Abstract
Bacterial colonization of the intestine is critical for the normal function of the mammalian immune system. However, the specific molecules produced by commensal bacteria that contribute to the modulation of the host immune system are largely uncharacterized. Polysaccharide A (PSA) produced by the ubiquitous human commensal, Bacteroides fragilis is a model symbiosis factor. PSA is capable of activating T cell-dependent immune responses that can affect both the development and homeostasis of the host immune system. Colonization of previously germ-free mice with B. fragilis alone is sufficient to correct the splenic Th1/Th2 imbalance found in germ-free mice. In addition, PSA can provide protection in animal models of colitis through repression of pro-inflammatory cytokines associated with the Th17 lineage. This review provides an overview of the immunologic properties of PSA including the mechanisms of immune system activation and the resulting immunomodulatory effects.
Keywords: Bacteroides fragilis, Colitis, Commensal, Germ-free, IL-10, Immune development, Polysaccharide, Th1, Th2, Th17, Review
2. INTRODUCTION
There are at least one hundred times more bacterial cells than eukaryotic cells in the human body. Most of these bacteria are found in the intestinal tract, where their density can reach 1012 organisms per gram of intestinal contents (1). The great majority of the interactions between these bacteria and the human host are mutualistic rather than pathogenic. The host-commensal relationship can be viewed as a co-evolutionary process where the fitness of the two participants becomes interdependent (2). Commensal bacteria enjoy a nutrient-rich and stable habitat while playing an integral role in host development and maintenance of proper physiologic function (3, 4). The benefits of bacterial colonization of the mammalian gut extend beyond the intestine and include far-reaching systemic effects. Commensal bacteria play an integral role in the development of both mucosal and systemic immunity (5–7).
Members of the Firmicutes and Bacteroidetes phyla make up a majority of the bacterial species present in the human intestinal microbiota (8). Within the Bacteriodetes phylum, members of the Bacteroidales order are the most abundant of the cultured Gram-negative organisms. Due to the fact that the mammalian gastrointestinal tract is the primary environmental reservoir of the Bacteroidales, it has been hypothesized that there is a co-evolutionary relationship between the Bacteroidales and the mammalian host. As such, Bacteroidales species have been used as models for studying the commensal bacteria-host interactions (9). One such species, Bacteroides fragilis, has been shown to exert profound beneficial effects on the host immune system (10, 11).
We are just beginning to elucidate the interactions between the gut flora and the host that lead to the establishment of immune system homeostasis. It has been proposed that symbiotic bacteria in the gastrointestinal tract produce molecules that mediate the normal maturation of the immune system and protect the host from disease (10, 12). Although B. fragilis constitutes less that 1% of the colonic microflora, it produces a capsular polysaccharide, polysaccharide A (PSA), that is sufficient to correct some of the immune system deficiencies found in the absence of bacterial colonization and to prevent intestinal inflammation in animal models of colitis (10, 11).
3. BACTEROIDES FRAGILIS
B. fragilis is a Gram-negative anaerobic bacterium whose primary known environmental reservoir is the human lower gastrointestinal tract. A distinctive feature of B. fragilis is the large proportion of the genome devoted to carbohydrate metabolism, including the degradation of dietary polysaccharides and the production of surface capsular polysaccharides (9, 13, 14). Thus far, eight different capsular polysaccharides, each synthesized from distinct genomic loci, have been identified within a single strain of B. fragilis. Each polysaccharide synthesis locus contains between 11 and 21 genes under the control of a single promoter. Seven of the eight loci are regulated by the reversible inversion of the DNA containing the promoter, which results in an ON-OFF expression phenotype known as phase variation. The loci are regulated independently, and a bacterium may simultaneously express any combination of polysaccharides; thus there are 256 distinct surface profiles (15, 16). It has been proposed that such large potential variation produces a population of bacteria most fit to survive in the intestinal milieu. The ability to express multiple polysaccharides has been shown to enhance intestinal colonization by B. fragilis (10).
Two of the capsular polysaccharides of B. fragilis, PSA and polysaccharide B (PSB), belong to a class of bacterial carbohydrates known as zwitterionic polysaccharides (ZPSs). ZPSs are characterized by the presence of both a positive and negative charge within each repeating unit (17). Traditionally, it was thought that only peptides could induce an adaptive T cell–mediated immune response. Carbohydrates had been characterized as classical T cell–independent antigens that induced a specific IgM response but failed to evoke an IgG or memory response (18, 19). However, it is now known that ZPSs are capable of inducing CD4+ T cell–dependent immune responses. In addition to PSA and PSB of B. fragilis, ZPSs have been identified from other bacterial species, including type 1 Streptococcus pneumoniae capsular polysaccharide (CP1) and types 5 and 8 Staphylococcus aureus capsular polysaccharide. All of the known ZPSs have immunomodulatory properties that depend on the presence of both positively and negatively charged groups within the repeating unit structures (17, 20, 21). Of all of the known ZPSs, PSA is the best characterized.
PSA is a unique and potent antigen. Among carbohydrates, this polysaccharide stands out as a T cell–dependent rather than a T cell–independent antigen. Although it is processed and presented along the same pathways as conventional peptide antigens, PSA modulates the host immune system more extensively than many other known T cell–dependent antigens. At this time, the impact of a single bacterial molecule on the host during commensalism is certainly best defined for PSA.
4. CORRECTION OF CD4+ T CELL DEFICIENCIES IN GERM-FREE MICE
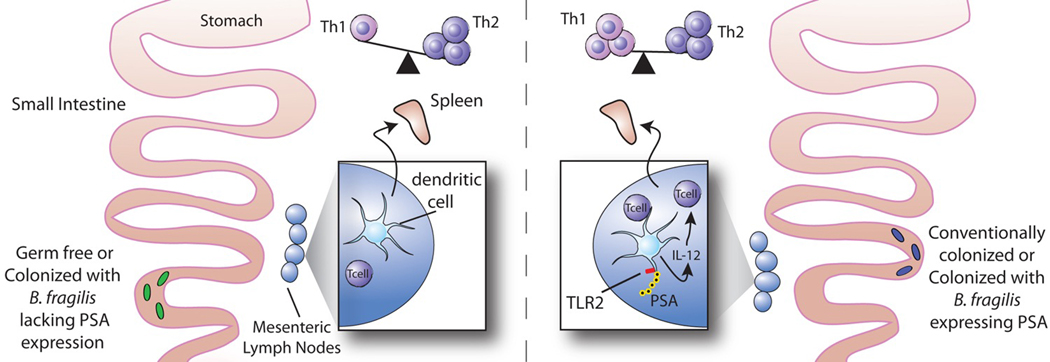
Although they are germ-free in utero, mammals are colonized with a complex microbiota soon after birth (22, 23). Germ-free (GF) animal models have been invaluable in investigations of the effects of commensal bacteria on health and disease (24). Studies of GF animals have demonstrated that the mammalian host has adapted over time to colonization with large numbers of microbes and has even become dependent on the presence of the gut flora. Compared with the mucosal and systemic immune systems of conventional specific-pathogen-free (SPF) mice colonized with a complex microbiota, both of these systems are functionally immature in GF mice. In the gut-associated lymphoid tissue of GF animals, the Peyer’s patches are small, with few germinal centers, and the number of IgA-producing plasma cells is reduced (25, 26). There is also a marked decrease in the number of CD4+ cells in the lamina propria of the small intestine (27). From a systemic perspective, the spleens and mesenteric lymph nodes (MLNs) of GF animals exhibit defects in structural development, with depletions of lymphocyte zones (10, 28). All of these abnormalities can be corrected after several weeks by colonization of GF mice with commensal bacteria (5, 29). Although the commensal bacterial products that are responsible for the correction of the immune abnormalities found in GF animals are largely unknown, the response to PSA from B. fragilis has been shown to be sufficient to correct some of the defects found in the spleen.
As described above, the spleens of GF mice have severe morphologic abnormalities. However, monocolonization of previously GF mice with B. fragilis alone results in the correction of all splenic architectural defects. The lymphoid follicles (white pulp) of these monocolonized animals are larger and more defined than those found in GF mice. This effect is mediated by PSA; GF mice colonized with an isogenic strain of B. fragilis lacking PSA production have lymphoid follicles that are small and fragmented, similar to those found in GF mice (10, 30). The observed difference is not due to a decrease in bacterial fitness in the absence of PSA as colonization levels of the two bacterial strains are comparable (10).
The restoration of splenic architecture is due to an expansion of CD4+ T cells in the spleens of the mice monocolonized with B. fragilis. Compared with the lymphocyte populations in the spleens of SPF mice, those in GF mice have a smaller proportion of CD4+ T cells. However, the percentage of CD4+ cells in the spleens is the same in B. fragilis monocolonized mice as in SPF mice. The effects are specific to CD4+ T cells, as the percentages of CD8+ T cells and CD19+ B cells remain unchanged. Again, the increase in the CD4+ population in previously GF mice monocolonized with B. fragilis is mediated by PSA; colonization of GF mice with B. fragilis lacking PSA does not enrich the splenic CD4+ T lymphocyte population, and treatment of GF mice with purified PSA is sufficient to restore this population. The ability of PSA to expand CD4+ T cell populations is not restricted to GF mice; treatment of SPF mice with PSA results in a splenic CD4+ T cell expansion of the same magnitude (10).
The ability to activate a CD4+ T cell response has long been considered to be limited to peptide antigens. However, the ability of PSA to induce a CD4+ T cell expansion in the spleen supports other data demonstrating that PSA is, in fact, a T cell–dependent antigen. A detailed molecular characterization of the immunomodulatory properties of PSA confirms that this polysaccharide induces an immune response analogous to that stimulated by conventional protein antigens.
5. ANTIGEN PRESENTATION OF PSA
Activation of CD4+ T cells requires the specific interaction of the alpha-beta T cell receptor (TCR) on the T cell with the antigen presented in the context of major histocompatibility complex II (MHCII) on a professional antigen-presenting cell (APC) (31). To induce an immune response, PSA must be processed in the endocytic pathway and presented on an MHCII molecule in a manner analogous to that by which peptide antigens are presented; the notable difference is that, in the endosome, the degradation of PSA to a molecular size small enough for presentation by the MHCII molecule is a chemical process, whereas for protein degradation it is an enzymatic process (31, 32).
Once taken up into an APC, PSA follows the same processing pathway as peptide antigens. Upon entry into the endosome of an APC, PSA is directed to a MHCII compartment, where it co-localizes with the lysosomal marker LAMP-1, the exocytic vesicle marker HLA-DM, and the MHCII protein HLA-DR. The up-regulation of inducible nitric oxide synthetase, which is associated with the oxidative burst, results in increased nitric oxide (NO) production. NO breaks down PSA through deamination, a chemical reaction in which PSA is oxidized and then depolymerized to a much smaller molecular size while retaining the zwitterionic motif and the overall structure of the repeating unit. Lastly, co-localization of PSA, MHCII, and alpha-beta TCRs is detectable on the cell surface of murine splenocytes. However, no PSA is found on cells lacking MHCII (32).
In the absence of direct contact between APCs and T cells, ZPSs cannot stimulate T cell proliferation or induce cytokine production in vitro (33). Furthermore, T cell activation requires the presence of HLA-DR and costimulatory molecules CD40 and CD86 on the surface of the APC (33, 34). The fact that major histocompatibility class I (MHCI) molecules are not required provides evidence for CD4+ specificity of the immune response to PSA (33).
After oral treatment of mice with fluorescently labeled PSA, the polysaccharide associates with CD11c+ dendritic cells, but not with CD4+ T cells or CD19+ B cells in the MLNs (10). PSA, through TLR2 stimulation, is able to activate CD11c+ dendritic cells; the consequence being the up-regulation of MHCII as well as the costimulatory molecule CD86. This up-regulation is critical, as activation of T cells requires presentation of the antigen on MHCII in the presence of the appropriate costimulatory molecules. Only a mature dendritic cell that has been exposed to an activating antigen fulfills these requirements (35). The implication is that dendritic cells sample PSA from the intestine and migrate to the MLNs to initiate an immune response. Despite the observed systemic T cell expansion, no PSA is found in the spleen (10).
6. ESTABLISHING A Th1/Th2 BALANCE
Three predominant subtypes of effector CD4+ cells—T-helper 1, T-helper 2, and T-helper 17 (Th1, Th2, and Th17)—induce distinct and opposing immune responses (36, 37). Th1 cells promote the development of cell-mediated immunity and host defense against viral and bacterial pathogens, especially intracellular organisms. Th2 cells mediate host defense against helminths (37, 38). Th17 cells are important mediators of inflammation and protect the host from extracellular bacteria and fungi (36, 39). All three lineages have also been implicated in pathogenic immune responses. Dysregulated Th1 or Th17 responses may result in autoimmune diseases, and an overactive Th2 response has been implicated in asthma and allergy. Therefore, a proper balance of T cell types is essential for the development and maintenance of immune system homeostasis (10, 36, 40, 41). Research in GF mice has revealed a Th1/Th2 imbalance that can be corrected through bacterial colonization (10).
Th1 and Th2 effector cells can be differentiated by their cytokine profiles. Th1 cells are characterized by the production of interferon-gamma (IFN-gamma) and Th2 cells by the production of interleukin 4 (IL-4). The immune system of GF mice is intrinsically biased toward Th2 responses (10). However, stimulation of the GF immune system with PSA generates a Th1 response sufficient to correct this imbalance. CD4+ T cells from the spleens of GF mice produce more of the Th2 cytokine IL-4 than cells from conventionally colonized mice, but monocolonization with a PSA-producing strain of B. fragilis decreases production of IL-4 and restores production of IFN-gamma to levels found in conventionally colonized mice (10).
The major pathway for Th1 cell differentiation is through dendritic cell production of IL-12, which binds the IL-12 receptor on T cells and signals to activate the Th1-specific transcription factors Stat-4 and T-bet (42, 43). The ability of PSA to stimulate IFN-gamma production by T cells represents a Th1 response for which signaling by both IL-12 and Stat-4 is required. Activated dendritic cells produce IL-12 after stimulation of TLR2 by PSA. PSA is the first polysaccharide known to signal dendritic cells to secrete IL-12. Chemical removal of the positive charge on the polysaccharide structure severely reduces the ability of PSA to induce IL-12 production; thus the zwitterionic motif on PSA is required for the activation of dendritic cells. However, given that the ZPS SP1 fails to activate IL-12 production, a zwitterionic motif clearly is not sufficient for carbohydrate stimulation of this cytokine (44).
Taken together, these results suggest that PSA from B. fragilis is sufficient to establish a proper systemic Th1/Th2 balance within the host and that CD11c+ dendritic cells associated with PSA in the MLNs play a role in mediating this response.
7. INNATE IMMUNITY AND PSA
TLRs play a critical role in host defense by sensing the presence of microbes. Upon stimulation, TLRs mediate inflammatory responses, coordinate innate and adaptive immunity, prime naive T cells, and induce memory to facilitate the elimination of pathogens (45, 46). TLRs recognize certain highly conserved microbial components known as pathogen-associated molecular patterns (PAMPs). Molecules recognized by TLRs include bacterial lipopolysaccharide, flagellin, bacterial DNA, and viral double-stranded RNA (47). However, both pathogenic and commensal microbes produce the microbial ligands recognized by TLRs. Although TLR signaling was traditionally associated with the sensing of pathogens, it is now appreciated that commensal bacteria are also recognized by TLRs and that this interaction is critical for protection against epithelial injury and intestinal homeostasis (48).
TLR2 is activated by several microbial products, including peptidoglycans and lipoproteins from both Gram-positive and Gram-negative bacteria (47). TLR2 is also the receptor that recognizes PSA. PSA-activated signaling through TLR2 is necessary for the differentiation of Th1 cells and the establishment of a proper Th1/Th2 balance. As discussed above, IFN-gamma is important in driving Th1 cell mediated immunity. In response to PSA stimulation, TLR2 signaling stimulates both innate and adaptive immune pathways resulting in the optimal induction of IFN-gamma production by T cells (44).
Although TLR2 is not necessary for endocytosis, TLR2 signaling is required for the optimal activation of dendritic cells. PSA stimulation of TLR2 on the APC results in activation of the transcription factor nuclear factor kappa B (NFkappaB), with subsequent production of NO, the pro-inflammatory cytokine tumor necrosis factor-alpha (TNF-alpha), and other immunologically important molecules such as IL-12, MHCII, CD86, and CC chemokine receptor 7 (CCR7) (44). CCR7 is associated with dendritic cell homing to secondary lymphoid organs (49). Bone marrow–derived dendritic cells (BMDCs) lacking TLR2 (TLR2−/−) produce significantly less IL-12 than wild-type dendritic cells, and the decrease in IL-12 results in a reduction of IFN-gamma produced by T cells in response to PSA (44).
The residual IFN-gamma production by T cells after elimination of signaling through TLR2 is the result of PSA processing in the adaptive immune pathway associated with antigen presentation on MHCII. Treatment of TLR2−/− BMDCs with chloroquine, which blocks the acidification of the MHCII compartment, eliminated IFN-gamma production by CD4+ T cells. No other innate immune pathway is thought to be involved in PSA-mediated production of IFN-gamma (44). Activation of TLR2 by PSA increases the efficiency of both the processing and presentation of PSA on MHCII. As described above, TLR2 stimulation by PSA increases NO production by dendritic cells. Since NO is necessary for the chemical degradation of PSA, the loss of TLR2 signaling results in inefficient processing of PSA in the MHCII compartment (32). Furthermore, the failure of TLR2−/− BMDCs to upregulate the expression of MHCII and CD86 results in decreased presentation of PSA to T cells (44).
The decrease in processing and presentation of PSA combined with the decreased production of IL-12 by dendritic cells activated by PSA in the absence of TLR2 signaling demonstrates that in addition to the stimulation of a specific CD4+ T cell immune response, innate immune pathways are required for PSA to establish a Th1/Th2 balance.
8. PREVENTION OF INFLAMMATORY DISEASE
Although the gut microflora is essential to the development of the immune system, it can also be detrimental to the host. Inflammatory bowel disease (IBD) is associated with uncontrolled inflammatory CD4+ T cell responses to bacteria in the gastrointestinal tract (50, 51). Inflammatory responses resulting in IBD are frequently directed against specific subtypes of bacteria, including Helicobacter, Clostridium, and Enterococcus. It is interesting that these bacterial subtypes can be abundant members of the gut microflora of healthy individuals (7, 52). The mechanisms behind initiating and maintaining an inflammatory immune response to commensal bacteria are largely unknown.
Symptoms of IBD are reduced if bacterial numbers in the intestine are decreased by means of antibiotic treatment (53, 54). Moreover, in rodent models, maintenance in a GF environment can protect animals genetically susceptible to IBD from intestinal inflammation (55–59). Consequently, it has been hypothesized that IBD can result from an imbalance among different types bacteria in the intestine (7, 60). In some IBD patients, entire classes of bacteria are lost from the gut flora (61). It may be that some bacteria in the gut minimize the pathogenic potential of other bacteria commonly associated with IBD. Recent research has demonstrated that PSA produced by B. fragilis in the gut may be capable of preventing IBD induced by the presence of bacterial agents of this disease (11).
As discussed above, the lymphocyte populations in the spleens of GF mice contain a reduced proportion of CD4+ T cells. However, colonization of GF mice with B. fragilis restores the CD4+ population to that found in conventional colonized mice (10). Further characterization of the CD4+ T cell population in the spleen reveals that GF mice have a lower proportion of CD4+CD45Rblow T cells than conventionally colonized mice. CD45Rblow T cells, which are thought to be antigen experienced, have anti-inflammatory properties that can provide protection in animal models of IBD (62). Remarkably, colonization of GF mice with B. fragilis increases the proportion of CD4+CD45Rblow cells in the spleen to conventional levels. This effect is PSA dependent; colonization of the GF mice with a strain of B. fragilis lacking PSA has no impact (11).
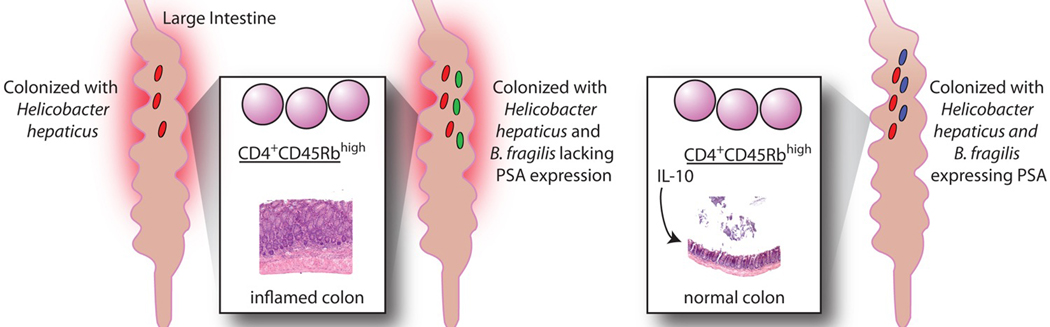
Furthermore, PSA can protect mice in an experimental model of colitis. Mice lacking recombination-activating genes (Rag−/−) have no T or B cells. If CD4+CD45Rbhigh T cells are transferred into Rag−/− mice and the animals are colonized with Helicobacter hepaticus, colitis develops within 2 months (63). The intestinal pathology of these mice is marked by a high degree of inflammation, colonic hyperplasia, and expression of pro-inflammatory cytokines. The mice also exhibit significant weight loss. If, at the time of colonization with H. hepaticus, the mice are co-colonized with a PSA-producing strain of B. fragilis, they are protected from disease and from the associated production of pro-inflammatory cytokines. However, if the mice are co-colonized with a B. fragilis mutant deficient in PSA production rather than with the wild-type strain, they develop full-blown colitis. Levels of colonization by all bacterial species remained constant; therefore, this protection is not due to differences in bacterial clearance. In addition, oral administration of purified PSA during H. hepaticus colonization almost completely protects mice against H. hepaticus–induced colitis by eliminating leukocyte infiltration and the resulting colonic hyperplasia (11). Thus, the presence of a single molecule produced by a single organism is sufficient to prevent colitis induced by colonization with a commensal bacterium that has pathogenic potential.
8.1. Repression of pro-inflammatory cytokines associated with the Th17 lineage
The protective effects of B. fragilis PSA in experimental models of colitis do not appear to be related directly to the role of the organism in establishing a Th1/Th2 balance in GF mice. Instead, PSA appears to dampen the pro-inflammatory response that leads to colitis by repressing Th17 cells (11). Classically, CD4+ T helper cells were thought to differentiate into only two lineages: Th1 and Th2. However, research has now shown that CD4+ T cells can also differentiate into Th17 cells, which are characterized by the production of IL-17 and not of IFN-gamma or IL-4 (36, 64). Because IL-17 is a potent chemoattractant for neutrophils, Th17 cells are key mediators of inflammation (39). Increased levels of IL-17 have been found in the inflamed mucosa and sera from patients with IBD (65). Th17 cells have a different developmental program and produce different cytokines than Th1 or Th2 cells. The induction of Th17 differentiation is independent of IL-12 or IL-4; rather, IL-6 and transforming growth factor-beta 1 (TGF-beta1) (in mice) and IL-1 (in humans) mediate Th17 differentiation from naive CD4+ T cells. IL-23 is necessary for the expansion and maintenance of the Th17 lineage. In addition to IL-17, Th17 cells produce the pro-inflammatory cytokines IL-21 and TNF-alpha (36).
Protection against colitis mediated by B. fragilis PSA results from modulation of cytokine production. Levels of cytokines critical in the Th17 lineage—i.e., IL-1beta, IL-23, and TNF-alpha are elevated in the colons of Rag−/− mice colonized with H. hepaticus. Colonization with B. fragilis in conjunction with H. hepaticus reduces levels of the pro-inflammatory cytokines to those found in SPF Rag−/− mice. In addition, documented decreases in levels of TNF-alpha and IL-23 in the spleens of co-colonized mice demonstrate that immune modulation in the intestinal compartment can affect the systemic immune system (11).
PSA has been given therapeutically to decrease pro-inflammatory cytokine production in an experimental model of colonic irritation (66). The cytokines induced by rectal administration of trinitrobenzene sulfonic acid (TNBS) mimic those found in the H. hepaticus colitis model. CD4+ T cells from the spleens and MLNs of TNBS-treated animals produce elevated levels of IL-23, TNF-alpha, and IL-17. Therapeutic administration of PSA to TNBS-treated animals significantly reduces levels of these pro-inflammatory cytokines. In addition, PSA treatment decreases the expression of RORgamma-t, the transcription factor that directs the Th17 differentiation pathway (11, 67).
8.2. IL-10 is required for the PSA mediated anti-inflammatory response
PSA suppression of the inflammatory immune response is dependent on IL-10, one of the most potent anti-inflammatory cytokines in the immune system. IL-10 plays a critical role in protection against inflammation in several animal models, including the H. hepaticus model of colitis (68, 69). Several experimental approaches support the assertion that IL-10 produced by CD4+ T cells is required for protection against inflammation by PSA in both TNBS and H. hepaticus models. First, mice that lack IL-10 (IL-10−/−) and are treated with TNBS develop symptoms of intestinal inflammation even in the presence of PSA. Second, if IL-10−/− mice are colonized either with H. hepaticus (to induce inflammation) or with H. hepaticus and B. fragilis together, the presence of B. fragilis does not decrease the levels of TNF-alpha or IL-17 produced by MLN cells stimulated in culture with H. hepaticus antigen. The level of IL-17 is actually higher in co-colonized mice than in mice colonized with H. hepaticus alone. Finally, if, in addition to PSA, an antibody to IL-10 is administered to Rag−/− mice after transfer of CD4+CD45Rbhigh cells, the polysaccharide no longer protects the mice from developing colitis during colonization with H. hepaticus. In addition, PSA cannot protect mice from colitis if the CD4+CD45Rbhigh cells used to induce experimental colitis are isolated from an IL-10−/− mouse (11). To date, PSA is the only bacterial molecule known to be sufficient to stimulate anti-inflammatory responses that can counteract the pro-inflammatory responses induced by commensals with pathogenic potential.
9. CONCLUSIONS
B. fragilis, a commensal bacterium that colonizes the lower gastrointestinal tract, is capable of mediating powerful effects on the host immune system. Most, if not all, of these effects are mediated by the capsular polysaccharide PSA, an immunomodulatory molecule that mediates T cell–dependent immune responses via the same pathways used by protein antigens. PSA is taken up into APCs, processed, and presented to T cells on the MHCII molecule (32). The activated T cells contribute to a variety of host cell immune responses. An immune response to PSA helps create a proper Th1/Th2 balance in the absence of any other bacteria (10). PSA can prevent the development of colonic inflammation and disease caused by potentially pathogenic commensal bacteria (11). In short, PSA is a model symbiosis factor—a molecule produced by commensal bacteria that modulates the host immune system in ways that benefit the host. The discovery of the powerful effects PSA on host immune development is spurring the search for other symbiosis factors produced by commensal bacteria. The identification of such factors will further elucidate the elaborate interactions between the host immune system and commensal bacteria.
Figure 1.
Impact of B. fragilis PSA on the development of the immune system. The immune system of GF mice is skewed towards Th2 immune responses. Colonizing GF mice with a PSA-producing strain of B. fragilis corrects this defect and restores a Th1/Th2 balance in the spleen. PSA produced by the intestinal bacteria is most likely sampled from the intestine by DCs, which then migrate to the MLNs. Within the APC, PSA is processed and presented to T cells. Recognition of PSA in the context of MHCII by the TCR triggers a CD4+ T cell immune response. PSA stimulates TLR2 signaling and IL-12 production by dendritic cells. The IL-12 produced by the APC binds to the IL-12 receptor on T cells and activates the Th1 transcription factor, Stat-4. In response to IL-12 and Stat-4, Th1 cells are generated that produce IFN-gamma. This process is dependent on PSA; colonization of GF mice with B. fragilis that lacks PSA production does not correct the Th1/Th2 imbalance found in the absence of bacterial colonization.
Figure 2.
PSA mediated protection from inflammatory disease in experimental models of colitis. Transfer of CD4+CD45Rbhigh T cells into Rag−/− mice colonized with Helicobacter hepaticus induces colitis. Rag−/− mice co-colonized with H. hepaticus and B. fragilis expressing PSA, but not B. fragilis lacking PSA expression, do not develop colitis. PSA mediated protection against colitis results from decreased levels of cytokines associated with the Th17 lineage including IL-1beta, IL-23, and TNF-alpha. Suppression of the inflammatory cytokines is dependent on IL-10. In the absence of IL-10, PSA cannot prevent colitis in co-colonized mice.
ACKNOWLEDGEMENTS
We thank Julie McCoy for her excellent editorial assistance. Work in Dennis Kasper’s lab was supported in part by funding from the NIH (NIH/NIAID AI-39576).
Abbreviations
- APC
antigen-presenting cell
- BMDC
bone marrow-derived dendritic cell
- CP1
type 1 Streptococcus pneumoniae capsular polysaccharide
- GF
germ-free
- IBD
inflammatory bowel disease
- IFN-gamma
interferon gamma
- IL
interleukin
- MHC
major histocompatibility complex
- MLN
mesenteric lymph node
- NO
nitric oxide
- PSA
polysaccharide A
- PSB
polysaccharide B
- SPF
specific-pathogen free
- TCR
T cell receptor
- TGF-beta1
transforming growth factor-beta 1
- Th
T helper cell
- TLR2
Toll-like receptor 2
- TNBS
trinitrobenzene sulfonic acid
- TNF-alpha
tumor necrosis factor-alpha
- ZPS
zwitterionic polysaccharide
REFERENCES
- 1.Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: the unseen majority. Proc Natl Acad Sci U S A. 1998;95:6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 3.Hooper LV, Falk PG, Gordon JI. Analyzing the molecular foundations of commensalism in the mouse intestine. Curr Opin Microbiol. 2000;3:79–85. doi: 10.1016/s1369-5274(99)00055-7. [DOI] [PubMed] [Google Scholar]
- 4.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 5.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 6.Noverr MC, Huffnagle GB. Does the microbiota regulate immune responses outside the gut? Trends Microbiol. 2004;12:562–568. doi: 10.1016/j.tim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Pamer EG. Immune responses to commensal and environmental microbes. Nat Immunol. 2007;8:1173–1178. doi: 10.1038/ni1526. [DOI] [PubMed] [Google Scholar]
- 8.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coyne MJ, Comstock LE. Niche-specific features of the intestinal bacteroidales. J Bacteriol. 2008;190:736–742. doi: 10.1128/JB.01559-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 12.Corr SC, Li Y, Riedel CU, O'Toole PW, Hill C, Gahan CG. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc Natl Acad Sci U S A. 2007;104:7617–7621. doi: 10.1073/pnas.0700440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wexler HM. Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev. 2007;20:593–621. doi: 10.1128/CMR.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, Hooper LV, Gordon JI. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science. 2003;299:2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 15.Coyne MJ, Weinacht KG, Krinos CM, Comstock LE. Mpi recombinase globally modulates the surface architecture of a human commensal bacterium. Proc Natl Acad Sci U S A. 2003;100:10446–10451. doi: 10.1073/pnas.1832655100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krinos CM, Coyne MJ, Weinacht KG, Tzianabos AO, Kasper DL, Comstock LE. Extensive surface diversity of a commensal microorganism by multiple DNA inversions. Nature. 2001;414:555–558. doi: 10.1038/35107092. [DOI] [PubMed] [Google Scholar]
- 17.Tzianabos AO, Onderdonk AB, Rosner B, Cisneros RL, Kasper DL. Structural features of polysaccharides that induce intra-abdominal abscesses. Science. 1993;262:416–419. doi: 10.1126/science.8211161. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Fernandez A, Faro J, Fernandez C. Immune responses to polysaccharides: Lessons from humans and mice. Vaccine. 2008;26:292–300. doi: 10.1016/j.vaccine.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 19.Janeway CA. Garland Science. New York: 2004. Immunobiology-the immune system in health and disease. [Google Scholar]
- 20.Tzianabos AO, Wang JY, Lee JC. Structural rationale for the modulation of abscess formation by Staphylococcus aureus capsular polysaccharides. Proc Natl Acad Sci U S A. 2001;98:9365–9370. doi: 10.1073/pnas.161175598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tzianabos AO, Finberg RW, Wang Y, Chan M, Onderdonk AB, Jennings HJ, Kasper DL. T cells activated by zwitterionic molecules prevent abscesses induced by pathogenic bacteria. J Biol Chem. 2000;275:6733–6740. doi: 10.1074/jbc.275.10.6733. [DOI] [PubMed] [Google Scholar]
- 22.Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69:1035S–1045S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 23.Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449:811–818. doi: 10.1038/nature06245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol. 2007;19:59–69. doi: 10.1016/j.smim.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Gordon HA. Morphological and physiological characterization of germfree life. Ann N Y Acad Sci. 1959;78:208–220. doi: 10.1111/j.1749-6632.1959.tb53104.x. [DOI] [PubMed] [Google Scholar]
- 26.Macpherson AJ, Hunziker L, McCoy K, Lamarre A. IgA responses in the intestinal mucosa against pathogenic and non-pathogenic microorganisms. Microbes Infect. 2001;3:1021–1035. doi: 10.1016/s1286-4579(01)01460-5. [DOI] [PubMed] [Google Scholar]
- 27.Macpherson AJ, Martinic MM, Harris N. The functions of mucosal T cells in containing the indigenous commensal flora of the intestine. Cell Mol Life Sci. 2002;59:2088–2096. doi: 10.1007/s000180200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauer H, Horowitz RE, Levenson SM, Popper H. The response of the lymphatic tissue to the microbial flora. Studies on germfree mice. Am J Pathol. 1963;42:471–483. [PMC free article] [PubMed] [Google Scholar]
- 29.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 30.Coyne MJ, Tzianabos AO, Mallory BC, Carey VJ, Kasper DL, Comstock LE. Polysaccharide biosynthesis locus required for virulence of Bacteroides fragilis. Infect Immun. 2001;69:4342–4350. doi: 10.1128/IAI.69.7.4342-4350.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watts C, Powis S. Pathways of antigen processing and presentation. Rev Immunogenet. 1999;1:60–74. [PubMed] [Google Scholar]
- 32.Cobb BA, Kasper DL. Zwitterionic capsular polysaccharides: the new MHCII-dependent antigens. Cell Microbiol. 2005;7:1398–1403. doi: 10.1111/j.1462-5822.2005.00591.x. [DOI] [PubMed] [Google Scholar]
- 33.Kalka-Moll WM, Tzianabos AO, Bryant PW, Niemeyer M, Ploegh HL, Kasper DL. Zwitterionic polysaccharides stimulate T cells by MHC class II-dependent interactions. J Immunol. 2002;169:6149–6153. doi: 10.4049/jimmunol.169.11.6149. [DOI] [PubMed] [Google Scholar]
- 34.Stephen TL, Niemeyer M, Tzianabos AO, Kroenke M, Kasper DL, Kalka-Moll WM. Effect of B7-2 and CD40 signals from activated antigen-presenting cells on the ability of zwitterionic polysaccharides to induce T-Cell stimulation. Infect Immun. 2005;73:2184–2189. doi: 10.1128/IAI.73.4.2184-2189.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect. 2004;6:1382–1387. doi: 10.1016/j.micinf.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 36.Chen Z, O'Shea JJ. Th17 cells: a new fate for differentiating helper T cells. Immunol Res. 2008 doi: 10.1007/s12026-007-8014-9. [DOI] [PubMed] [Google Scholar]
- 37.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 38.Anthony RM, Rutitzky LI, Urban JF, Jr, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuzaki G, Umemura M. Interleukin-17 as an Effector Molecule of Innate and Acquired Immunity against Infections. Microbiol Immunol. 2007;51:1139–1147. doi: 10.1111/j.1348-0421.2007.tb04008.x. [DOI] [PubMed] [Google Scholar]
- 40.Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223–246. [PubMed] [Google Scholar]
- 41.Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat Rev Immunol. 2001;1:69–75. doi: 10.1038/35095579. [DOI] [PubMed] [Google Scholar]
- 42.Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM, O'Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 43.Trinchieri G. Interleukin-12andtheregulationofinnateresistanceandadaptiveimmunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 44.Wang Q, McLoughlin RM, Cobb BA, Charrel-Dennis M, Zaleski KJ, Golenbock D, Tzianabos AO, Kasper DL. A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J Exp Med. 2006;203:2853–2863. doi: 10.1084/jem.20062008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 46.Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21:733–741. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 48.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 50.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 51.Cong Y, Brandwein SL, McCabe RP, Lazenby A, Birkenmeier EH, Sundberg JP, Elson CO. CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J Exp Med. 1998;187:855–864. doi: 10.1084/jem.187.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balish E, Warner T. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am J Pathol. 2002;160:2253–2257. doi: 10.1016/S0002-9440(10)61172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Videla S, Vilaseca J, Guarner F, Salas A, Treserra F, Crespo E, Antolin M, Malagelada JR. Role of intestinal microflora in chronic inflammation and ulceration of the rat colon. Gut. 1994;35:1090–1097. doi: 10.1136/gut.35.8.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoentjen F, Harmsen HJ, Braat H, Torrice CD, Mann BA, Sartor RB, Dieleman LA. Antibiotics with a selective aerobic or anaerobic spectrum have different therapeutic activities in various regions of the colon in interleukin 10 gene deficient mice. Gut. 2003;52:1721–1727. doi: 10.1136/gut.52.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 56.Mombaerts P, Mizoguchi E, Grusby MJ, Glimcher LH, Bhan AK, Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:274–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- 57.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M, Fernandez-Sueiro JL, Balish E, Hammer RE. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–2364. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veltkamp C, Tonkonogy SL, De Jong YP, Albright C, Grenther WB, Balish E, Terhorst C, Sartor RB. Continuous stimulation by normal luminal bacteria is essential for the development and perpetuation of colitis in Tg (epsilon26) mice. Gastroenterology. 2001;120:900–913. doi: 10.1053/gast.2001.22547. [DOI] [PubMed] [Google Scholar]
- 60.Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:119–129. doi: 10.1016/j.chom.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 61.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 63.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ T (R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T (H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elson CO, Beagley KW, Sharmanov AT, Fujihashi K, Kiyono H, Tennyson GS, Cong Y, Black CA, Ridwan BW, McGhee JR. Hapten-induced model of murine inflammatory bowel disease: mucosa immune responses and protection by tolerance. J Immunol. 1996;157:2174–2185. [PubMed] [Google Scholar]
- 67.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of pro-inflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 68.Xavier R, Podolsky DK. Commensal flora: wolf in sheep's clothing. Gastroenterology. 2005;128:1122–1126. doi: 10.1053/j.gastro.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 69.Kullberg MC, Ward JM, Gorelick PL, Caspar P, Hieny S, Cheever A, Jankovic D, Sher A. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect Immun. 1998;66:5157–5166. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]