Abstract
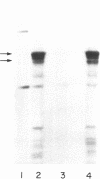
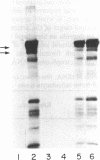
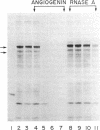
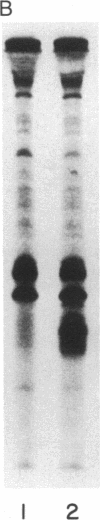
Angiogenin is a potent inhibitor of cell-free protein synthesis. When incubated with rabbit reticulocyte lysate at a concentration of 40-60 nM, it completely abolishes the capacity of the lysate to support protein synthesis. The inhibition appears to be due to its ribonucleolytic activity since it (i) generates limited cleavage products from reticulocyte RNA and (ii) is prevented from both cleaving RNA and inhibiting protein synthesis by placental RNase inhibitor. The ribonucleolytic activity of angiogenin toward the reticulocyte RNA system is highly specific. Thus, under conditions where angiogenin totally abolishes protein synthesis, an equivalent concentration of pancreatic RNase A inhibits it only partially. In contrast, RNase A is a much more effective enzyme than angiogenin using isolated RNA as substrate. Angiogenin inhibits protein synthesis by cleaving rRNA, thereby inactivating the protein synthesis machinery. Addition of isolated reticulocyte ribosomes to an angiogenin-treated lysate restores the capacity for protein synthesis, whereas addition of tRNA or mRNA does not. This potent effect on protein synthesis suggests a possible physiological function of angiogenin whose overall relevance and implications should become evident as the mechanisms of neovascularization are deciphered. The use of angiogenin may also further elucidate ribosome structure and its role in protein synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C. M., Sidikaro J., Nomura M. Specific inactivation of ribosomes by colicin E3 in vitro and mechanism of immunity in colicinogenic cells. Nat New Biol. 1971 Dec 1;234(48):133–137. doi: 10.1038/newbio234133a0. [DOI] [PubMed] [Google Scholar]
- Brewer E. N., Foster L. B., Sells B. H. A possible role for ribonuclease in the regulation of protein synthesis in normal and hypophysectomized rats. J Biol Chem. 1969 Mar 25;244(6):1389–1392. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Fett J. W., Strydom D. J., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985 Sep 24;24(20):5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Grove B. K., Johnson T. C. The role of ribosomal ribonucleic acid in the structure and function of mammalian brain ribosomes. Biochem J. 1974 Nov;143(2):419–426. doi: 10.1042/bj1430419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood S. M. tcRNA as a naturally occurring antisense RNA in eukaryotes. Nucleic Acids Res. 1986 Aug 26;14(16):6771–6772. doi: 10.1093/nar/14.16.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi K., Davie E. W., Strydom D. J., Riordan J. F., Vallee B. L. Sequence of the cDNA and gene for angiogenin, a human angiogenesis factor. Biochemistry. 1985 Sep 24;24(20):5494–5499. doi: 10.1021/bi00341a032. [DOI] [PubMed] [Google Scholar]
- Marx J. L. Angiogenesis research comes of age. Science. 1987 Jul 3;237(4810):23–24. doi: 10.1126/science.2440104. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Plesner P., Goodchild J., Kalckar H. M., Zamecnik P. C. Oligonucleotides with rapid turnover of the phosphate groups occur endogenously in eukaryotic cells. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1936–1939. doi: 10.1073/pnas.84.7.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELA M., ANFINSEN C. B. Some spectrophotometric and polarimetric experiments with ribonuclease. Biochim Biophys Acta. 1957 May;24(2):229–235. doi: 10.1016/0006-3002(57)90186-5. [DOI] [PubMed] [Google Scholar]
- Schindler D. G., Davies J. E. Specific cleavage of ribosomal RNA caused by alpha sarcin. Nucleic Acids Res. 1977 Apr;4(4):1097–1110. doi: 10.1093/nar/4.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen G. C., Lebleu B., Brown G. E., Kawakita M., Slattery E., Lengyel P. Interferon, double-stranded RNA and mRNA degradation. Nature. 1976 Nov 25;264(5584):370–373. doi: 10.1038/264370a0. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Fett J. W., Strydom D. J., Vallee B. L. Isolation and characterization of a human colon carcinoma-secreted enzyme with pancreatic ribonuclease-like activity. Biochemistry. 1986 Nov 18;25(23):7255–7264. doi: 10.1021/bi00371a002. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Riordan J. F., Vallee B. L. Characteristic ribonucleolytic activity of human angiogenin. Biochemistry. 1986 Jun 17;25(12):3527–3532. doi: 10.1021/bi00360a008. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Strydom D. J., Olson K. A., Vallee B. L. Isolation of angiogenin from normal human plasma. Biochemistry. 1987 Aug 11;26(16):5141–5146. doi: 10.1021/bi00390a037. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Vallee B. L. Human placental ribonuclease inhibitor abolishes both angiogenic and ribonucleolytic activities of angiogenin. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2238–2241. doi: 10.1073/pnas.84.8.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strydom D. J., Fett J. W., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Amino acid sequence of human tumor derived angiogenin. Biochemistry. 1985 Sep 24;24(20):5486–5494. doi: 10.1021/bi00341a031. [DOI] [PubMed] [Google Scholar]
- Takehara K., LeRoy E. C., Grotendorst G. R. TGF-beta inhibition of endothelial cell proliferation: alteration of EGF binding and EGF-induced growth-regulatory (competence) gene expression. Cell. 1987 May 8;49(3):415–422. doi: 10.1016/0092-8674(87)90294-7. [DOI] [PubMed] [Google Scholar]
- Wreschner D. H., James T. C., Silverman R. H., Kerr I. M. Ribosomal RNA cleavage, nuclease activation and 2-5A(ppp(A2'p)nA) in interferon-treated cells. Nucleic Acids Res. 1981 Apr 10;9(7):1571–1581. doi: 10.1093/nar/9.7.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wreschner D., Melloul D., Herzberg M. Interaction between membrane functions and protein synthesis in reticulocytes. Isolation of RNase M, a membrane component inhibiting protein synthesis through specific endonucleolytic activity. Eur J Biochem. 1978 Sep 1;89(2):341–352. doi: 10.1111/j.1432-1033.1978.tb12535.x. [DOI] [PubMed] [Google Scholar]