOI] [PubMed] [Google Scholar]


