Abstract
Dendrimers are a class of synthetically produced highly branched, spherical nanostructures that can be used as carrier molecules for imaging agents. A variety of dendrimers exist and each has biological properties that will alter its biodistribution. Dendrimers are composed of combinations of core types such as ethylene diamine (EDA), diaminobutyl (DAB), polyamidoamine (PAMAM) and polypropylimine (PPI) and different surface residues such as amine, carboxyl, and alcoholic groups. Increasing the number of primary amine groups attached to the core will increase the size of the dendrimer, which is known by the term ‘generation’ of the dendrimer. Because dendrimers are highly structured in size and shape and have a low poly-dispersity index, each dendrimer generation has distinct pharmacokinetic and pharmacodynamic properties which may prove advantageous for particular medical applications. Research has centered on developing these macromolecules as imaging agents for numerous modalities including magnetic resonance imaging, X-ray computed tomography, optical imaging and nuclear medicine. Another prospective function of dendrimers is as drug delivery vectors, whereby therapeutic payloads are encapsulated within the shell, or incorporated onto their multivalent surface, and targeted to tumor cells using ligands that specifically bind to cancer cells or in normal cells altered by nearby cancer cells. Furthermore, the larger size of high generation dendrimers offers potential to develop dual purpose agents that can act both as imaging agents and as delivery vectors, or can be imaged with more than one modality. Herein, we discuss the current and future applications of dendrimers in medicine and the central role they play in the emerging field of nanotechnology.
1. Introduction
Dendrimers are a class of highly branched synthetic polymers that form spherical macromolecules which can be reliably synthesized to a specific physical size in a highly reproducible manner. Dendrimers of different generations are highly related to each other chemically, allowing the study of molecular size without the interfering variable of different chemical structures.
The name “dendrimer” originates from the Greek word ‘dendron’, meaning tree and references the structure of these molecules which ‘branch’ outwards from their core molecule. Dendrimers can be divided in three distinct regions: the core, the interior (or branches) and the periphery (surface groups). Two main strategies for synthesis of dendrimers have been described. The first method, based on the “divergent” approach pioneered by Tomalia and Newkome, relies on the growth of a dendron from a polyfunctional core to the periphery by incorporation of monomeric modules in a radial fashion [1-3]. Further conjugation of monomers to the core site results in the production of different dendrimer ‘generations’, which increase in size and molecular weight in proportion to their generation [1]. Yet, excessive monomer loading and lengthy chromatography separations during synthesis may lead to imperfections and increased polydispersity in the higher generation dendrimers [4]. The second method named the “convergent” approach described by Hawker and Frechet involves the construction of macromolecules starting at the periphery of the dendrimer [5]. Due to the relatively small number of active sites per reaction, this method generates dendrimers with fewer architectural defects. Assembly of dendrimers based on the convergent approach is simpler but limited to the synthesis of lower generation dendrimers [5]. Many of the issues relating to dendrimer construction have been reviewed elsewhere [6]. Several core types such as ethylene diamine (EDA), diaminobutyl (DAB), and various interiors such as polyamidoamine (PAMAM) and diaminobutane core polypropylimine (DAB), consisting of a pure aliphatic polyamine core, polypropylimine (PPI) and different surface residues such as amine, carboxyl, and alcoholic groups exist [5-7]. Another related class of dendrimer-based agents is the Gadomers developed by Schering AG. Since their core structure incorporates an aromatic ring and the interior is made up of lysines, they are simpler and smaller compared to more typical dendrimers such as the PAMAM or DAB dendrimer-based agents of the same generation [8].
Dendrimers have a vital role to play in the developing field of medical nanotechnology. Dendrimers have been developed for use as contrast agents in the field of medical imaging. The exterior primary amine groups can be functionalized, allowing attachment of contrast agents for Computed Tomography (CT), nuclear medicine, optical imaging and Magnetic Resonance Imaging (MRI). Targeting ligands, such as antibodies can also be attached. Two classes of dendrimers with MR imaging capabilities have been developed: dendrimers containing paramagnetic iron oxide particles (magneto-dendrimers), and those incorporating high numbers of gadolinium-chelates. Furthermore, integration of targeting ligands such as monoclonal antibodies offers potential for highly specific imaging agents, and targeted drug delivery vehicles, or even dual-purpose dendrimers, incorporating both types of ‘payload’ [9]. Dendrimers have been investigated as carrier molecules for therapeutic drugs [10], gene therapy [11] and radiation sensitizers [12]. Therapeutic agents can either be encapsulated within the particle, or bound to its multivalent surface. Physical entrapment has the advantage of shielding potentially harmful drugs within the interior shell of the dendrimer, by non-covalent, ionic, or hydrophobic interactions. Even without specific cell surface targeting, the enhanced permeability and retention (EPR) effect of tumors is the property by which macromolecules tend to accumulate in tumor tissue significantly more than in normal tissues due to the leaky vessels supplying tumors [13]. Internalized drug release is dependent on either the differences present in the tumor micro-environment such as a lower pH, or via the EPR effect where the increased peri-tumoral accumulation eventually leads to the release of the enclosed therapeutic agents [14, 15].
The physical size, which can be controlled by the generation of a dendrimer, the charges, and the hydrophilicity, affect to its pharmacokinetics and pharmacodynamics. Dendrimers are spherical, thus their molecular size (diameter) is directly proportional to their molecular weight, with only minimal conformational change possible. Increasing dendrimer size, will decrease permeability across the vascular wall, alter the excretion route, and their recognition and uptake by the reticulo-endothelial system (RES) [16]. Clearly, these properties will influence the success of dendrimers as imaging agents or as platforms for therapy delivery. Indeed, these differences may be used advantageously: smaller diameter dendrimers are predominantly excreted via the kidneys, thus these agents may be used for renal imaging; larger sized dendrimers will be taken up by the RES and so can be used in targeted imaging of, or drug delivery to, the liver and spleen. Cationic dendrimers including PAMAM interior with amine surface are generally very sticky and easy to aggregate, so that the molecules are difficult to be purified or concentrated. In terms of in vivo biodistribution of cationic dendrimers showed strong avidity to the liver. In addition, cationic dendrimers tend to show cytotoxicity or renal tocixicity as other cationic macromolecules including polylysine do [7]. Therefore, a dendrimer with PAMAM interior and amine surface is not an appropriate molecule for in vivo application by itself, but a good platform molecule for conjugation chemistry. Hydrophilicity of dendrimer can also be controlled by changing its interior and surface. Less hydrophilic dendrimer tend to accumulated in the liver [16]. Herein, we discuss the current and potential applications of dendrimers in bio-medical imaging and the central role they play in the emerging field of nanotechnology.
2. Contrast agents
Dendrimers have the distinct advantage of low poly-dispersity and therefore, can be fine tuned to the necessary applications. In this section, the synthesis and practical usage of dendrimer-based contrast agents are discussed for the major imaging modalities.
2.1 Magnetic Resonance Imaging agents
Dendrimer molecules are not inherently paramagnetic; thus, either iron oxide or gadolinium must be added to create MRI imaging agents. Dendrimers incorporating gadolinium-chelates for injectable contrast agents, and those containing paramagnetic iron oxide particles, so called ‘magneto-dendrimers’ for labeling and tracking cells have both been developed [17, 18]. The larger generation dendrimers can integrate more imaging moieties, and hence produce a higher MR signal, e.g. G4 dendrimers have 64 primary amine groups available for binding conjugates, whereas G6 molecules have 256 sites available. Thus, a G6 labeled with Gadolinium chelates will likely be a better contrast agent, assuming the larger size of the G6 does not interfere with the intended function of the contrast agent. Gadolinium chelates must be added on the outer shell of the dendrimer in order to allow the interaction of the Gadolinium ion with water protons. Gadolinium-Diethylene triamine pentaacetic acid (Gd-DTPA) chelates that are bound to dendrimers have less rotational movement than individual molecules of Gd-DTPA (Figure 1), and thus produce increased relaxivity per Gadolinium than Gd-DTPA alone. On MR images that translates into increased signal.
Figure 1.
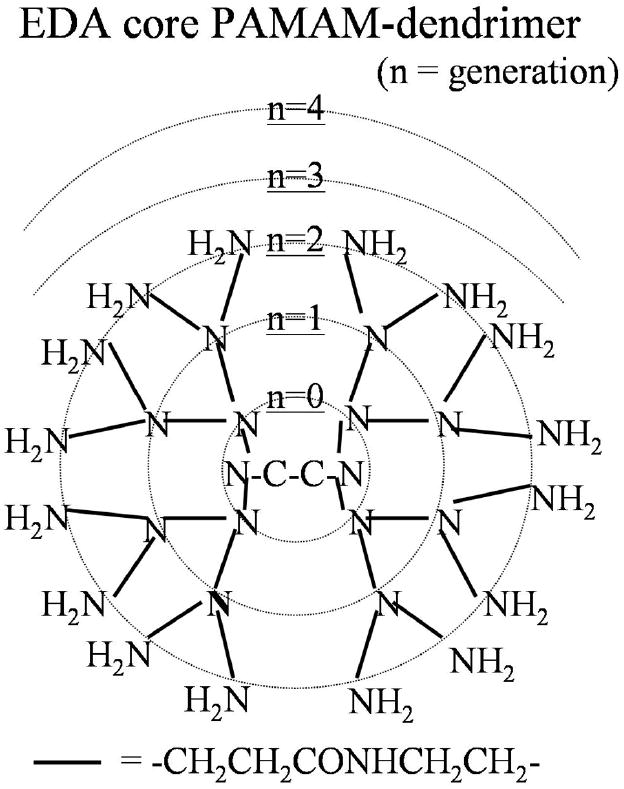
A schema of EDA core PAMAM dendrimer-based MRI contrast agent is shown.
One concern about intravenously administered dendrimer-based Gadolinium contrast agents has arisen recently. There is now some theoretical concern that the prolonged retention within the vasculature owing to the large size of these molecules may lead to a dissociation of toxic Gd ions from chelates. Recent case reports of nephrogenic systemic fibrosis (NSF) following administration of Gadolinium contrast agents in patients with renal failure highlight these concerns [19]. The registry at Yale University, USA cites over 200 cases of NSF; all patients had either end-stage renal failure or significant renal impairment, and 75 had a documented prior exposure to Gadolinium [20]. Prolonged retention and the resulting likelihood of Gadolinium dissociation is a popular theory for the pathogenesis of this condition [21]. The use of strong chelates (e.g. DOTA) or cages for incorporating and constraining gadolinium ions and the minimizing the injection dose of the agent should be considered to avoid this problem. Fortunately, dendrimer-based macromolecular contrast agents have up to 10-fold relaxivities compared with Gd-DTPA agent in the magnetic field of 1-1.5 Tesla range [16, 22].
Iron oxides are superparamagnetic and create an MR signal predominantly by shortening the T2 relaxation time to produce signal drop-out, hence produce a ‘negative’ enhancement. Iron oxides when added to dendrimers usually significantly increase the overall size of the molecule, often doubling it. The larger size of the higher generation dendrimers especially incorporating iron oxides results in delayed renal excretion and preferential excretion through the liver. Therefore, the combination of iron oxide and dendrimer has been used only as an excellent agent for cell labeling.
Dendrimers were first used as MR contrast agents by Wiener et al in 1994 [23]. To date, dendrimers have not been used in human studies, but their efficacy has been demonstrated in numerous animal models. The low polydispersity of dendrimers means that they can be reliably and consistently re-produced. Moreover, the increase in generations produces a predictable and accurate increase in both diameter and weight of the molecule which can be tailored to specific imaging requirements. For example, MR imaging with smaller generation agents (<5 nm diameter) shows them to leak rapidly from even normal vasculature in a similar, but slightly slower, manner to the low molecular weight gadolinium chelates (i.e. Gd-DTPA). These agents are excreted rapidly via the kidneys, primarily during the first pass, and may be useful in assessing renal function [24]. Conversely, the intermediate size generation dendrimers (G4 or G5; 5-8 nm in diameter) are able to selectively leak through hyperpermeable tumor vessels, but not through normal vessels. G9 and G10 dendrimer (> 13 nm) agents are preferentially taken up by, and can be used to enhance, the reticulo-endothelial system within the liver and spleen. These, and larger generation agents demonstrate only minimal leakage through tumor vessels, but are excellent for MR-enhancement of the blood-pool vasculature [23].
Yordanov et al used varying generations of gadolinium-dendrimers to determine the optimal dendrimer for imaging tumors in murine models [25]. Using dynamic-contrast enhanced MRI techniques, G8 dendrimers enhanced the tumor vasculature, whereas the smaller G6 agents, were able to leak through these vessels and better delineate the tumor tissue itself. The ability of PAMAM-G8 dendrimers to demonstrate tumor vessel permeability and the early effects of radiation therapy have also been established [26]. Thus, it may be possible to monitor the effects of radiation and/or cytotoxic drug therapy following a treatment course, in order to demonstrate treatment response or resistance at an early time-point and potentially aid clinical decision making.
Molecular weight and hydrodynamic diameter are not the only determinants of dendrimer pharmacokinetics; altering the hydrophilic or hydrophobic nature of the dendrimers also influences pharmacokinetics. Increasing the hydrophilicity of PAMAM-G4 dendrimers by PEG-lation was shown to reduce the renal excretion of these relatively small dendrimers and increases their liver accumulation, hence prolonging their half-life and enhancing blood vessel visualization by MRI [17]. The DAB/PPI dendrimers are inherently more hydrophobic than their PAMAM counterparts, thus they preferentially accumulate in the liver, potentially making them more suitable as hepatic imaging agents [27] (Figure 2). Dendrimer agents have also been shown to be efficacious for MR lymphangiography via interstitial injection, enabling visualization of the lymphatic system. Again the hydrophilicity of the agent will help determine its characteristics: Gadolinum-conjugated PAMAM-G4 can be used to visualize the lymphatics, whereas DAB-G5 has been shown to be better suited for the identification of individual lymph nodes [28].
Figure 2.
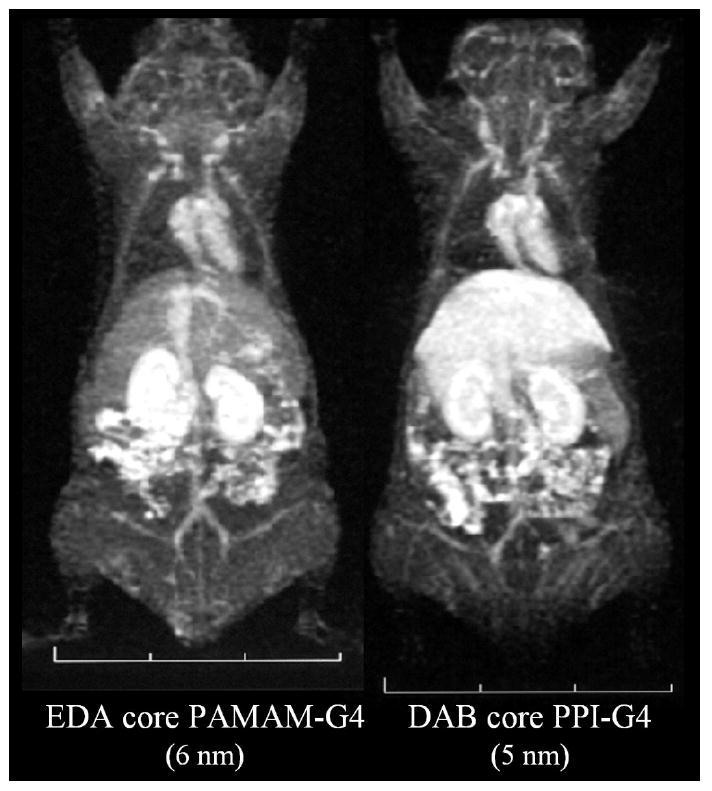
Contrast enhanced whole body 3D-MRIs of a normal mouse using EDA core PAMAM-G4 dendrimer-based MRI contrast agent (left) and DAB core PPI-G5 dendrimer-based MRI contrast agent (right) are shown. Despite of similar physical sizes, a little less hydrophilic DAB core PPI-G5 based agent auumulated in the liver significantly more than EDA core PAMAM-G4.
Gadolinium-based dendrimer agents for MRI hold the advantage of being ‘positive’ contrast agents as compared to iron oxide-based agents which produce a differential enhancement by signal reduction. However, concerns remain over the theoretical risk of dendrimer breakdown and resulting gadolinium toxicity following prolonged retention of these agents. Nevertheless, MR-based dendrimer contrast agents hold great promise, however, these and other long term safety concerns need to be addressed before such dendrimer agents are made available in the clinic.
2.2 Computed Tomography agents
There has been relatively little research into the use of dendrimers as CT contrast agents. One reason is the high required dose of iodinated contrast agents suitable for CT and the concern over toxicity. Another one of the reasons for less emphasis on CT is the high dose of ionizing radiation that small animals are exposed to during a CT scan. This is particularly problematic when repeated scans are necessary; for instance in relation to dynamic CT, where multiple scans are required, and/or in the monitoring tumor response following anti-angiogenic therapy. This makes it difficult to test in pre-clinical settings. However, there are certain circumstances where iodinated CT dendrimer agents may be beneficial. Traditional contrast-enhanced CT scans use low-molecular weight iodinated agents (<2,000 Da); these agents readily leak from hyperpermeable tumor vasculature, however, they also have a high first-pass extravasation through normal, non-cerebral vessels. CT angiography is used to visualize arterial and venous blood flow; indications include pulmonary angiography (suspected pulmonary embolus), and neuro-angiography (suspected stroke). Contrast agents of a larger molecular weight have the benefit of remaining within the intravascular compartment, thus, are more suitable as blood pool enhancing agents than their low-molecular weight counterparts.
Macromolecular agents have started to be developed with this aim in mind. P743, a non-ionic iodinated macromolecule with a molecular weight of 13 kD, was shown to have pharmacokinetic and imaging profiles that were consistent with those of a rapid-clearance blood-pool agent [29]. Efforts to develop larger macromolecular agents for CT enhancement (>30 kDa), that could attain greater blood pool retention are yet to advance to the stage of clinical trials. Examples include polymeric micelles formed from iodine-containing amphiphilic block-copolymers [30], and iopromide-containing liposomes (both PEG-ylated and non-PEG-ylated) [31]. However, both these compounds display a high degree of polydispersity, which may compromise reproducibility and accuracy. Thus, a low polydispersed dendrimer family of compounds may be more appealing as macromolecular CT contrast agents. As with the other macromolecules developed for CT imaging, dendrimers are not inherently radio-opaque, thus need to be bound to iodinated compounds on their surface. X-ray CT is much less sensitive than MRI so high concentrations of iodine are needed and that makes chemical synthesis of macromolecular contrast agents challenging. As with Gadolinium-dendrimers, prolonged retention of iodinated dendrimer agents may prove problematic. The main side-effects of iodinated agents are anaphylactoid reactions, and damage to the kidneys causing acute renal failure [32, 33]. For this reason iodinated agents are contra-indicated in patients with existing renal insufficiency, or at significant risk of such (e.g. patients with diabetes mellitus, multiple myeloma); patients on dialysis are exempt, because dialysis can be used to remove the agent [34].
Fu et al have recently produced a CT compatible, paired dendrimer particle based on a poly-ethylene glycol (PEG) core [35]. They synthesized paired G3, G4 and G5 dendrimers (molecular weight: 35 – 143 kDa) with 16, 32, and 64 amino groups, respectively, available for conjugation with reactive tri-iodophthalamide moieties. Using rat models, they were able to achieve successful in vivo imaging with intravascular enhancement, and a half-life of 35 minutes (G4 molecules). The molecules all demonstrated high water-solubility and hydrophilicity, low osmolality, and good chemical stability. Although CT has the distinct disadvantage of ionizing radiation exposure, macromolecular agents may prove beneficial for certain types of CT investigation, and the early research into using dendrimer agents to achieve this goal certainly appears promising.
2.3 Optical Imaging agents
Optical imaging has a number of advantages over the other imaging modalities: cameras are portable and relatively inexpensive, additionally optical techniques have a high sensitivity and specificity, with excellent temporal and spatial resolution, but without exposure to ionizing radiation [36]. However, they are limited in their ability to penetrate through tissue, which is a particular problem for in-vivo human studies. A lot of research has centered on using dendrimers for optical imaging, although the majority of these studies use in-vitro as opposed to in-vivo models. It has previously been observed that PAMAM dendrimers with carboxyl and amino terminals have a weak, but detectable intrinsic fluorescence [37]. In addition, a simple oxidation of OH-terminated PAMAM dendrimers produces intrinsic blue fluorescence with high quantum yield [38]. Wang et al also observed strong fluorescence emission from different kinds of dendrimers under acidic condition [39]. Shi et al reported on dendrimer-stabilized gold particles which displayed strong blue emission intensity at 458 nm [40]. Al-Jamal et al have exploited this inherent fluorescence, using G6-dendrimers, to monitor their uptake and trafficking within the cytoplasm of target cells, without the need for linkage of external optical contrast agents [41]. Using the inherent fluorescence of dendrimers has its advantages. Primarily, it means that incorporation of potentially toxic optical agents, such as the inorganic, cadmium-based optical dyes is unnecessary. Additionally, the size and mobility of the unmodified dendrimer will be unaffected, and the risk of the attached optical probe dissociating before the dendrimer reaches its target is also avoided.
Jevprasesphant et al have shown binding and uptake of fluorescein isothiocyanate (FITC)-conjugated G3-PAMAM dendrimers into cells, using both flow-cytometry and confocal laser scanning microscopy [42]. Transmission electron microscopic analysis of the cells following incubation with gold-labeled G3 PAMAM dendrimers confirmed their endocytosis-mediated cellular internalization. Shi et al demonstrated that G5 dendrimer-entrapped gold nanoparticles can be covalently linked with folic acid and FITC for cancer cell targeting and optical imaging [43]. Striebel et al have used dendrimers to amplify the DNA microarray fluorescent signal for the detection of viral pathogens [44]. G4-PAMAM dendrimers were conjugated to Cy3-flourophores and the relevant complementary oligonuclide probes in order to produce significantly enhanced fluorescence intensity when detecting viruses from the herpes simplex group, using their microarray hybridization technique. Thomas et al have used FITC-labeled, monoclonal antibody conjugated G5-PAMAM dendrimers to successfully monitor the targeting of, and uptake by, the cell surface receptors CD14 and prostate-specific membrane antigen in vitro [45]. Ibey et al developed an Alexa Fluor 594-labeled G4-PAMAM for the near continuous detection of glucose levels in diabetic patients, as an implantable chemical assay, which would have a clear clinical use [46]. Similar dextran-based assays, also using optical imaging, work through a competitive binding reaction between the protein concanavalin A, dextran, and glucose. The dendrimer-based assay was shown to be advantageous in terms of having a larger response to physiological glucose concentrations, and incorporating longer wavelength dyes to improve signal penetration. Hill et al synthesized G5-PAMAM dendrimers conjugated to FITC and peptides containing the Arg-Gly-Asp(RGD) motif targeting integrin receptors αv/β3 [47]. αv/β3 receptors are preferentially expressed on proliferating endothelial cells during angiogenesis [48]. Such dendrimeric platforms with demonstrated specificity to αv/β3 integrins, may be useful for in-vivo evaluation of tumor-related angiogenesis and as well as in monitoring response to anti-angiogenic medications [49]. Shukla et al tested a G5-PAMAM dendrimer labeled with alexaFluor 488 and conjugated to anti-HER2 monoclonal antibody (mAb) [50]. Overexpression of HER2 receptors has been observed in a variety of epithelial tumors including 20% of breast cancers, where it is associated with a more aggressive tumor behavior [51-53]. High levels of HER2 expression serve to identify patients who are most likely to benefit from therapy with the anti-HER2 monoclonal antibody trastuzumab [54]. In-vivo and in-vitro studies demonstrated not only targeting of this dendrimer-mAb conjugate to HER2 receptors but a faster and more efficient internalization in comparison with anti-HER2 antibody alone [50]. Future applications of such compounds may include the optimization of drug delivery directed against HER2 positive tumors. Optically-labeled G4 dendrimers have also been developed for industrial use, as sensors for detection of bacterial contamination in water supplies, where the probes have the advantage of being sensitive, rapid, and robust, with a long operational lifetime of > 64 hours [55].
To date, relatively few groups have managed to successfully adapt ‘optical’ dendrimers for use in in-vivo models. McIntyre et al developed a G4-PAMAM dendrimer core covalently coupled to PB-M7VIS, a novel fluorescein-labeled, polymer-based substrate, which targets matrix metalloproteinase-7 (MMP-7) [56]. MMP-7 is known to be important in the progression of a number of tumors (e.g. colon, breast) since it is an initial enzyme involved in remodeling of neoplastic vessels, thus may serve as a suitable target for the treatment of such tumors. After binding, MMP-7 cleaves the peptide to produce a several-fold enhancement in the optical signal of fluorescein. They used a murine xenograft model of SW480 human colon cancer cells which were either positive or negative for MMP-7. PB-M7VIS-dendrimers produced a significantly higher fluorescence in the MMP-7-positive tumors, compared to the control MMP-7 negative tumors. Additionally, prior treatment of MMP-7 positive tumors with an MMP inhibitor decreased the relative fluorescence of MMP-7 positive tumors by 60%, demonstrating the selective nature of this optically-labeled dendrimer. El-Sayed et al used in-vivo models to study the extravasation process of the fluorescently labeled PAMAM dendrimers (G0-G4), fluorescently labeled with FITC [57]. In this study, cremaster muscles were visualized for extravasation of the various optically-labeled dendrimer preparations, using intravital microscopy techniques. Results showed that an increase in dendrimer size resulted in an exponential increase in the time needed for extravasation. Optically-labeled dendrimers have also been used for monitoring dendrimer-based delivery of gene therapy to the nucleus of cells and in dual-modality imaging, vide infra.
Optical imaging has its own distinct advantages, yet significant problems need to be overcome before it can be successfully translated into the clinic. However, it is clear that optical imaging has a strong role to play in the development and testing of new dendrimer-based therapies and imaging agents in the laboratory.
2.4 Nuclear Medicine agents
Nuclear medicine is a medical specialty that uses the properties of radioactive compounds for the diagnosis and treatment of disease. Radionuclides, or radio-pharmaceuticals (pharmaceuticals labeled with radionuclides) are administered and the radiation they emit is detected by sensors. A gamma camera is an example of a detector that can be used; in scintigraphic imaging the camera detects emission of the tracer in a planar form, whereas in Single Photon Emission Tomography (SPECT), a tomographic technique, the gamma camera is able to detect and reconstruct the tracer emissions as 3-Dimensional images [58]. Positron Emission Tomography (PET) uses compounds labeled with positron-emitting radionuclides to provide images of physiologic processes. PET is based on the detection of two photons produced when the positron is emitted from the nucleus of an unstable radionuclide and annihilates with its antiparticle (an electron). PET has a 10-fold higher sensitivity than SPECT and is able to detect picomolar concentrations of a tracer. However, both SPECT and PET are limited by a low spatial resolution. PET and SPECT can be concurrently acquired with CT scans in the same session, in order to provide attenuation correction of the emission images, and to allow a more accurate anatomic correlation of tracer uptake [59-61]. Depending on the efficiency of targeting, low signal-to-noise ratios may make it difficult to distinguish targets from the background. As a result the majority of research into the use of dendrimers in the field of nuclear medicine has concentrated less on the primary diagnosis of tumors, and more on their treatment, i.e. dendrimers delivering cytotoxic radiation via radioimmunotherapy, which will be discussed later.
2.5 Transmission electron microscopy agents
The development of dendrimer- entrapped metal nanoparticles (DENP) has been an area of recent interest due to their unique structure and functionality. The high electron density properties of noble metals such as gold and silver allow imaging with transmission electron microscopy (TEM) while the interactions with the surrounding biological environment are determine by the properties of the hosting dendrimer. Bielinska et al [62] studied gold/PAMAM dendrimer composites in-vitro and in-vivo. Results demonstrated that a few atoms of gold per PAMAM dendrimer already make possible the visualization of the amorphous single and multiple nanocomposite units in tissues and in cells by TEM. Lesniak et al [62, 63] synthesized silver/dendrimer nanocomposites with potential for TEM imaging. When polycationic and polyanionic Ag/dendrimers were incubated with various cell lines, particles were detected on the surface of cellular membranes, in the cytoplams, or trapped by the phagocytic or endocytic vesicles. However, cellular uptake of neutral composite was low. Shi et al [43] also utilized TEM to demonstrate in-vitro binding and internalization of dendrimer-entrapped gold nanoparticles (Au DENP) linked to folic acid and FITC in KB cells that overexpressing folate receptors.
2.6 Multi-modality agents
Each imaging modality has its own distinct advantages and limitations. The simultaneous use of two or more modalities may help to overcome the disadvantages of the individual techniques, improving the information obtained during a single session. As previously mentioned, the combined use of PET and CT is a successful example of multi-modal imaging; the two modalities combined can help identify and localize functional abnormalities [64]. Generally speaking, imaging agents can only be detected by one particular modality, and the use of two separate agents concurrently is rarely attempted. For instance, PET-CT scans are acquired with a PET agent alone; thus, depending on the clinical indication, the unenhanced CT images may be sub-optimal. Dendrimers have multiple binding sites and have the potential to be loaded with multiple, separate imaging agents, thus enabling their detection by two, or more, modalities. Such probes may further improve the information derived from multi-modality scans.
To date there are only few instances of dual-modality probes that have been developed. Examples include peptide-based probes for recognition by both radionuclide and optical imaging [65, 66]. An example based on macromolecular agents is the probe produced by Dafni et al, using the prototype macromolecular MR contrast agent Albumin-GdDTPA, and linking fluorescent probes for combined MR/optical imaging monitoring of lymph nodal metastases [67]. Talanov et al, was the first group to use dendrimers for the purpose of incorporating separate imaging moieties [68]. A G6-PAMAM dendrimer nanoprobe was complexed to Gd ions and Cy5.5 molecules to allow dual modality recognition by both magnetic resonance and near infrared fluorescence imaging. Fluorescence studies demonstrated that the conjugation of Gd ions did not affect the quantum yield of the probe, however, increases in the Cy5.5 dye content did result in partial quenching of the fluorophore. The dendrimer-based dual modality probe was successfully used in-vivo to visualize sentinel lymph nodes (SLNs) in murine models by both modalities. Progressing this work, Koyama et al, used a similar probe to both identify and resect SLNs during near-infra red (NIR) optical image-guided surgery [69]. Due to the high background signal, the NIR optical imaging was unavailable to identify some SLNs located close to the injection site of the agent, however, MR lymphography imaging was able to overcome this problem, allowing accurate localization of all SLNs, regardless of location. Such a probe may prove useful for the peri-operative detection and removal of SLNs in cases of primary breast cancer or malignant melanoma.
Another combination of dual modality, dendrimer-based imaging agents is a radioisotope- and 5-color NIR fluorophore-labeled G6-PAMAM dendrimer which have been synthesized and used for lymphatic imaging [70] (Figure 3). Similar sensitivity of radionuclide and fluorescence allowed the minimization of the injected dose of the agent down to 1/100 compared with the above mentioned MRI and optical agent for visualizing the lymphatic system.
Figure 3.
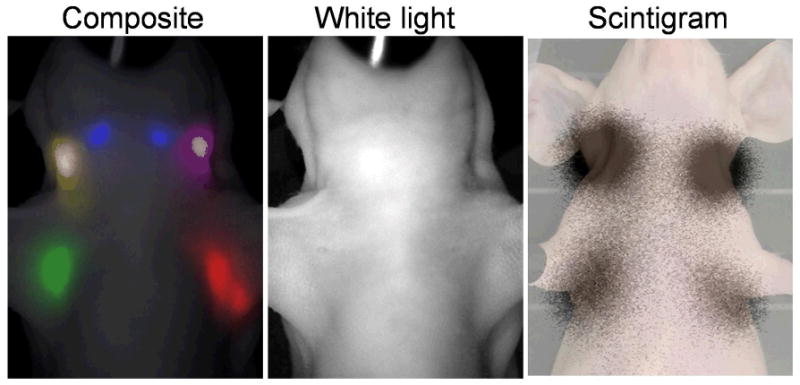
5-color NIR fluorescence and radionuclide lymphatic drainage imaging of the head and neck region obtained with dendrimer-based dual modality contrast agents are shown. Five different lymphatic drainages are separately shown in 5-color NIR fluorescence imaging (left) and quantitatively shown in a schintigram (right).
Multi-modality contrast agents have clear advantages, with macromolecular nanoprobes, and dendrimers in particular, being ideally suited for the incorporation of multiple imaging beacons on each carrier molecule. There are both advantages (convenience) and disadvantages (difficult regulatory approval, expense) to dual modality agents so their future is still uncertain.
3. Therapeutic delivery vectors for cancer treatment
Dendrimers can serve as delivery vehicles, or nano-capsules, which can be tracked with imaging methods such as radionuclides or fluorophores. Multifunctional property of dendrimer to carry the drug and monitor its delivery is a great advantage. However, complexity of synthetic chemistry can be a disadvantage. For example, too intense modification can compromise the ability of dendrimer to holding or releasing drugs. In this section, the use of dendrimers as therapeutic delivery vectors is summarized and discussed especially concerning the types of therapies with imaging potential for monitoring drug delivery.
3. 1. Non-covalent encapsulation of drugs
Initial studies focused on the use of dendrimers for noncovalent encapsulation of drug molecules in the so-called ‘dendritic box’ which was probably more conceptual than real [71-73]. One of the main objectives for incorporation of drugs into the dendrimer was to improve the solubilization of otherwise hydrophobic therapeutic drugs. Initial preparations demonstrated suboptimal retention and release of encapsulated drugs from the dendrimer core [74, 75] Recently PEGylated dendrimers were introduced in attempts to reduced drug leakage in these systems. For example, Bhadra et al. [76] used a G4 PAMAM dendrimer and PEG-5000 to synthesize PEGylated dendrimers for the delivery of 5-fluorouracil (5-FU). After the intravenous administration of PEGylated and non-PEGylated 5-FU containing dendrimers into rats, the maximum drug concentration from free drugs, non-PEGylated dendrimers, and PEGylated dendrimers, was 200-220, 21-23, and 6-7 μg/mL, respectively. However, the blood level of 5-FU was still detectable up to 12 h after the PEGylated dendrimer-5-FU formulation was administrated indicating a lower but more sustained drug exposure. Results demonstrated that PEG coating increased stability and drug loading capacity of PAMAM dendrimers and decrease their rate of 5-FU release.
Further studies by Kojima et al extended the use of PEGylated dendrimers for delivery of doxorubicin and methrotexate [75]. PEG monomethyl ether with the average molecular weight of 550 or 2000 was combined to essentially every chain end of the G3 or G4 dendrimers via urethane bond. Of the two PEGylated dendrimers, G4 dendrimers demonstrated the highest ability to retain doxorubicin and methotrexate (6.5 and 26 molecules respectively). The methotrexate-loaded PEGylated dendrimers released the drug slowly in an aqueous solution of low ionic strength.
Due to the strong cationic properties of some types of dendrimer such as PAMAM dendrimers with amino surfaces, dendrimer can be a good delivery vehicle for the oligo-nucleotides such as anti-sense DNA or small and inhibitory RNA (siRNA). Sato et al. reported that PAMAM G4 dendrimer could efficiently deliver an anti-sense DNA to the target cancer cell and protect DNA from degradation by the serum endonucleotidase. By adding the In-111 to the dendrimer, the delivery of the anti-sense DNA was successfully imaged [77] (Figure 4).
Figure 4.
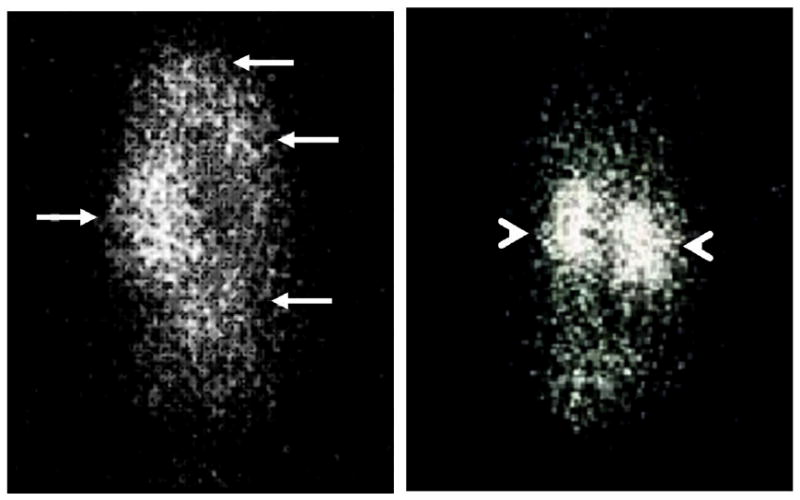
A scintigram, which depicted the delivery of anti-sense oligo-nucleotide with (left) or without (right) dendrimer as a delivery vehicle, is shown. Arrows indicate tumors and arrowheads indicate kidneys.
Even though the introduction of PEG for stabilization of the drug-dendrimer conjugates can slow the leakage of the drugs from dendrimers, covalent dendrimer-drug conjugates offer a better platform for drug delivery.
3.2 Covalent dendrimer-drug conjugates
A favored method for delivery of anti-cancer drugs to a target tumor is to covalently attach these agents to the well defined multivalent surface of the dendrimers. The drug loading can be adjusted by increasing the generation of the dendrimer, and release can be controlled by incorporating degradable linkages between the drug and the dendrimer. Furthermore, the high density of surface groups permits attachment of additional targeting groups or functionality that may modify the solution behavior or toxicity of dendrimers.
In an early study, Malik et al [78] conjugated a G3.5 PAMAM dendrimer to cisplatin giving a dendrimer-platinate which was highly soluble in water and released platinum slowly in-vitro. Intravenous administration of these conjugates to mice bearing B16F10 tumors demonstrated increased efficacy of the dendrimer-platinum complex relative to cisplatin alone (50-fold increase compared to i.v. cisplatin alone).
Padilla De Jesus et al [79] evaluated the application of various dendritic architectures composed of 2,2-bis (hydroxymethyl) propanoic acid as biocompatible drug carriers to improve the therapeutic efficiency of anti-cancer drugs such as doxurubicin. Doxorubicin was covalently bound to this carrier via an acid-labile hydrazone linkage. In-vitro studies showed that the cytotoxicity of doxorubicin was significantly reduced (80–98%), and the drug was successfully taken up by several cancer cell lines.
Patri et al [80] compared the drug release kinetics of covalently coupled methotrexate-dendrimer-folic acid conjugates to complexed methotrexate within the interior of the dendrimer-folic acid complex. Cytotoxicity tests showed that methotrexate as the dendrimer inclusion complex had an activity identical to the free drug in-vitro. In contrast, folic acid targeted dendrimer with covalently conjugated methotrexate specifically killed receptor-expressing cells by intracellular delivery of the drug through receptor-mediated endocytosis. This study demonstrates that while drug as a dendrimer inclusion complex is readily released and active in-vitro, covalently conjugated drug to dendrimer is better suited for specifically targeted drug delivery. Further investigation by Thomas et al demonstrated improved cellular uptake and cytotoxicity in-vitro of a covalently coupled fluorescein-methotrexate-G5 dendrimer-folic acid conjugate against folic acid receptor-expression KB cells. The targeted dendrimer containing folic acid inhibited cell growth in KB cells, whereas the non-targeted methotrexate-G5 dendrimer did not [81]. This dendrimer conjugate was also tested in mice bearing human xenographs overexpressing folic acid receptors demonstrated increased methotrexate anti-tumor activity and markedly decreased its toxicity, allowing improvements in therapeutic response in comparison with free drug [82, 83].
A recent study reported the use of dendrimers as building blocks in the construction of new ‘bow-tie’ structures for delivery of drugs [84]. In this construct, the peripheral hydroxyl groups of one hemisphere of the dendrimer are functionalized with PEO chains and the peripheral hydroxyl groups on the opposite hemisphere are left unfunctionalized for the subsequent attachment of a drug or reporter payload. Lee et al [85] evaluated the effect of doxorubicin (DOX) conjugated to a bow-tie dendrimer in mice bearing C-20 colon carcinoma. In culture, dendrimer-DOX was > 10 × less toxic than free DOX. In vivo studies demonstrated that the anti-cancer activity of the conjugate was 9 fold higher compared to free DOX. The remarkable antitumor activity of dendrimer–DOX results from the ability of the dendrimer to favorably modulate the pharmacokinetics of the attached DOX.
3.3 Dendrimers in radioimmunotherapy (RIT)
Radiolabeled antibodies have been extensively evaluated for therapy of cancer [86]. As one of many promising therapeutic strategies that are encompassed by the ‘magic-bullet’ concept historically linked to antibodies, radioimmunotherapy (RIT) uses an antibody that recognizes tumor-associated antigens and carries cytotoxic radionuclides to target cancer cells [87]. The approval of 2 radiolabeled anti-CD 20 antibodies for the treatment of non-Hodgkin's lymphoma (90Y-labeled ibritumomab tiuxetan and 131I-labeled tositumomab) has rekindled interest in RIT [88].
131I is the most widely understood radionuclide in use for RIT [89]. Despite a simple protein labeling process, 131I has the disadvantages of a long half-life and high γ-emissions requiring patient isolation or at least proper outpatient precautions to avoid unnecessary radiation of patient's close contacts. Furthermore, 131I is not optimal when conjugated to antibodies that internalize via the lysosomes due to dehalogenation. Methods for attaching metal chelating groups have facilitated the study of metallic radionuclides (e.g., 90Y, 177Lu and 67Cu) that are potentially better suited for RIT. In such cases, the radiometals require a chelate to bind the radionuclide, in order to avoid their release and subsequent effects of either inappropriate targeting and/or adverse dosimetry [90]. RIT is ideally preceded by a diagnostic study establishing target and favorable biodistribution for dosimetry. An interesting approach is the take advantage of the superior spatial resolution of PET, matching a positron emitter radionuclide (β+ emission) with an analogous therapeutic radionuclide which decays by β- emission. Examples of matched β+/β− (imaging/therapy) pairs include 124I/131I, 86Y/90Y and 64Cu/67Cu.
The use of dendrimers for potentially improving delivery of radioimmunotherapy conjugates is in its early phases of development. In an early report, Roberts et al successfully attached starburst dendrimers to IgG-antibodies in a two step reaction [91]. The immunoreactivity of antibodies were shown to be largely unaffected by dendrimer conjugation as determined via sensitive enzyme linked immunoassays (ELISA). In an effort directed towards testing appropriate dendrimer labeled radionuclides to establish targeting and adequate biodistribution, Kobayashi et al evaluated the in-vivo biodistribution of diagnostic radionuclides- 111I and 88Y labeled G2-PAMAM dendrimers conjugated with 1B4M (a derivative of DTPA) [92]. They found high accumulation in the liver, kidney, and spleen, which was noted to significantly decrease when the chelates were saturated with cold radiometals. Additionally, clearance times of unsaturated preparations were significantly slower than those of saturated preparations, suggesting that charge and molecular weight are not the only factors affecting biodistribution (the final charge and molecular weight should be similar following the saturation process). 125Iodine-labeled G3- and G4-PAMAM dendrimers have been successfully developed in-vivo and in-vitro as imaging agents for nuclear medicine [7, 93]. Further studies are necessary to establish the clinical usefulness of dendrimer-conjugates for radioimmunotherapy.
3.4. Dendrimers in boron neutron capture therapy
Boron neutron capture therapy (BNCT) is based on the selective uptake of 10B, a non-radioactive constituent of the natural element boron by a tumor, and the subsequent irradiation of the area with an appropriate low-energy neutron beam. 10B is then activated to 11B, which will immediately decay releasing alpha-particles (4He) and recoiled lithium nuclei (7Li). These particles deposit large amounts of energy along their very short path (< 10 μm of range), causing highly localized ionization and damage to tumor cells. Clinical interest in BNCT has focused mainly on the treatment of high grade gliomas [94], and either cutaneous primaries [95] or cerebral metastases of melanoma. More recently, it also has been used to treat patients with head and neck [96, 97] and metastatic liver cancer [98].
For BNCT to be successful a sufficient number of 10B atoms (∼ 109 atoms/cell) must be delivered to tumor cells and a sufficient number of thermal neutrons must be absorbed by them to sustain a lethal 10B (n, α) 7Li capture reaction. Delivery of high levels of boron to tumors can be accomplished by using boronated monoclonal antibodies (mAbs) targeted to tumor associated epitopes. To be effective, mAbs must recognize a surface membrane epitope that is highly expressed on tumor cells and a large number of boron-10 atoms must be attached to each antibody molecule. However, direct immunoconjugation of boron atoms to mAbs is a difficult task and may impair the solubility and immunoreactivity of the antibodies [99].
Dendrimers have been studied as a potential platform for the delivery of boronated monoclonal antibodies to tumor cells due to their well-defined structure and large number of reactive terminal groups. In an early study by Bath et al [12], attempts were made to react second and fourth generation PAMAM dendrimers with water soluble isocyanato polyhedral borane [Na(CH3)3NB10H8NCO] and to link them to the mAb IB16-6 directed against murine B16 melanoma cells. Unfortunately, after the intravenous injection of these conjugates, loss of reactivity and altered biodistribution was detected. In subsequent studies, the same group reported heavy boronation of fifth generation PAMAM dendrimers to anti-EGFR mAb cetuximab and the EGFRvIII mAb L8A4 [100]. The bioconjugates were injected intratumorally in rats bearing F98 glioma that had been genetically engineered to express either wild-type EGFR [101] or EGFRv111 [102]. Results demonstrated tumor boron concentrations that were in the therapeutic range (∼ 20 μg/g wet weight tumor).
Recent studies have focused on the poly(ethylene glycol) (PEG) modification of boronated PAMAM dendrimers in order to improve biodistribution and decrease uptake by the reticuloendothelial system [103]. Folic acid moieties were also conjugated to the ends of the PEO chains to enhance the uptake of the dendrimers by tumors. The expression of the folate receptor is amplified in a variety of human tumors which may serve as a molecular target for BNCT [104]. Shukla et al, evaluated a folate receptor-targeted boronated G3 PAMAM dendrimer containing approximately 13 decaborate clusters, approximately 1 PEG(2000) unit, and approximately 1 PEG(800) unit with folic acid attached to the distal end. Biodistribution studies with this conjugate in C57BL/6 mice bearing folate receptor (+) murine 24JK-FBP sarcomas resulted in selective tumor uptake (6.0% ID/g tumor), but also high hepatic (38.8% ID/g) and renal (62.8% ID/g) uptake. The authors concluded that even though the strategy was successful in increasing tumor selectivity, further investigation is necessary to optimize biodistribution.
An alternative technique to optimize delivery of boron compounds to tumor cells is to incorporate carborane cages within the dendrimer. Parrot et al [105], synthesized a biocompatible aliphatic polyester dendrimer that incorporated as many as 16 p-carboranes in their interior. Water solubility has been achieved by modification of their periphery, making them possible candidates for use in BNCT.
4. Summary
Dendrimers are a class of synthetically produced spherical macromolecules; their low polydispersed nature means that they can be reliably and accurately synthesized and evaluated with consistent results. Dendrimers have been developed for use as contrast agents for Computed Tomography, nuclear medicine, optical imaging, and Magnetic Resonance Imaging. Although dendrimers could emit fluorescence by themselves, this cannot yet be adapted for clinical use, however, exogenous fluorophores can be conjugated to the exterior primary amine groups to enable detection by the respective imaging modalities.
Increasing generation size affects the pharmacokinetic and pharmacodynamic properties of dendrimers, altering their permeability across the vascular wall, excretion route, and their recognition and uptake by the reticulo-endothelial system. These differences may be used advantageously: smaller diameter dendrimers may be used for renal imaging, whereas larger sized dendrimers are predominantly sequestered by the RES, thus can preferentially image the liver and spleen. The majority of dendrimer research has centered on their use as MR contrast agents. Dendrimers can either incorporate gadolinium-chelates, which have the advantage of producing a ‘positive’ contrast signal, or superparamagnetic iron oxide particles for cell tracking. Contrast agents of a larger molecular weight have the benefit of remaining within the intravascular compartment, thus are more suitable as blood pool enhancing agents, to this end dendrimers incorporating iodinated contrast may prove beneficial for the purposes of CT angiography. However, prolonged vascular half-life carriers with it the potential risk of more toxicities including degradation of the Gadolinium-chelate leading to the release of free gadolinium, which is a hypothetical risk.
Another prospective function of dendrimers is as a traceable drug delivery vectors, whereby therapeutic payloads are encapsulated within the shell of the dendrimer. Integration of targeting epitopes such as monoclonal antibodies offers potential for the targeted delivery of such agents directly to tumor cells. Dendrimers have successfully been developed to deliver cytotoxic drugs, gene therapy, and radiation sensitizers (for external radiation therapy) to tumors in animal models.
The simultaneous use of two or more modalities may help to overcome the disadvantages of the individual techniques. The larger size of higher dendrimer generations offers potential to develop dual-modality agents that can be imaged in more than one modality (e.g. combined optical and MRI or optical and radionuclide imaging), or even dual-purpose agents that can both image and treat a tumor following administration. Multi-modality contrast agents have clear advantages, and their multiple binding sites make dendrimers ideally suited for the incorporation of multiple separate agents. Although still largely at an experimental stage, it is clear that now and in the future, dendrimers have a vital role to play in the rapidly developing field of medical nanotechnology and in both the diagnosis and treatment of tumors. The road to the clinical application of dendrimer-based macromolecular imaging/therapeutic agents, has become a hot topic, which remains to be investigated and discussed by scientific researchers and administrative sides in numbers of aspects including synthesis, purity of agents, toxicity, pharmacokinetics and excretion, immunogenecity.
5. References
- 1.Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P. A New Class of Polymers - Starburst-Dendritic Macromolecules. Polymer Journal. 1985;17:117–132. [Google Scholar]
- 2.Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P. Dendritic Macromolecules - Synthesis of Starburst Dendrimers. Macromolecules. 1986;19:2466–2468. [Google Scholar]
- 3.Newkome GR, Yao Z, Baker GR, Gupta VK. Micelles. Part 1. Cascade molecules: a new approach to micelles. A [27]-arborol. J Org Chem. 1985;50:2003–2004. [Google Scholar]
- 4.Tomalia DA, Naylor AM, Goddard WA., Iii Starbust dendrimers: Molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angewandte Chemie - International Edition in English. 1990;29:138–175. [Google Scholar]
- 5.Hawker CJ, Frechet JMJ. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. Journal of the American Chemical Society. 1990;112:7638–7647. [Google Scholar]
- 6.Tomalia DA, Frechet JMJ. Discovery of dendrimers and dendritic polymers: A brief historical perspective. Journal of Polymer Science Part a-Polymer Chemistry. 2002;40:2719–2728. [Google Scholar]
- 7.Malik N, Wiwattanapatapee R, Klopsch R, Lorenz K, Frey H, Weener JW, Meijer EW, Paulus W, Duncan R. Dendrimers: relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J Control Release. 2000;65:133–48. doi: 10.1016/s0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- 8.Misselwitz B, Schmitt-Willich H, Ebert W, Frenzel T, Weinmann HJ. Pharmacokinetics of Gadomer-17, a new dendritic magnetic resonance contrast agent. Magma. 2001;12:128–34. doi: 10.1007/BF02668094. [DOI] [PubMed] [Google Scholar]
- 9.Wu CC, Brechbiel MW, Kozak RW, Gansow OA. Metal-Chelate-Dendrimer-Antibody Constructs for Use in Radioimmunotherapy and Imaging. Bioorganic & Medicinal Chemistry Letters. 1994;4:449–454. [Google Scholar]
- 10.Gillies ER, Frechet JM. Dendrimers and dendritic polymers in drug delivery. Drug Discov Today. 2005;10:35–43. doi: 10.1016/S1359-6446(04)03276-3. [DOI] [PubMed] [Google Scholar]
- 11.Shah DS, Sakthivel T, Toth I, Florence AT, Wilderspin AF. DNA transfection and transfected cell viability using amphipathic asymmetric dendrimers. Int J Pharm. 2000;208:41–8. doi: 10.1016/s0378-5173(00)00534-2. [DOI] [PubMed] [Google Scholar]
- 12.Barth RF, Adams DM, Soloway AH, Alam F, Darby MV. Boronated starburst dendrimer-monoclonal antibody immunoconjugates: evaluation as a potential delivery system for neutron capture therapy. Bioconjug Chem. 1994;5:58–66. doi: 10.1021/bc00025a008. [DOI] [PubMed] [Google Scholar]
- 13.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–92. [PubMed] [Google Scholar]
- 14.Beezer AE, King ASH, Martin IK, Mitchel JC, Twyman LJ, Wain CF. Dendrimers as Potential Drug Carriers; Encapsulation of Acidic Hydrophobes within Water Soluble PAMAM Derivatives. Tetrahedron. 2003;59:8. [Google Scholar]
- 15.Bosman AW, Janssen HM, Meijer EW. About Dendrimers: Structure, Physical Properties, and Applications. Chem Rev. 1999;99:1665–1688. doi: 10.1021/cr970069y. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi H, Brechbiel MW. Nano-sized MRI contrast agents with dendrimer cores. Adv Drug Deliv Rev. 2005;57:2271–86. doi: 10.1016/j.addr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi H, Kawamoto S, Saga T, Sato N, Hiraga A, Ishimori T, Konishi J, Togashi K, Brechbiel MW. Positive effects of polyethylene glycol conjugation to generation-4 polyamidoamine dendrimers as macromolecular MR contrast agents. Magn Reson Med. 2001;46:781–8. doi: 10.1002/mrm.1257. [DOI] [PubMed] [Google Scholar]
- 18.Kiessling F, Morgenstern B, Zhang C. Contrast agents and applications to assess tumor angiogenesis in vivo by magnetic resonance imaging. Current Medicinal Chemistry. 2007;14:77–91. doi: 10.2174/092986707779313516. [DOI] [PubMed] [Google Scholar]
- 19.Grobner T. Gadolinium--a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104–8. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 20.Cowper SE. Nephrogenic fibrosing dermopathy. Yale University. 2007 NFD/NFS Website. [Google Scholar]
- 21.Rosenkranz AR, Grobner T, Mayer GJ. Conventional or Gadolinium containing contrast media: the choice between acute renal failure or Nephrogenic Systemic Fibrosis? Wien Klin Wochenschr. 2007;119:271–5. doi: 10.1007/s00508-007-0801-8. [DOI] [PubMed] [Google Scholar]
- 22.Bryant LH, Jr, Brechbiel MW, Wu C, Bulte JW, Herynek V, Frank JA. Synthesis and relaxometry of high-generation (G = 5, 7, 9, and 10) PAMAM dendrimer-DOTA-gadolinium chelates. J Magn Reson Imaging. 1999;9:348–52. doi: 10.1002/(sici)1522-2586(199902)9:2<348::aid-jmri30>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 23.Wiener EC, Brechbiel MW, Brothers H, Magin RL, Gansow OA, Tomalia DA, Lauterbur PC. Dendrimer-based metal chelates: a new class of magnetic resonance imaging contrast agents. Magn Reson Med. 1994;31:1–8. doi: 10.1002/mrm.1910310102. [DOI] [PubMed] [Google Scholar]
- 24.Anzai Y. Superparamagnetic iron oxide nanoparticles: nodal metastases and beyond. Top Magn Reson Imaging. 2004;15:103–11. doi: 10.1097/01.rmr.0000130602.65243.87. [DOI] [PubMed] [Google Scholar]
- 25.Yordanov A, Kobayashi H, English S, Reijnders K, Milenic D, Krishna M, Mitchell J, Brechbiel M. Gadolinium-labeled dendrimers as biometric nanoprobes to detect vascular permeability. Journal of Materials Chemistry. 2003;13:3. [Google Scholar]
- 26.Kobayashi H, Reijnders K, English S, Yordanov AT, Milenic DE, Sowers AL, Citrin D, Krishna MC, Waldmann TA, Mitchell JB, Brechbiel MW. Application of a macromolecular contrast agent for detection of alterations of tumor vessel permeability induced by radiation. Clin Cancer Res. 2004;10:7712–20. doi: 10.1158/1078-0432.CCR-04-1175. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi H, Kawamoto S, Saga T, Sato N, Hiraga A, Ishimori T, Akita Y, Mamede MH, Konishi J, Togashi K, Brechbiel MW. Novel liver macromolecular MR contrast agent with a polypropylenimine diaminobutyl dendrimer core: comparison to the vascular MR contrast agent with the polyamidoamine dendrimer core. Magn Reson Med. 2001;46:795–802. doi: 10.1002/mrm.1259. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi H, Kawamoto S, Choyke PL, Sato N, Knopp MV, Star RA, Waldmann TA, Tagaya Y, Brechbiel MW. Comparison of dendrimer-based macromolecular contrast agents for dynamic micro-magnetic resonance lymphangiography. Magn Reson Med. 2003;50:758–66. doi: 10.1002/mrm.10583. [DOI] [PubMed] [Google Scholar]
- 29.Idee JM, Port M, Robert P, Raynal I, Prigent P, Dencausse A, Le Greneur S, Tichkowsky I, Le Lem G, Bourrinet P, Mugel T, Benderbous S, Devoldere L, Bourbouze R, Meyer D, Bonnemain B, Corot C. Preclinical profile of the monodisperse iodinated macromolecular blood pool agent P743. Invest Radiol. 2001;36:41–9. doi: 10.1097/00004424-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Trubetskoy VS, Gazelle GS, Wolf GL, Torchilin VP. Block-copolymer of polyethylene glycol and polylysine as a carrier of organic iodine: design of long-circulating particulate contrast medium for X-ray computed tomography. J Drug Target. 1997;4:381–8. doi: 10.3109/10611869709017895. [DOI] [PubMed] [Google Scholar]
- 31.Sachse A, Leike JU, Schneider T, Wagner SE, Rossling GL, Krause W, Brandl M. Biodistribution and computed tomography blood-pool imaging properties of polyethylene glycol-coated iopromide-carrying liposomes. Invest Radiol. 1997;32:44–50. doi: 10.1097/00004424-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Schild HH, Kuhl CK, Hubner-Steiner U, Bohm I, Speck U. Adverse Events after Unenhanced and Monomeric and Dimeric Contrast-enhanced CT: A Prospective Randomized Controlled Trial. Radiology. 2006;240:56–64. doi: 10.1148/radiol.2393050560. [DOI] [PubMed] [Google Scholar]
- 33.Finn WF. The clinical and renal consequences of contrast-induced nephropathy. Nephrol Dial Transplant. 2006;21:i2–10. doi: 10.1093/ndt/gfl213. [DOI] [PubMed] [Google Scholar]
- 34.Toprak O. Conflicting and New Risk Factors for Contrast Induced Nephropathy. The Journal of Urology. 2007;178:2277–2283. doi: 10.1016/j.juro.2007.08.054. [DOI] [PubMed] [Google Scholar]
- 35.Fu Y, Nitecki DE, Maltby D, Simon GH, Berejnoi K, Raatschen HJ, Yeh BM, Shames DM, Brasch RC. Dendritic iodinated contrast agents with PEG-cores for CT imaging: synthesis and preliminary characterization. Bioconjug Chem. 2006;17:1043–56. doi: 10.1021/bc060019c. [DOI] [PubMed] [Google Scholar]
- 36.Graves EE, Weissleder R, Ntziachristos V. Fluorescence molecular imaging of small animal tumor models. Curr Mol Med. 2004;4:419–30. doi: 10.2174/1566524043360555. [DOI] [PubMed] [Google Scholar]
- 37.Larson CL, Tcker SA. Intrinsic fluorescence of carboxylateterminated polyamidoamine dendrimers. Applies Spectroscopy. 2001;55 [Google Scholar]
- 38.Lee WI, Bae Y, Bard AJ. Strong blue photoluminescence and ECL from OH-terminated PAMAM dendrimers in the absence of gold nanoparticles. J Am Chem Soc. 2004;126:8358–9. doi: 10.1021/ja0475914. [DOI] [PubMed] [Google Scholar]
- 39.Wang D, Imae T. Fluorescence emission from dendrimers and its pH dependence. J Am Chem Soc. 2004;126:13204–5. doi: 10.1021/ja0454992. [DOI] [PubMed] [Google Scholar]
- 40.Shi X, Ganser TR, Sun K, Balogh LP, Baker JR., Jr Characterization of crystalline dendrimer-stabilized gold nanoparticles. Nanotechnology. 2006;17:1072–1078. [Google Scholar]
- 41.Al-Jamal KT, Ruenraroengsak P, Hartell N, Florence AT. An intrinsically fluorescent dendrimer as a nanoprobe of cell transport. J Drug Target. 2006;14:405–12. doi: 10.1080/10611860600834441. [DOI] [PubMed] [Google Scholar]
- 42.Jevprasesphant R, Penny J, Attwood D, D'Emanuele A. Transport of dendrimer nanocarriers through epithelial cells via the transcellular route. J Control Release. 2004;97:259–67. doi: 10.1016/j.jconrel.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 43.Shi X, Wang S, Meshinchi S, Van Antwerp ME, Bi X, Lee I, Baker JR., Jr Dendrimer-entrapped gold nanoparticles as a platform for cancer-cell targeting and imaging. Small. 2007;3:1245–52. doi: 10.1002/smll.200700054. [DOI] [PubMed] [Google Scholar]
- 44.Striebel HM, Birch-Hirschfeld E, Egerer R, Foldes-Papp Z, Tilz GP, Stelzner A. Enhancing sensitivity of human herpes virus diagnosis with DNA microarrays using dendrimers. Exp Mol Pathol. 2004;77:89–97. doi: 10.1016/j.yexmp.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Thomas TP, Patri AK, Myc A, Myaing MT, Ye JY, Norris TB, Baker JR., Jr In vitro targeting of synthesized antibody-conjugated dendrimer nanoparticles. Biomacromolecules. 2004;5:2269–74. doi: 10.1021/bm049704h. [DOI] [PubMed] [Google Scholar]
- 46.Ibey BL, Beier HT, Rounds RM, Cote GL, Yadavalli VK, Pishko MV. Competitive binding assay for glucose based on glycodendrimer-fluorophore conjugates. Anal Chem. 2005;77:7039–46. doi: 10.1021/ac0507901. [DOI] [PubMed] [Google Scholar]
- 47.Hill E, Shukla R, Park SS, Baker JR., Jr Synthetic PAMAM-RGD conjugates target and bind to odontoblast-like MDPC 23 cells and the predentin in tooth organ cultures. Bioconjug Chem. 2007;18:1756–62. doi: 10.1021/bc0700234. [DOI] [PubMed] [Google Scholar]
- 48.Cox D, Aoki T, Seki J, Motoyama Y, Yoshida K. The Pharmacology of the Integrins. Medicinal Research Reviews. 1994;14:195–228. doi: 10.1002/med.2610140203. [DOI] [PubMed] [Google Scholar]
- 49.Hsu AR, Veeravagu A, Cai W, Hou LC, Tse V, Chen XY. Integrin alpha(v)beta(3) antagonists for anti-angiogenic cancer treatment. Recent Patents on Anti-Cancer Drug Discovery. 2007;2:143–158. doi: 10.2174/157489207780832469. [DOI] [PubMed] [Google Scholar]
- 50.Shukla R, Thomas TP, Peters JL, Desai AM, Kukowska-Latallo J, Patri AK, Kotlyar A, Baker JR., Jr HER2 specific tumor targeting with dendrimer conjugated anti-HER2 mAb. Bioconjug Chem. 2006;17:1109–15. doi: 10.1021/bc050348p. [DOI] [PubMed] [Google Scholar]
- 51.Koeppen HK, Wright BD, Burt AD, Quirke P, McNicol AM, Dybdal NO, Sliwkowski MX, Hillan KJ. Overexpression of HER2/neu in solid tumours: an immunohistochemical survey. Histopathology. 2001;38:96–104. doi: 10.1046/j.1365-2559.2001.01084.x. [DOI] [PubMed] [Google Scholar]
- 52.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5:63–9. doi: 10.3816/cbc.2004.n.011. [DOI] [PubMed] [Google Scholar]
- 53.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 54.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 55.Ji J, Schanzle JA, Tabacco MB. Real-time detection of bacterial contamination in dynamic aqueous environments using optical sensors. Anal Chem. 2004;76:1411–8. doi: 10.1021/ac034914q. [DOI] [PubMed] [Google Scholar]
- 56.McIntyre JO, Fingleton B, Wells KS, Piston DW, Lynch CC, Gautam S, Matrisian LM. Development of a novel fluorogenic proteolytic beacon for in vivo detection and imaging of tumour-associated matrix metalloproteinase-7 activity. Biochem J. 2004;377:617–28. doi: 10.1042/BJ20030582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.El-Sayed M, Kiani MF, Naimark MD, Hikal AH, Ghandehari H. Extravasation of poly(amidoamine) (PAMAM) dendrimers across microvascular network endothelium. Pharm Res. 2001;18:23–8. doi: 10.1023/a:1011066408283. [DOI] [PubMed] [Google Scholar]
- 58.Miller TR. The AAPM/RSNA physics tutorial for residents. Clinical aspects of emission tomography. Radiographics. 1996;16:661–668. doi: 10.1148/radiographics.16.3.8897630. [DOI] [PubMed] [Google Scholar]
- 59.Blankespoor SC, Wu X, Kalki K, Brown JK, Tang HR, Cann CE, Hasegawa BH. Attenuation correction of spect using x-ray ct on an emissiontransmission ct system: myocardial perfusion assessment. IEEE Transactions on Nuclear Science. 1996;43:2263–2274. [Google Scholar]
- 60.Townsend DW, Beyer T, Blodgett TM. PET/CT scanners: A hardware approach to image fusion. Seminars in Nuclear Medicine. 2003;33:193–204. doi: 10.1053/snuc.2003.127314. [DOI] [PubMed] [Google Scholar]
- 61.Yeung H, Schöder H, Smith A, Gonen M, Larson S. Clinical Value of Combined Positron Emission Tomography/Computed Tomography Imaging in the Interpretation of 2-Deoxy-2-[F-18]fluoro-d -glucose–Positron Emission Tomography Studies in Cancer Patients. Molecular Imaging and Biology. 2005;7:229–235. doi: 10.1007/s11307-005-4113-y. [DOI] [PubMed] [Google Scholar]
- 62.Bielinska A, Eichman JD, Lee I, Baker JR, Jr, Balogh L. Imaging {Au0-PAMAM} Gold-dendrimer Nanocomposites in Cells. Journal of Nanoparticle Research. 2002;4 [Google Scholar]
- 63.Lesniak W, Bielinska AU, Sun K, Janczak KW, Shi X, Baker JR, Jr, Balogh LP. Silver/dendrimer nanocomposites as biomarkers: fabrication, characterization, in vitro toxicity, and intracellular detection. Nano Lett. 2005;5:2123–30. doi: 10.1021/nl051077u. [DOI] [PubMed] [Google Scholar]
- 64.Blodgett TM, Meltzer CC, Townsend DW. PET/CT: form and function. Radiology. 2007;242:360–85. doi: 10.1148/radiol.2422051113. [DOI] [PubMed] [Google Scholar]
- 65.Bullok KE, Dyszlewski M, Prior JL, Pica CM, Sharma V, Piwnica-Worms D. Characterization of novel histidine-tagged Tat-peptide complexes dual-labeled with (99m)Tc-tricarbonyl and fluorescein for scintigraphy and fluorescence microscopy. Bioconjug Chem. 2002;13:1226–37. doi: 10.1021/bc025573a. [DOI] [PubMed] [Google Scholar]
- 66.Houston JP, Ke S, Wang W, Li C, Sevick-Muraca EM. Quality analysis of in vivo near-infrared fluorescence and conventional gamma images acquired using a dual-labeled tumor-targeting probe. J Biomed Opt. 2005;10:054010. doi: 10.1117/1.2114748. [DOI] [PubMed] [Google Scholar]
- 67.Dafni H, Cohen B, Ziv K, Israely T, Goldshmidt O, Nevo N, Harmelin A, Vlodavsky I, Neeman M. The role of heparanase in lymph node metastatic dissemination: dynamic contrast-enhanced MRI of Eb lymphoma in mice. Neoplasia. 2005;7:224–33. doi: 10.1593/neo.04433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Talanov VS, Regino CA, Kobayashi H, Bernardo M, Choyke PL, Brechbiel MW. Dendrimer-based nanoprobe for dual modality magnetic resonance and fluorescence imaging. Nano Lett. 2006;6:1459–63. doi: 10.1021/nl060765q. [DOI] [PubMed] [Google Scholar]
- 69.Koyama Y, Talanov VS, Bernardo M, Hama Y, Regino CA, Brechbiel MW, Choyke PL, Kobayashi H. A dendrimer-based nanosized contrast agent dual-labeled for magnetic resonance and optical fluorescence imaging to localize the sentinel lymph node in mice. J Magn Reson Imaging. 2007;25:866–71. doi: 10.1002/jmri.20852. [DOI] [PubMed] [Google Scholar]
- 70.Kobayashi H, Koyama Y, Barrett T, Hama Y, Regino CAS, Shin IS, Jang BS, Le N, Paik CH, Choyke PL, Urano Y. Multimodal nanoprobes for radionuclide and five-color near-infrared optical lymphatic imaging. Acs Nano. 2007;1:258–264. doi: 10.1021/nn700062z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jansen J, Debrabandervandenberg EMM, Meijer EW. Encapsulation of Guest Molecules into a Dendritic Box. Science. 1994;266:1226–1229. doi: 10.1126/science.266.5188.1226. [DOI] [PubMed] [Google Scholar]
- 72.Newkome GR, Moorefield CN, Baker GR, Saunders MJ, Grossman SH. Chemistry of Micelles .13. Unimolecular Micelles. Angewandte Chemie-International Edition in English. 1991;30:1178–1180. [Google Scholar]
- 73.Hawker CJ, Wooley KL, Frechet JMJ. Unimolecular Micelles and Globular Amphiphiles - Dendritic Macromolecules as Novel Recyclable Solubilization Agents. Journal of the Chemical Society-Perkin Transactions 1. 1993:1287–1297. [Google Scholar]
- 74.Jansen J, Meijer EW, Debrabandervandenberg EMM. The Dendritic Box - Shape-Selective Liberation of Encapsulated Guests. Journal of the American Chemical Society. 1995;117:4417–4418. [Google Scholar]
- 75.Kojima C, Kono K, Maruyama K, Takagishi T. Synthesis of polyamidoamine dendrimers having poly(ethylene glycol) grafts and their ability to encapsulate anticancer drugs. Bioconjug Chem. 2000;11:910–7. doi: 10.1021/bc0000583. [DOI] [PubMed] [Google Scholar]
- 76.Bhadra D, Bhadra S, Jain S, Jain NK. A PEGylated dendritic nanoparticulate carrier of fluorouracil. Int J Pharm. 2003;257:111–24. doi: 10.1016/s0378-5173(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 77.Sato N, Kobayashi H, Saga T, Nakamoto Y, Ishimori T, Togashi K, Fujibayashi Y, Konishi J, Brechbiel MW. Tumor targeting and imaging of intraperitoneal tumors by use of antisense oligo-DNA complexed with dendrimers and/or avidin in mice. Clin Cancer Res. 2001;7:3606–12. [PubMed] [Google Scholar]
- 78.Malik N, Evagorou EG, Duncan R. Dendrimer-platinate: a novel approach to cancer chemotherapy. Anticancer Drugs. 1999;10:767–76. [PubMed] [Google Scholar]
- 79.Padilla De Jesus OL, Ihre HR, Gagne L, Frechet JM, Szoka FC., Jr Polyester dendritic systems for drug delivery applications: in vitro and in vivo evaluation. Bioconjug Chem. 2002;13:453–61. doi: 10.1021/bc010103m. [DOI] [PubMed] [Google Scholar]
- 80.Patri AK, Kukowska-Latallo JF, Baker JR., Jr Targeted drug delivery with dendrimers: comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv Drug Deliv Rev. 2005;57:2203–14. doi: 10.1016/j.addr.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 81.Thomas TP, Majoros IJ, Kotlyar A, Kukowska-Latallo JF, Bielinska A, Myc A, Baker JR., Jr Targeting and inhibition of cell growth by an engineered dendritic nanodevice. J Med Chem. 2005;48:3729–35. doi: 10.1021/jm040187v. [DOI] [PubMed] [Google Scholar]
- 82.Majoros IJ, Thomas TP, Mehta CB, Baker JR., Jr Poly(amidoamine) dendrimer-based multifunctional engineered nanodevice for cancer therapy. J Med Chem. 2005;48:5892–9. doi: 10.1021/jm0401863. [DOI] [PubMed] [Google Scholar]
- 83.Kukowska-Latallo JF, Candido KA, Cao Z, Nigavekar SS, Majoros IJ, Thomas TP, Balogh LP, Khan MK, Baker JR., Jr Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005;65:5317–24. doi: 10.1158/0008-5472.CAN-04-3921. [DOI] [PubMed] [Google Scholar]
- 84.Gillies ER, Frechet JM. Designing macromolecules for therapeutic applications: polyester dendrimer-poly(ethylene oxide) “bow-tie” hybrids with tunable molecular weight and architecture. J Am Chem Soc. 2002;124:14137–46. doi: 10.1021/ja028100n. [DOI] [PubMed] [Google Scholar]
- 85.Lee CC, Gillies ER, Fox ME, Guillaudeu SJ, Frechet JM, Dy EE, Szoka FC. A single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomas. Proc Natl Acad Sci U S A. 2006;103:16649–54. doi: 10.1073/pnas.0607705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Witzig TE, White CA, Gordon LI, Wiseman GA, Emmanouilides C, Murray JL, Lister J, Multani PS. Safety of yttrium-90 ibritumomab tiuxetan radioimmunotherapy for relapsed low-grade, follicular, or transformed non-hodgkin's lymphoma. J Clin Oncol. 2003;21:1263–70. doi: 10.1200/JCO.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 87.Ehrlich P. The collected papers of Paul Ehrlich. Pergamon. 1960;3 [Google Scholar]
- 88.Sharkey RM, Burton J, Goldenberg DM. Radioimmunotherapy of non-Hodgkin's lymphoma: a critical appraisal. Expert Rev Clin Immunol. 2005;1:47–62. doi: 10.1586/1744666X.1.1.47. [DOI] [PubMed] [Google Scholar]
- 89.DeNardo SJ, DeNardo GL, O'Grady LF, Hu E, Sytsma VM, Mills SL, Levy NB, Macey DJ, Miller CH, Epstein AL. Treatment of B cell malignancies with 131I Lym-1 monoclonal antibodies. Int J Cancer Suppl. 1988;3:96–101. [PubMed] [Google Scholar]
- 90.Chong HS, Milenic DE, Garmestani K, Brady ED, Arora H, Pfiester C, Brechbiel MW. In vitro and in vivo evaluation of novel ligands for radioimmunotherapy. Nucl Med Biol. 2006;33:459–67. doi: 10.1016/j.nucmedbio.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 91.Roberts JC, Adams YE, Tomalia D, Mercer-Smith JA, Lavallee DK. Using starburst dendrimers as linker molecules to radiolabel antibodies. Bioconjug Chem. 1990;1:305–8. doi: 10.1021/bc00005a001. [DOI] [PubMed] [Google Scholar]
- 92.Kobayashi H, Wu C, Kim MK, Paik CH, Carrasquillo JA, Brechbiel MW. Evaluation of the in vivo biodistribution of indium-111 and yttrium-88 labeled dendrimer-1B4M-DTPA and its conjugation with anti-Tac monoclonal antibody. Bioconjug Chem. 1999;10:103–11. doi: 10.1021/bc980091d. [DOI] [PubMed] [Google Scholar]
- 93.Wilbur DS, Pathare PM, Hamlin DK, Buhler KR, Vessella RL. Biotin reagents for antibody pretargeting. 3. Synthesis, radioiodination, and evaluation of biotinylated starburst dendrimers. Bioconjug Chem. 1998;9:813–25. doi: 10.1021/bc980055e. [DOI] [PubMed] [Google Scholar]
- 94.Nakagawa Y, Pooh K, Kobayashi T, Kageji T, Uyama S, Matsumura A, Kumada H. Clinical review of the Japanese experience with boron neutron capture therapy and a proposed strategy using epithermal neutron beams. J Neurooncol. 2003;62:87–99. doi: 10.1007/BF02699936. [DOI] [PubMed] [Google Scholar]
- 95.Wadabayashi N, Honda C, Mishima Y, Ichihashi M. Selective boron accumulation in human ocular melanoma vs surrounding eye components after 10B1-p-boronophenylalanine administration. Prerequisite for clinical trial of neutron-capture therapy. Melanoma Res. 1994;4:185–90. doi: 10.1097/00008390-199406000-00007. [DOI] [PubMed] [Google Scholar]
- 96.Kato I, Ono K, Sakurai Y, Ohmae M, Maruhashi A, Imahori Y, Kirihata M, Nakazawa M, Yura Y. Effectiveness of BNCT for recurrent head and neck malignancies. Appl Radiat Isot. 2004;61:1069–73. doi: 10.1016/j.apradiso.2004.05.059. [DOI] [PubMed] [Google Scholar]
- 97.Rao M, Trivillin VA, Heber EM, Cantarelli Mde L, Itoiz ME, Nigg DW, Rebagliati RJ, Batistoni D, Schwint AE. BNCT of 3 cases of spontaneous head and neck cancer in feline patients. Appl Radiat Isot. 2004;61:947–52. doi: 10.1016/j.apradiso.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 98.Koivunoro H, Bleuel DL, Nastasi U, Lou TP, Reijonen J, Leung KN. BNCT dose distribution in liver with epithermal D-D and D-T fusion-based neutron beams. Appl Radiat Isot. 2004;61:853–9. doi: 10.1016/j.apradiso.2004.05.043. [DOI] [PubMed] [Google Scholar]
- 99.Alam F, Soloway AH, Barth RF, Mafune N, Adams DM, Knoth WH. Boron neutron capture therapy: linkage of a boronated macromolecule to monoclonal antibodies directed against tumor-associated antigens. J Med Chem. 1989;32:2326–30. doi: 10.1021/jm00130a017. [DOI] [PubMed] [Google Scholar]
- 100.Wu G, Barth RF, Yang W, Chatterjee M, Tjarks W, Ciesielski MJ, Fenstermaker RA. Site-specific conjugation of boron-containing dendrimers to anti-EGF receptor monoclonal antibody cetuximab (IMC-C225) and its evaluation as a potential delivery agent for neutron capture therapy. Bioconjug Chem. 2004;15:185–94. doi: 10.1021/bc0341674. [DOI] [PubMed] [Google Scholar]
- 101.Yang W, Barth RF, Adams DM, Ciesielski MJ, Fenstermaker RA, Shukla S, Tjarks W, Caligiuri MA. Convection-enhanced delivery of boronated epidermal growth factor for molecular targeting of EGF receptor-positive gliomas. Cancer Res. 2002;62:6552–8. [PubMed] [Google Scholar]
- 102.Yang W, Barth RF, Wu G, Ciesielski MJ, Fenstermaker RA, Moffat BA, Ross BD, Wikstrand CJ. Development of a syngeneic rat brain tumor model expressing EGFRvIII and its use for molecular targeting studies with monoclonal antibody L8A4. Clin Cancer Res. 2005;11:341–50. [PubMed] [Google Scholar]
- 103.Luo D, Haverstick K, Belcheva N, Han E, Saltzman WM. Poly(ethylene glycol)-Conjugated PAMAM Dendrimer for Biocompatible, High-Efficiency DNA Delivery. Macromolecules. 2002;35:3456–3462. [Google Scholar]
- 104.Shukla S, Wu G, Chatterjee M, Yang W, Sekido M, Diop LA, Muller R, Sudimack JJ, Lee RJ, Barth RF, Tjarks W. Synthesis and biological evaluation of folate receptor-targeted boronated PAMAM dendrimers as potential agents for neutron capture therapy. Bioconjug Chem. 2003;14:158–67. doi: 10.1021/bc025586o. [DOI] [PubMed] [Google Scholar]
- 105.Parrott MC, Marchington EB, Valliant JF, Adronov A. Synthesis and properties of carborane-functionalized aliphatic polyester dendrimers. J Am Chem Soc. 2005;127:12081–9. doi: 10.1021/ja053730l. [DOI] [PubMed] [Google Scholar]


