Abstract
Doxycycline hydrogels containing reversible disulfide crosslinks were investigated for a dermal wound healing application. Nitrogen mustard (NM) was used as a surrogate to mimic the vesicant effects of the chemical warfare agent sulfur mustard. An 8-arm-poly(ethylene glycol) (PEG) polymer containing multiple thiol (-SH) groups was crosslinked using hydrogen peroxide (H2O2 hydrogel) or 8-arm-S-thiopyridyl (S-TP hydrogel) to form a hydrogel in situ. Formulation additives (glycerin, PVP and PEG 600) were found to promote dermal hydrogel retention for up to 24 h. Hydrogels demonstrated high mechanical strength and a low degree of swelling (<1.5%). Doxycycline release from the hydrogels was biphasic and sustained for up to 10-days in vitro. Doxycycline (8.5 mg/cm3) permeability through NM-exposed skin was elevated as compared to non vesicant-treated controls at 24, 72 and 168 h post exposure with peak permeability at 72 h. The decrease in doxycycline permeability at 168 h correlates to epidermal reepithelialization and wound healing. Histology studies of skin showed that doxycycline-loaded (0.25% w/v) hydrogels provided improved wound healing response on NM-exposed skin as compared to untreated skin and skin treated with placebo hydrogels in a SKH-1 mouse model. In conclusion, PEG-based doxycycline hydrogels are promising for dermal wound healing application of mustard injuries.
1 Introduction
Sulfur Mustard (1, 5-dichloro-3-thiapentane; SM) is a blistering/vesicating agent that has been used in chemical warfare. Injuries induced by SM are more pronounced in the eye, lung and skin [1]. SM rapidly penetrates the skin causing edema, inflammation and blistering. The clinical signs of injury are delayed 2–24 h after exposure depending on dose, temperature, moisture, and the anatomical site of exposure [2]. Although the molecular mechanisms of injury are unclear, SM can alkylate DNA, RNA, proteins and lipids; this can result in tissue damage and cell death [1, 3]. In recent years, the targeting of civilian populations by groups willing to employ chemical warfare agents has intensified the need to develop countermeasures. The most devastating aspect of SM exposure is that wound healing occurs over a prolonged time period (chronic wounds) as compared to other blister-forming injuries resulting from, for example ultraviolet (UV) light exposure [2]. SM exposure on the skin elicits an inflammatory response that results in increased production of several inflammatory cytokines including interleukins (IL-8, IL-6, IL-1α, IL-1β), tumor necrosis factor α (TNF-α), nuclear transcription factor kappa B (NF-κB), along with induction of proteases like matrix metalloproteinases (MMPs) [2, 4, 5], all of which can contribute to toxicity.
Chronically elevated levels of TNF-α induce synthesis of IL-1 [6] and impairs wound healing by increasing the production of MMPs while decreasing the synthesis of collagen [7] and tissue inhibitors of metalloproteinases (TIMPs) [8]. Increased ratio of MMP/TIMP leads to degradation of components of the extra cellular matrix (ECM), growth factors and their receptors in the wound prolonging the time for wound healing [9]. Therefore, inhibition of inflammatory cytokines and proteases can promote healing of chronic wounds such as those seen with SM injury. Doxycycline is a Food and Drug Administration (FDA) approved tetracycline analog that, in addition to its antimicrobial properties, acts as a protease (MMP) inhibitor [10]. Doxycycline also has shown potential to inhibit TNF-α converting enzyme (TACE) and prevent secretion of TNF-α into serum [11]. Hence doxycycline can potentially promote dermal wound healing by reducing both protease activity and inflammation caused by TNF-α at the wound site.
Hydrogels, which are a crosslinked network of hydrophilic polymers [12–14], have been extensively studied for dermal wound healing applications during the past decade. They have the ability to absorb large amounts of water and swell, while maintaining their three-dimensional structure. Their advantages compared to other wound dressings are fluid absorption, hydration of the wound bed, and cooling of the wound surface, which may relieve the symptoms of SM exposure such as erythema, burning, itching, pain and cutaneous lesions [15, 16]. Hydrogels are permeable to water vapor and oxygen, but do not leak liquid water [17]. Depending on the state of hydration of the tissue, hydrogels can absorb or donate water to the wound environment [18]. Hydrogels leave no residue, are malleable and improve reepithelialization of wounds [19]. The maintenance of a moist wound bed has been widely accepted as the most ideal environment for effective wound healing [20]. Many clinical studies attest to the benefits of moist wound healing [21] and demonstrate that hydrogel dressings led to quicker healing, reduced pain, and cost savings when compared to saline dressings [22].
The current studies focus on the development of topical doxycycline hydrogel formulations for the treatment of mustard-injured skin. Nitrogen mustard (mechlorethamine hydrochloride; NM) was used as a surrogate to simulate SM skin injury since it does not require a specialized containment facility [23, 24]. Poly(ethylene glycol) (PEG) based hydrogels are used since PEG is FDA approved, non toxic, non immunogenic, water soluble, and highly stable to pH [10, 25]. The hydrogels evaluated in the current study are formed in situ by covalent inter- and intra-molecular crosslinking of polymer chains through reversible disulfide bonds. Fast forming doxycycline hydrogels are designed and developed for the treatment of simulated mustard injuries using surrogate vesicants and evaluated for their wound healing efficacy in an SKH-1 hairless mouse model.
2 Materials and methods
2.1 Materials
The polymer 8-arm-PEG-SH (20 kDa) was custom synthesized by NOF Corporation (White Plains, NY). Doxycycline hyclate, Poly Vinyl Pyrrolidone (PVP) and PEG 600 were purchased from Sigma-Aldrich (St. Louis, MO). Methanol, acetonitrile, oxalic acid and HPLC grade solvents were obtained from Thermo Fisher Scientific (Pittsburgh, PA). Rheological evaluations were performed on a SR-2000 rheometer from Rheometric Scientific Inc (Piscataway, NJ) equipped with RSI orchestrator software. Drug release and permeation studies were performed using a Franz diffusion cell apparatus from Permgear, Hellertown, PA. Doxycycline was quantified using a Waters HPLC system equipped with a UV detector and an Eclipse XDB-C8 column (Agilent, Zorbax, 4.6 × 150 mm). Human cadaver skin was obtained from New York Firefighter’s skin bank (New York, NY). Tape stripping was done by Scotch magic tape from 3M (St. Paul, MN). A Discovery C18 column (Sigma-Aldrich, St. Louis, MO; 125 × 4 mm; 5 μm) was used for Liquid Chromatography and Mass Spectroscopy (LC-MS) analysis of doxycycline. The LC pump Spectra System P4000, auto sampler Spectra System AS300 and LCQ Deca Mass spectrometer and Xcalibur software were purchased from Thermo Finnigan (Thermo Fisher Scientific, Pittsburgh, PA). Differential Scanning Calorimetry (DSC) was performed on a Q10 differential scanning calorimeter and analyzed using Universal Analysis software (TA instruments, New Castle, DE).
2.2 Synthesis and characterization of 8-arm-PEG-S-thiopyridyl
To a stirred solution of 8-arm-PEG-SH (20 kDa, 1 g) in acetic acid/methanol (5 mL, 1:20), 2-aldrithiol (0.210 g) was added and the reaction mixture was stirred for 12 h at room temperature (RT) to obtain 8-arm-PEG-S-TP (thiopyridyl terminated 8-arm-PEG). After completion of the reaction, the solvent was removed under reduced pressure. The crude 8-arm-PEG-S-TP was purified by size exclusion column chromatography using Sephadex G-25 and water as eluent. Pure compound, obtained after lyophilization of the 8-arm-PEG-S-TP (Yield: 71 % (0.74 g)), was characterized by 1H-NMR, DSC and X ray photoelectron spectroscopy (XPS).
NMR
The 1H NMR spectrum was recorded in CDCl3 on a Varian NMR 500 MHz spectrometer facilitated with AutoX probe and VnmrJ software.
8-arm-PEG-S-TP, 1H-NMR
(CDCl3, 500MHz) δ 3.01 (t, 16H, J = 2, 4 Hz, CH2-S-) 3.55–3.75 (brm, -CH2-CH2O-PEG chain), 7.15 (t, 8H, J = 2, 4Hz, TP-H), 7.78 (t, 8H, J =2, 4 Hz, TP-H) 7.81 (d, 8H, J = 4Hz, TP-H), 8.46 (d, 8H, J = 2Hz, TP-H).
XPS
XPS was performed using an XSAM 800 KRATOS apparatus with a 127 mm radius concentric hemispherical analyzer (CHA). An Al Kα radiation monchromator with photon energy of 1486.6 eV was used as the X-ray source; photoelectrons were detected by the CHA operated in the fixed retarding ratio mode FRR5 (survey scans), and in the fixed analyzer transmission mode FAT80 (detail scans) with pass energies of 80 eV. One scan was conducted to record the survey spectra, and subsequent scans were used to record the detailed spectra. The XPS quantification method, based on the comparison of relative intensities of photoelectron peaks, allows the calculation of the atomic fraction for each component, assuming their total intensities to be 100% and using the corresponding sensitivity factors. The measurements were performed under UHV conditions with a residual pressure of about 10−9 Torr. The solid samples were mounted using double-sided adhesive carbon tape, whereas the powder samples were pressed into tin (Sn) foil to eliminate charging problems.
Differential scanning calorimetry (DSC)
Thermograms for 8-arm-PEG-SH (20 kDa) and 8-arm-PEG-S-TP (~20 kDa) were recorded using nitrogen as purge gas (flow rate of 50 mL/min). Indium was used to calibrate the enthalpy and temperature values. The thermograms were recorded on crimped sealed aluminum pans for samples weighing 4 – 6 mg. The heat cool heat cycles were used to record the thermograms. The samples were equilibrated at −10°C, ramped at 5°C/min to 200°C, quench cooled to −10°C and equilibrated before the second heat cycle at 5°C/min to 200°C.
2.3 Hydrogel formation
Hydrogels were prepared by crosslinking of the 8-arm-PEG-SH (20 kDa) branched thiol terminated PEG polymer. 8-arm-PEG-SH was crosslinked using either hydrogen peroxide (H2O2 hydrogel) or 8-arm-PEG-S-TP (S-TP hydrogel) in the stoichiometric ratio of 1:1 in phosphate buffer (PB, pH 8) at RT. The hydrogel compositions were varied using different concentrations of polymers as shown in Table 1. Hydrogel formation was determined by the “inverted tube method” and hydrogels were considered to have formed once the solution ceased to flow from the inverted tube [26]. Hydrogel disks (200 μL in volume, 9 mm in diameter, and 0.3 mm in thickness) were used for evaluating the degree of swelling, drug loading efficiency and in vitro release studies.
Table 1.
Composition of PEG hydrogel formulations evaluated in the current study
| Ratio of components in the hydrogel (w/w) | Hydrogels | Hydrogel Composition | ||||
|---|---|---|---|---|---|---|
| Gelation time (s) | 8-arm-PEG-SH (% w/v) | H2O2 volume (μl) | 8-arm-PEG-S-TP (% w/v) | Total wt of polymers (% w/v) | ||
| (1:1) | 4 % H2O2 | 75 | 4 | 1.9 | -------- | 4 |
| (1:1) | 8 % H2O2 | 55 | 8 | 5.4 | -------- | 8 |
| (1:1) | 5 % S-TP | < 10 | 5 | -------- | 5 | 5 |
| (1:1) | 8 % S-TP | < 10 | 8 | -------- | 8 | 8 |
2.4 Determination of physicochemical properties of the hydrogel
2.4.1 Swelling studies
The degree of swelling was evaluated for 4 and 8 % H2O2 hydrogels and 5 and 8 % S-TP hydrogels. Hydrogels were placed in a vial and weighed prior to being suspended in 5 mL phosphate buffer saline (PBS, pH 7.4) and placed in an incubator at 37 °C. The degree of swelling of the hydrogels was calculated by weighing the vials after removing the entire PBS at predetermined time intervals. The same amount of PBS was replaced after each measurement and the hydrogels were allowed to swell until equilibrium was reached. The degree of swelling for each hydrogel was determined by using the equation below:
| (1) |
Where Ws is the weight of the swollen hydrogel at time t and W0 is the initial weight. All measurements were made in triplicate for each hydrogel using separate samples and the mean ± SEM of the values was reported. Two-way analysis of variance (ANOVA) was used to determine the effect of hydrogel composition on its degree of swelling.
2.4.2 Effect of formulation additives
Glycerin, PVP and PEG 600 were used as additives in the hydrogel formulation. The hydrogels comprising these additives are referred hereafter as hydrogels with additives. The optimal concentration of additives was determined by measuring the degree of dehydration and retention time of hydrogel formulation on mice skin. Rheometry and DSC were used to evaluate the effect of additives on the mechanical strength and crosslinking properties of the hydrogels, respectively. The retention time (24 h) of hydrogels on SKH-1 mouse skin was investigated by visual examination.
2.4.2.1 Rheology
The rheological measurements of 4 and 8 % H2O2 hydrogels and 5 and 8 % S-TP hydrogels in the presence and absence of formulation additives were performed using a rheometer with cone plate geometry at 37°C (plate diameter: 25 mm, gap: 3 mm, 2° angle) [27, 28]. The hydrogel samples were equilibrated on the plate for 5 min to reach the running temperature before each measurement. Rheological test parameters, storage/elasticity (G′) and loss (G″) moduli were obtained under dynamic conditions of non-destructive oscillatory tests. The strain sweep test was performed at a constant frequency of 1 Hz with percent strain ranging from 10−1 to 101. The frequency sweep test was carried out at a constant strain of 1 % (linear viscoelastic region) with frequency ranging from 10−1 to 101 Hz. All the rheological studies were done in triplicate and the mean ± SEM reported. Two-way ANOVA was used to determine the effect of hydrogel composition and formulation additives on its rheological properties.
2.4.2.2 DSC
Thermograms for the 4 and 8 % H2O2 hydrogels and 5 and 8 % S-TP hydrogels with and without formulation additives were recorded using DSC as described above in section 2.2.
2.5 Reversible nature of hydrogels
The time for reduction of disulfide bonds in presence of reducing agent was evaluated using different concentrations of glutathione (GSH) in PBS pH 7.4.
2.6 In vitro release studies
2.6.1 Drug loading efficiency
4 and 8 % H2O2 hydrogels and 5 and 8 % S-TP hydrogels were used for drug loading and release studies. The hydrogel discs containing 0.25 % w/v of doxycycline were dissected into small pieces and suspended into 5 mL PBS (pH 7.4). The suspension was sonicated for 30 min to completely extract doxycycline from the hydrogel. The amount of doxycycline extracted was quantified by reverse phase (RP) HPLC analysis at a wavelength of 350 nm. 0.01M oxalic acid, acetonitrile and methanol (70:18:12) were used as mobile phase at a flow rate of 1 mL/min. The retention time for doxycycline was 6 min. After extraction, the suspension containing the hydrogel was stored for several days at 4°C and then reanalyzed to ensure the complete extraction of doxycycline from the hydrogel. Doxycycline was stable under the storage conditions used, as determined by HPLC analysis.
2.6.2 In vitro doxycycline release
In vitro release studies were performed as previously described [10] using a Franz diffusion cell apparatus with a diameter of 5 mm and a diffusional area of 0.636 cm2. A polycarbonate membrane (0.45 μ) was sandwiched between the lower cell reservoir and the glass cell-top containing the sample for doxycycline release studies. The receiving compartment (volume 5.1 mL) was filled with PBS (pH 7.4). The system was maintained at 37 °C using a circulating water bath and a jacket surrounding the cell. The receiving medium was continuously stirred (600 rpm) with a magnetic bar to avoid stagnant aqueous diffusion layer effects. 200 μL of each hydrogel formulation containing 0.25 % w/v doxycycline was prepared and placed in the donor compartment, which was then sealed with parafilm and aluminum foil to prevent evaporation. Aliquots (200 μL) were collected from the receiver compartment at predetermined intervals and replaced with an equal volume of PBS to maintain sink conditions throughout the study. The concentration of doxycycline in the release medium was determined using a RP HPLC as described above. The cumulative amount of doxycycline released from the hydrogel was determined using a calibration curve. All experiments were done in triplicate and the results are reported as mean ± SEM. The release data were fitted using a two-phase exponential association equation in GraphPad Prism 4 software. Two way ANOVA was used to determine the effect of the hydrogel composition on the in vitro doxycycline release.
2.7 In vivo studies
2.7.1 Formation of wounds
SKH-1 hairless mice were used for permeability and wound healing efficacy studies. Animals were treated according to the Principles of Animal Care by National Institutes of Health and an animal protocol approved by the Rutgers University Institutional Animal Care and Use Committee. Dermal wounds were created by topical application of 5 μmoles of NM dissolved in acetone to the dorsal skin of mice. A standard circular template (15 mm) was used to ensure uniform exposure area on all mice. The mice were left in the hood overnight to degas after NM exposure. The mice were euthanized by CO2 gas at predetermined time intervals for collection of wounded skin or punch biopsies to be used in permeability or wound healing efficacy studies, respectively.
2.7.2 Permeation of doxycycline through NM-exposed skin
Doxycycline permeability on mice skin exposed to NM for 0 (control), 24, 72 and 168 h was studied using a similar procedure to the in vitro release studies using a Franz diffusion cell set up. Mouse skin was placed in PBS (pH 7.4) for 1 h prior to being sandwiched between the lower cell reservoir and the glass cell-top. Samples of doxycycline (200 μL) in PBS (pH 7.4) were placed in the donor compartment. Aliquots (200 μL) were collected from the receiver compartment at predetermined intervals and replaced with equal volumes of PBS to maintain sink conditions throughout the study. The concentration of doxycycline in the release medium was determined using a RP HPLC as described above. The cumulative amount of doxycycline permeated through skin was determined using a calibration curve. All permeation experiments were done in triplicate and the results reported as mean ± SEM. Student’s t-test was used to determine the effects of NM on the permeation of doxycycline through mouse skin.
2.7.3 In vivo wound healing efficacy of doxycycline hydrogels
2.7.3.1 Application of doxycycline hydrogels
150 μl of 8 % 8-arm-PEG-SH in PB (pH 8.0) containing 0.25 % w/v doxycycline and 5.4 μl of H2O2 were applied simultaneously on wounded areas of mouse to result in 8 % H2O2 hydrogels. 80 μl of 8 % 8-arm-PEG-SH in PB (pH 8.0) containing 0.25 % w/v doxycycline and 80 μl of 8 % 8-arm-PEG-S-TP in PB (pH 8.0) were applied simultaneously on wounded area of mouse to result in 8 % S-TP hydrogels. Both the H2O2 and S-TP hydrogels were applied using a double barrel syringe and contain the formulation additives 5 % v/v glycerin, 4 % w/v PVP and 5 % v/v PEG 600.
2.7.3.2 Wound healing efficacy
Five treatment groups (n=5 per group) were evaluated to measure the wound healing efficacy of doxycycline loaded H2O2/S-TP hydrogels on NM wounds. Two hours after exposure to NM, the wounded site was either left untreated (control) or treated with placebo H2O2/S-TP hydrogels or doxycycline (0.25 % w/v) loaded H2O2/S-TP hydrogels. Skin biopsies from the wounded site were collected at 0 (control), 24, 72, 168 and 240 h after exposure to NM. For histology analysis, the skin biopsies were fixed in 10 % formalin overnight before sectioning and analyzing by hematoxylin and eosin (H & E) staining. Digital images were captured with a light microscope at 40 × magnification.
3 Results and Discussion
3.1 Synthesis of 8-arm-PEG-S-TP
8-arm-PEG-S-TP was prepared by reaction of 8-arm-PEG-SH and 2-aldrithiol in acetic acid/methanol. The formation of 8-arm-PEG-S-TP was confirmed by 1H NMR. The signals corresponding to the aromatic proton of thiopyridyl groups appear at 7.15, 7.78, 7.81 and 8.46 ppm as seen from the proton spectrum of 8-arm-PEG-S-TP. The peaks for the PEG chain in 8-arm-PEG-S-TP appear at 3.55-3.75 (-CH2-CH2O-) and 3.01 (-CH2-S-) ppm. The results were further affirmed by DSC analysis of the 8-arm-PEG-SH and 8-arm-PEG-S-TP. Figure 1 shows the melting temperatures for 8-arm-PEG-SH and 8-arm-PEG-S-TP at 53.37°C and 45.81°C. The shift of −7.56°C in the melting of 8-arm-PEG-SH when converted to 8-arm-PEG-S-TP confirms the substitution of the TP groups on PEG. These observations were consistent with those reported in the past [29]. Furthermore, XPS analysis was conducted to confirm the number of thiol and nitrogen species present on 8-arm-PEG-S-TP. The atoms have valence and core electrons, and for each atom the core level binding provides a unique signature of that element. The binding energy 399 corresponds to nitrogen 1s/2 and 164 corresponds to sulfur 2p/1 (Figure 2). These studies confirmed that the ratio between sulfur and nitrogen atoms was 2:1, indicative of two sulfurs and one nitrogen species present in the star shaped 8-arm-PEGS-TP (Figure 2).
Figure 1.

The DSC thermograms show the melting of 8-arm-PEG-SH and 8-arm-PEG-S-TP at 53.37°C and 45.81°C respectively, indicative of conversion of thiols to thiopyridine.
Figure 2.

The XPS spectrum for the 8-arm-PEG-S-TP. A) The peak centered at 399eV corresponds to N1s confirming the incorporation of nitrogen in the 8-arm-PEG-S-TP polymer; B) The peak centered at 164eV represents S 2p of sulfur corresponding to the dithiopyridine groups. The XPS analysis of 8-arm-PEG-TP, shows the diffractograms for nitrogen and sulfur atoms. The ratio between sulfur and nitrogen was found to be 2:1.
3.2 Hydrogel formation
Hydrogels with different compositions were investigated for their controlled drug delivery application on skin. 8-arm-PEG-SH was crosslinked in presence of either H2O2 (H2O2 hydrogel) or 8-arm-PEG-S-TP (S-TP hydrogel) resulting in the formation of a hydrogel network through disulfide bridges (Scheme 1 and Scheme 2). The hydrogel network in H2O2 hydrogels could result from inter- or intramolecular disulfide bridges in 8-arm-PEG-SH formed due to the oxidation of thiol groups, while the hydrogel network resulting from the crosslinking of 8-arm-PEG-SH by 8-arm-PEG-S-TP is exclusively through the formation of intermolecular disulfide bridges. 4 and 8 % H2O2 hydrogels and 5 and 8 % S-TP hydrogels each containing 1:1 stoichiometric ratios of these components to 8-arm-PEG-SH were evaluated in the current study. The gelation times for 4 and 8 % H2O2 hydrogels are 75 and 55 s, respectively. The faster formation of hydrogels with the increased concentration of polymers might be due to formation of rapid and intense crosslinking networks, reducing the time for gelation. The 5 and 8 % S-TP hydrogels formed in less than 10 s indicating that total polymer concentration has little effect on the gelation time of S-TP hydrogels.
Scheme 1.

Schematic representation of H2O2 hydrogels. Intra and inter molecular crosslinking of the 8-arm-PEG-SH by H2O2 in PB (pH 8) via disulfide bridges.
Scheme 2.

Schematic representation of thiopyridyl terminations appended on the 8-arm-PEG-SH to form 8-arm-PEG-S-TP. Thiopyridine is a good leaving group and the 8-arm-PEG-S-TP forms disulfide bridges with the 8-arm-PEG-SH in PB (pH 8) resulting in S-TP hydrogels.
3.3 Reversible nature of hydrogels
Reversible crosslink hydrogels were developed based on a disulfide exchange reaction that involves the transfer of existing disulfide bonds to free thiol containing moieties that are present in the reducing agent. In the current study, the reduction of disulfide bonds in the hydrogels was investigated using GSH as reducing agent. GSH acts as a thiolate moiety at basic and mild acidic conditions becoming oxidized in the process while cleaving existing disulfide bonds. Exchange reactions do not change the total number of disulfide bonds but rather shuffle the species that form them. The possible products in the presence of excess GSH are 8-arm-PEG-SH, 8-arm-PEG-S-S-G, G-S-S-G and 8-arm-PEG-SH, which may have a few arms bearing either ‘SH’ or ‘S-S-G’ termini as shown in Scheme 3. GSH solutions (1, 3 and 5 % w/v) in PBS (pH 7.4) were used to study the reversibility of disulfide bridges in hydrogels. It was observed that, 5 % GSH solution cleaved the disulfide bonds in 10 – 15 min as compared to the 1 and 3 % GSH solutions that required 30 – 40 min and 15 – 20 min, respectively. These results demonstrate that crosslinks in the H2O2 and S-TP hydrogels can be reversed facilitating easy removal of hydrogels from the damaged tissue without the necessity of peeling them off.
Scheme 3.

Schematic representation of the reversible nature of hydrogels. GSH acts as a thiolate moiety and attacks the disulfide bonds resulting in the breakdown of the hydrogel network (gel to sol transition). The possible products are 8-arm-PEG-SH, 8-arm-PEG-(SH)-S-SG, 8-arm-PEG-S-SG and GS-SG.
3.4 Degree of swelling
The swelling behavior of a hydrogel influences its surface and mechanical properties as well as drug release kinetics. Hydrogels are swelling controlled systems and the degree of swelling is a measure of its crosslinking density, which is important for regulating its pore size. Figure 3 shows the degree of swelling expressed as percent swelling plotted against time for 4 and 8 % H2O2 hydrogels and 5 and 8 % S-TP hydrogels. Both the H2O2 and S-TP hydrogels swelled initially and then gradually reached equilibrium. Compared to hydrophilic hydrogels reported in the literature, a relatively lower degree of swelling (< 1 % for H2O2 hydrogels and < 1.5 % for S-TP hydrogels) was observed for the currently investigated hydrogels [30]. Furthermore, the degree of swelling decreased with an increase in polymer concentrations. The decrease in hydrogel swelling with increasing polymer concentrations is due to the smaller pore size and higher crosslinking density [31, 32].
Figure 3.

Effect of the concentration of polymers on swelling kinetics of 4, 8 % H2O2 hydrogels and 5, 8 % S-TP hydrogels. As the concentration of polymers is increased, the degree of swelling is lowered.
3.5 Effect of formulation additives
The hydrogels in the current study were developed for topical application on the skin without the presence of adhesive backing materials. Formulation additives such as glycerin, PVP and PEG 600 were used in the hydrogels to decrease the brittleness caused by water evaporation/dehydration and to increase their adhesiveness on skin. Glycerin is a well known humectant and plasticizer that was used to prevent hydrogel dehydration[33]. PEG 600 was used as a plasticizer and humectant [34]. PVP was used to increase hydrogel adhesiveness on the skin and to impart viscosity and film forming ability [35]. The optimal concentration of the additives to prevent brittleness and increase retention time on mouse skin for up to 24 h was found to be 5 % glycerin, 4 % PVP and 5 % PEG 600.
3.6 Rheology
The mechanical strength and viscoelastic properties of the hydrogels were investigated using rheological measurements [28] to assess their physical integrity in vivo. Viscoelastic properties were investigated because hydrogels with good mechanical strength are expected to maintain their integrity and help prevent physical drug loss from disintegration of the hydrogel [36]. The rheological studies were performed on 4 and 8 % H2O2 hydrogels (Figure 4) and 5 and 8 % S-TP hydrogels (Figure 5), with and without formulation additives. The strain sweep test was performed on all the hydrogels in order to establish the range of linear viscoelasticity (LVE) and to determine if the elasticity of the formulations differed, as expressed by the storage/elastic modulus (G′). The strain sweep test results (Figures 4A and 5A) suggest that G′ dominates in all the hydrogels, both with and without formulation additives and this is supported by the results obtained from the frequency sweep test (Figures 4B and 5B). G′ for all the hydrogels is one order higher magnitude than G″ (loss modulus), suggesting that the hydrogels are more elastic than viscous in the investigated frequency range.
Figure 4.

Influence of strain (A) and frequency (B) on G′ and G″ of 4 and 8 % H2O2 hydrogels with and without formulation additives. The strain sweep test establishes the regime of linear viscoelasticity (LVE). The frequency sweep test shows that the hydrogels are elastic than viscous and that they have the ability to resist structural changes under strain.
Figure 5.

Influence of strain (A) and frequency (B) on G′ and G″ of 5 and 8 % S-TP hydrogels with and without formulation additives. The strain sweep test establishes the regime of linear viscoelasticity (LVE). The frequency sweep test shows that the hydrogels are elastic than viscous and that they have the ability to resist structural changes under strain.
Figure’s 4A and 4B show that G′ is independent of frequency and strain for all the H2O2 hydrogels with and without additives. However, G″ is independent of frequency and strain only in the H2O2 hydrogels without additives but is weakly dependent on frequency and strain in the H2O2 hydrogels with additives. The G′ and G″ values are almost similar for the hydrogels with and without additives suggesting that formulation additives have minimal influence on the rheological properties and hence the physical strength of H2O2 hydrogels.
Figures 5A and 5B show that G′ is independent of frequency and strain for all the S-TP hydrogels with and without additives. G″ is also found to be independent of frequency and strain in all the S-TP hydrogels with an exception of the 8 % S-TP hydrogel without additives that was found to depend on frequency. The total polymer concentration in the hydrogels influenced both G′ and G″ values for all of the hydrogels regardless of the presence or absence of formulation additives.
The loss tangent values (tanδ= G″/G′) indicate that the storage modulus is the dominant feature in all the hydrogels, as a small tanδ indicates an elastic material. The rheological data show that both the H2O2 and S-TP hydrogels have good mechanical properties as evident by higher G′ values, which might help prolong their contact time on the skin and prevent structural breakage.
3.7 Differential scanning calorimetry (DSC)
The difference in crystallization behavior of polymer networks from that of linear polymers is well known [29]. The crosslinking of polymer chains result in a reduction of crystallinity. Figure 6 shows the influence of polymer concentration and presence of additives on melting temperatures of 4 and 8 % H2O2 hydrogels (Figure 6A) and 5 and 8 % S-TP hydrogels (Figure 6B). Both the H2O2 and S-TP hydrogels with additives showed lower crystallization temperatures when compared to hydrogels without additives. Also, the crystallization temperatures decreased slightly with increasing polymer concentration indicating higher crosslinking density. In case of S-TP hydrogels, the crosslinks are highly defined as they result from intermolecular network formed between the two polymers, 8-arm-PEG-SH and 8-arm-PEG-S-TP. Since the PEG chains are bound, there is reduced mobility and a highly ordered crystalline structure is not achieved, as reflected by the lower melting temperature for S-TP hydrogels compared to the H2O2 hydrogels. The main reason for the observed behavior is the restricted diffusion and orientation of the molten polymer chains as a result of crosslinking, since the crosslinks are excluded from the crystalline phase [29]. The addition of formulation additives further restricts the movement of the PEG chains so that they orient in the crystalline phase resulting in additional lowering of the melting temperature.
Figure 6.

The DSC thermograms for the 4 and 8 % H2O2 hydrogels (A) and 5 and 8 % S-TP hydrogels (B) with and without formulation additives. With an increase in polymer concentration of the hydrogels, the melting of the PEG shifts to lower temperatures. The presence of formulation additives further restricts the motion of PEG chains lowering their melting temperature.
3.8 In vitro doxycycline loading and release
Doxycycline loading efficiency results show that 4 and 8 % H2O2 hydrogels entrapped 44.38 and 36.42 % w/w of doxycycline. The 5 and 8 % S-TP hydrogels showed 46.17 and 43.94 % w/w doxycycline loading. The S-TP hydrogels appear to have higher drug loading efficiencies than the H2O2 hydrogels. The H2O2 hydrogels result in a hydrogel with collapsed structure as compared to S-TP hydrogels where the polymer branches (or arms) are stretched to form crosslinks with other polymer molecules. This could attribute for the difference in pore size and hence the drug loading efficiencies between the two hydrogels.
The doxycycline release profiles from four different hydrogels were studied in vitro using a Franz diffusion cell apparatus. A plot of cumulative amount of doxycycline released (μg/cm2) as a function of time (h) (Figure 7) demonstrates that doxycycline entrapped in both the H2O2 and S-TP hydrogels showed sustained release for 10 days (240 h) with 73 to 84 % of doxycycline being released from different formulations. A slower drug release is observed for H2O2 hydrogels compared to S-TP hydrogels. The swelling data shows that the volume of hydrodynamic water associated with 4 % H2O2 hydrogels is lower as compared to the 5 % S-TP hydrogels, which correlates with the collapsed structure of H2O2 hydrogels arising from intramolecular crosslinking. Also, slower doxycycline release was observed for hydrogels with higher concentrations of polymers, which might be due to the formation of a tighter hydrogel network and decreased pore size. The release data were fitted using two-phase exponential association equation in GraphPad Prism 4 software and the goodness of fit for the different hydrogels varied from 0.83 to 0.96.
Figure 7.

Cumulative amount of doxycycline released as a function of time for 4 and 8 % H2O2 hydrogels and 5 and 8 % S-TP hydrogels. The release data were fitted using a two-phase exponential association equation using GraphPad Prism 4 software. The goodness of fit for the different hydrogels varied from 0.83 to 0.96. The release mechanism for the H2O2 hydrogels is non-fickian or anomalous involving both diffusion and polymer relaxation (0.5<n<1). The release mechanism for the S-TP hydrogels is super case II transport involving relaxation of the polymer as the hydrogel swells (n>1).
The relative influence of diffusion and polymer relaxation on the mechanism of doxycycline release was determined by fitting the experimental data (first 60 % of the total amount released) to the Ritger-Peppas equation [37].
| (2) |
In Equation 2, Mt/M∞ is the fractional release of the drug, ‘k’ is the proportionality constant, ‘n’ is the diffusion exponent and ‘t’ is the time. The diffusion exponent ‘n’ was calculated from the slope of the natural logarithmic values (ln) of the fractional release as a function of time (Table 2). The release mechanism for both the H2O2 hydrogels was found to be Non-Fickian or anomalous involving both diffusion and polymer relaxation (0.5<n<1). The release mechanism for both the S-TP hydrogels was found to be Super Case II transport, which is due to the relaxation of the polymer as the hydrogel swells [38, 39]. This correlates well with the swelling data, where the 5 % S-TP hydrogel with higher polymer content shows higher swelling as compared to the 4 % H2O2 hydrogel.
Table 2.
Estimation of flux and diffusion exponents (n) for various hydrogel formulations.
| % w/v Hydrogels | Flux (J) (mg cm−2 s−1) × 10−6 | Diffusion exponent ( n ) |
|---|---|---|
| 4 % H2O2 | 4.91 | 0.65 |
| 8 % H2O2 | 3.83 | 0.59 |
| 5 % S-TP | 5.45 | 2.04 |
| 8 % S-TP | 4.43 | 1.60 |
For the drug to diffuse through the hydrogel, the polymeric chains must first relax to allow the diffusion process. The hydrogels described in the present study are based on PEG, which is a highly hydrophilic polymer with low glass transition temperature (Tg) [40]. The Tg of PEG hydrogels was found to be 27–28°C (data not shown). As compared to the glassy polymeric materials which exhibit a moving boundary of water phase in hydrogel layers, the diffusion of water is rapid in PEG hydrogels thereby attaining a rapid equilibrium[41]. This contributes to the observed Super Case II transport, as the hydrogel exhibits the ability to imbibe water and swell, and the whole polymer system is relaxed. Typically in glassy polymers, Super Case II transport occurs due to a plasticization process in the hydrogel layer which arises from a reduction of the attractive forces among polymeric chains that increase the mobility of macromolecules [42]. Thus diffusion rate increases with an increase in chain mobility and relaxation rate of the polymeric chains [43]. Both the non-Fickian and Super Case II transport mechanisms of drug diffusion are time dependent (tn−1) [44]. Doxycycline release from both the H2O2 and S-TP hydrogels was dependent on two simultaneous processes-migration of water into the hydrogel and drug diffusion through continuously swelling hydrogels.
The flux (J) and diffusion exponent (n) for various hydrogel formulations is shown in Table 2. Flux was calculated from the slope of the linear portion of the cumulative amount of doxycycline released (μg/cm2) as a function of time. The flux and diffusion exponents decreased with an increase in polymer concentrations for both the H2O2 and S-TP hydrogels. This indicates a decrease in swelling and pore size of the hydrogels due to the formation of a tighter hydrogel. The in vitro release studies show that by changing the polymers and their concentrations, drug release from hydrogels can be tailored.
3.9 Doxycycline permeation in NM exposed skin
Permeability studies were performed to evaluate the barrier function (transdermal drug permeability) of vesicant-exposed skin. The stratum corneum is generally considered to be the rate-limiting barrier to dermal penetration of topically applied drugs [45]. The permeability of vesicant-exposed skin is expected to increase by the loss of stratum corneum and separation of the epidermis and dermis after vesicant exposure [2]. Since the biological membrane (i.e., stratum corneum) controlling drug influx would be less or no longer functional, the hydrogel delivery system would have to provide the rate controlling properties.
The permeation of doxycycline through skin exposed to NM for 0 (control), 24, 72 and 168 h was evaluated using a Franz diffusion cell apparatus. Figure 8 shows a plot of cumulative amount of doxycycline permeated (μg/cm2) as a function of time. Table 3 shows the lag times and permeability coefficients of doxycycline permeation through NM-exposed skin. The lag time for doxycycline permeation was determined by extrapolating the linear portion of the permeation curve to x-axis. Flux (J) was obtained from the slope of the linear portion of the permeation curve and permeability coefficient (P) was calculated from flux using the equations below.
Figure 8.
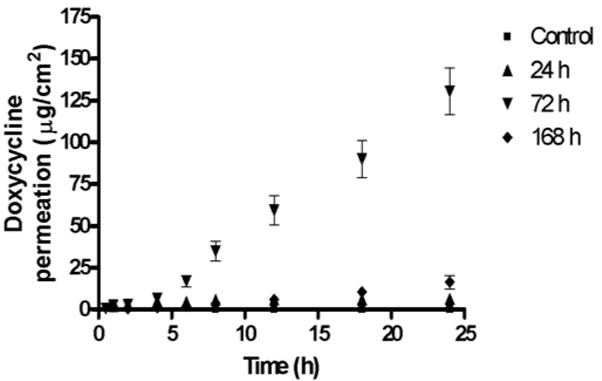
Cumulative amount of doxycycline permeated as a function of time through NM-exposed skin. The permeability of doxycycline through NM-exposed skin for variable time periods was found to increase significantly (p<0.01) compared to the control. The order of permeation of doxycycline is 72 h> 168 h> 24 h>control.
Table 3.
Estimation of lag time and permeability coefficients for doxycycline through NM-exposed skin.
| NM exposure time (h) | Lag time (h) | Permeability Coefficient (P) (cm. h−1) × 10−6 |
|---|---|---|
| 0 (Control) | 6.998 | 0.5717 ± 0.0664 |
| 24 | 1.792 | 17.95 ± 5.87 |
| 72 | 1.620 | 661.9 ± 28.4 |
| 168 | 1.608 | 78.86 ± 6.83 |
| (3) |
| (4) |
Where ‘J’ indicates the steady state flux, ‘dQ’ the amount of drug permeated, ‘A’ the dermal area exposed, ‘dt’ the time of permeation and ‘C0’ represents the initial drug concentration in the donor compartment.
The permeability of doxycycline through NM-exposed skin for variable time points was found to increase significantly (p<0.01) compared to the control. The order of permeation of doxycycline through NM-exposed skin is 72 h> 168 h> 24 h> control (Table 3) indicating that the barrier function of the stratum corneum is compromised after vesicant exposure. The order of permeation of doxycycline suggests that the greatest damage after NM exposure is evident at 72 h and the barrier property of skin seems to improve between 72 and 168 h, which is in accordance with the results published by others [46]. The two major factors that determine transdermal drug absorption are transdermal drug permeability and contact time of the delivery system on skin. Since the barrier properties of skin are compromised when exposed to vesicants, the current hydrogel system can be expected to promote wound healing not only by prolonging drug contact time, but also by providing continuous drug release at the injured site.
3.10 In vivo wound healing efficacy of doxycycline hydrogels
For wound healing efficacy five mice were evaluated in each treatment group at 24, 72, 168 and 240 h after NM exposure and representative H & E stained histological sections are shown in Figures 9, 10, 11 and 12, respectively. The treatments were initiated 2 h after NM exposure and this time period approximately simulates the time that would pass before an exposure is recognized (based on the delayed times for symptoms) and medical help is secured. Two hours after exposure to NM, the wounded site was either left untreated (control) or treated with placebo H2O2/S-TP hydrogels or doxycycline (0.25 % w/v) loaded H2O2/S-TP hydrogels. The hydrogels described in the present study are in situ forming, i.e., they form a hydrogel within a few seconds after the two polymer solutions are mixed and applied to the skin. A thin film of hydrogel was formed and retained on the skin, due to the presence of formulation additives, for up to 24 h. The hydrogel dressing was changed every 24 h by washing with a solution of 5 % GSH in PBS, which facilitated hydrogel dissolution and easy removal without the need for peeling it off from wounded skin. Glutathione is used in many skin products. Glutathione (GSH) is the most abundant thiol species in the cytoplasm and the major reducing agent for almost all biochemical processes. The biosynthesis and metabolism of GSH, which proceeds through the γ-glutamyl cycle, has been studied and thoroughly characterized. Oxidized glutathione (GS-SG) is the product that is obtained in the biosynthesis and metabolism of GSH. Poly(ethylene glycol) (PEG) is FDA approved, nontoxic, nonimmunogenic compound, therefore the degradation products obtained after hydrogel degradation are substantially nontoxic. Therefore, GSH or products obtained after hydrogel degradation should not cause any damage in the open wound bed. This is consistent with the results in the current study.
Figure 9.

Histology of mouse skin 24 h post exposure to NM and hydrogels (Magnification 40x). Two h after topical application of NM or control, either placebo- or doxycycline-containing H2O2 or S-TP hydrogels were applied to the skin. After 24 h, NM was found to cause a marked inflammatory cell infiltration and edema; areas of epidermal/dermal separation were also evident. Epidermal separation was not evident in skin treated with either placebo or doxycycline hydrogels indicating that the hydrogels were acting as an occlusive bandage.
Figure 10.

Histology of mouse skin 72 h post exposure to NM and hydrogels (Magnification 40x). NM caused degradation of the dermis, epidermal necrosis and extensive epidermal/dermal separation. The placebo and doxycycline containing H2O2- or S-TP hydrogels suppressed dermal degradation as well as necrosis and blistering. There was marked improvement in the epidermis in doxycycline containing hydrogels. Significant acanthosis, a marker of wound repair, was noted in the skin treated with both doxycycline-containing hydrogels.
Figure 11.

Histology of mouse skin 168 h post exposure to NM and hydrogels (Magnification 40x). NM caused extensive necrosis of the skin. Both placebo H2O2- or S-TP hydrogels provided some protection; skin treated with the H2O2-hydrogel retained epidermis, although nuclear degradation was evident. A significant improvement in the skin was found post-treatment with both doxycycline containing hydrogels. This included marked epidermal hyperplasia and hyperkeratosis, which extended into the remnant hair follicles. Significant hypergranulosis was also observed.
Figure 12.
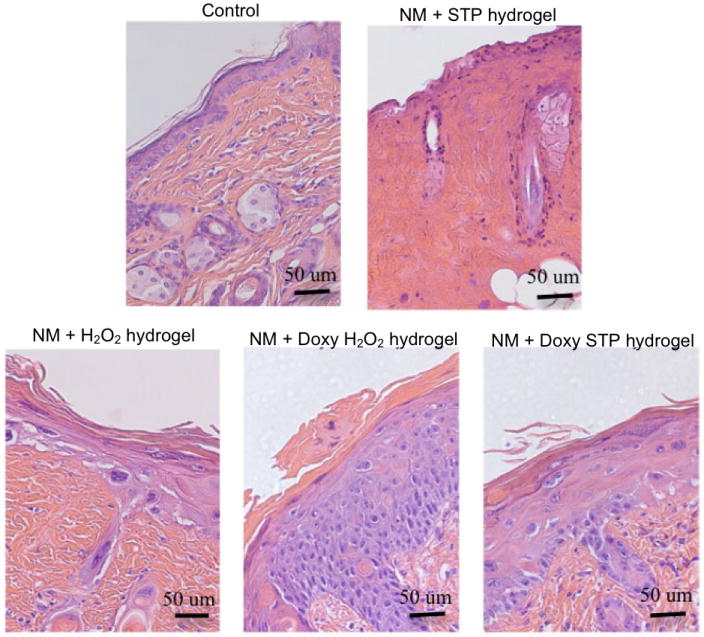
Histology of mouse skin 240 h post exposure to NM and hydrogels (Magnification 40x). Mice did not survive 240 h post exposure to NM without treatments. Although the epidermis appeared damaged by NM when skin was treated with the placebo H2O2-hydrogel, its basic structure was retained indicating that the hydrogel patch alone can suppress tissue injury. S-TP hydrogels were significantly less effective in protecting against NM-induced skin damage. In contrast, doxycycline-containing H2O2- and S-TP-hydrogels were highly effective in protecting the skin from NM-induced injury. Following treatment with either hydrogel, marked acanthosis was noted. A thickening of the stratum corneum, parakeratosis and extensive hyperplasia were evident in skin treated with the doxycycline containing H2O2-hydrogel following exposure to NM.
Figure 9 shows the histology of mice skin exposed to NM and subsequently treated with H2O2/S-TP placebo or doxycycline hydrogels for 24 h. The histology of control skin shows an intact epidermis and dermis, healthy nuclei in the epidermis and fibroblasts in dermis. The common histological features observed in all treatment groups 24 h after NM exposure, were edema (i.e., abundance of clear areas in the dermis), shrinkage/condensation of nuclei in the epidermis (pyknosis) and infiltration of inflammatory cells (blue colored) in the dermis. However, the untreated NM-exposed skin also shows signs of epidermal separation from the dermis and higher infiltration of inflammatory cells compared to the placebo or doxycycline hydrogel treated groups. The placebo or doxycycline hydrogel treated groups do not show any signs of epidermal separation from the dermis. Hence the hydrogel acted as a bandage and prevented the separation of epidermis and dermis. Not much difference in histology was seen between the H2O2/S-TP placebo or doxycycline hydrogels.
Figure 10 shows the histology of mice skin exposed to NM and subsequently treated with H2O2/S-TP placebo or doxycycline hydrogels for 72 h. Edema, complete separation of epidermis from the dermis, death of epidermal cells (absence of nuclear staining) and infiltration of inflammatory cells in the dermis were observed in untreated skin at 72 h after NM exposure. The histology of skin treated with both the H2O2 and S-TP placebo hydrogels showed edema, pyknotic nuclei in the epidermis and areas where epidermis and dermis are slightly separated. The histology of skin treated with both the H2O2 and S-TP doxycycline hydrogels showed pyknotic nuclei in the epidermis but no separation of epidermis and dermis. The placebo and doxycycline hydrogels showed a significant improvement over untreated NM-exposed skin. H2O2/S-TP doxycycline hydrogels prevented the separation of epidermis from the dermis and hence represent an improvement over the H2O2/S-TP placebo hydrogels.
Figure 11 shows the histology of mice skin exposed to NM and subsequently treated with H2O2/S-TP placebo or doxycycline hydrogels for 168 h. The histology of untreated NM-exposed skin showed necrosis all over the dermis and no nuclear staining in the epidermis implying that the tissue is dying or dead. Only sections from two mice showed signs of reepithelialization (i.e., an indication that wound healing is occurring) from the wound edges in the epidermis (data not shown). The histology of skin treated with both the H2O2 and S-TP placebo hydrogels showed an absence of nuclear staining in the epidermis and fibroblasts in the dermis. The histology of skin treated with both the H2O2 and S-TP doxycycline hydrogels showed reepithelialization characterized by epidermal hyperplasia (increased keratinocyte proliferation) and hyperkeratosis (thickening of the stratum corneum). Reepithelialization is essential for wound repair to restore the intact epidermal barrier and it occurs by the migration of epithelial cells from the edge of the unwounded tissue across the site of injury [47, 48]. The H2O2/S-TP doxycycline hydrogels showed a significant improvement in NM-exposed wounds compared to the untreated and H2O2/S-TP placebo hydrogel treated groups.
Figure 12 shows the histology of mice skin exposed to NM and subsequently treated with H2O2/S-TP placebo or doxycycline hydrogels for 240 h. 100% of the mice treated with NM, but neither placebo nor doxycycline hydrogels, died between 168 and 240 h post-exposure. Hence no representative histological sections are shown for this group. 80 and 100% of the mice treated with H2O2/S-TP placebo and doxycycline hydrogels survived for 240 h after NM exposure implying that hydrogels in general have a beneficial effect and that doxycycline hydrogels are better than the placebo hydrogels. The histology of S-TP placebo hydrogel treated NM-exposed group looked like a dying tissue. The histology of H2O2 placebo hydrogel treated NM-exposed group showed hyperplasia and hyperkeratosis, signs of wound healing. The histology of H2O2/S-TP doxycycline hydrogel treated NM-exposed groups showed a second occurrence of reepithelialization (the first occurrence was observed 168 h after NM exposure). The reason for this could possibly be the retention of NM in skin that may then be released at later times resulting in a reoccurrence of reepithelialization. This is similar to SM, which forms a reservoir in skin from which there is continual uptake of SM in the blood during the first few days following exposures [2, 49].
H2O2/S-TP doxycycline hydrogels showed improved wound healing efficacy compared to untreated and H2O2/S-TP placebo hydrogel treated groups after NM exposure, evidenced by increased survival rates and signs of wound healing. Among the two placebo hydrogels, H2O2 hydrogel showed a beneficial effect at 240 h. H2O2 is well known to oxidize mustard [50] and hence, the H2O2 hydrogel might also be acting as a decontaminant to oxidize NM depot in skin resulting in a dermal wound healing effect. The increased pharmacological efficacy of doxycycline hydrogels could be due to increased doxycycline permeation in NM-exposed skin and continuous influx of doxycycline from the hydrogels. Hence, doxycycline hydrogels should be pursued as a potential treatment option for healing of dermal mustard injuries.
4 Conclusions
The in situ forming PEG hydrogels investigated in the current study utilize disulfide bonds, which also facilitates their removal. This can be accomplished by spraying a reducing agent that reverses the disulfide crosslinks resulting in a gel to sol conversion. Formulation additives decrease dehydration and increase adhesiveness of the hydrogel on skin for up to 24 h. The hydrogels have a low degree of swelling, good mechanical strength and provide sustained doxycycline release for up to 10 days. The permeability studies show that the barrier properties of the skin are compromised when exposed to vesicants, allowing increased transdermal drug influx. The in vivo wound healing efficacy results show that doxycycline hydrogels provide a superior wound healing response on NM-exposed skin compared to untreated skin and skin treated with placebo hydrogels. Overall, the doxycycline loaded PEG-based hydrogels are promising for dermal mustard-induced wound healing applications.
Acknowledgments
This research is supported by the CounterACT Program, National Institutes of Health Office of the Director, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Grant number U54AR055073. The Parke-Davis Endowed Chair in Pharmaceutics and Controlled Drug Delivery is also acknowledged. We would like to thank Dr. Boris Yakshinskiy and Dr. Raghavendra Navath for their assistance and fruitful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kehe K, Balszuweit F, Emmler J, Kreppel H, Jochum M, Thiermann H. Sulfur mustard research-strategies for the development of improved medical therapy. Eplasty. 2008;8:e32. [PMC free article] [PubMed] [Google Scholar]
- 2.Graham JS, Chilcott RP, Rice P, Milner SM, Hurst CG, Maliner BI. Wound healing of cutaneous sulfur mustard injuries: strategies for the development of improved therapies. J Burns Wounds. 2005;4:e1. [PMC free article] [PubMed] [Google Scholar]
- 3.Shohrati M, Peyman M, Peyman A, Davoudi M, Ghanei M. Cutaneous and ocular late complications of sulfur mustard in Iranian veterans. Cutan Ocul Toxicol. 2007;26(2):73–81. doi: 10.1080/15569520701212399. [DOI] [PubMed] [Google Scholar]
- 4.Isidore MA, Castagna MP, Steele KE, Gordon RK, Nambiar MP. A dorsal model for cutaneous vesicant injury by 2-chloroethyl ethyl sulfide using C57BL/6 mice. Cutan Ocul Toxicol. 2007;26(3):265–276. doi: 10.1080/15569520701521914. [DOI] [PubMed] [Google Scholar]
- 5.Paromov V, Suntres Z, Smith M, Stone WL. Sulfur mustard toxicity following dermal exposure: role of oxidative stress, and antioxidant therapy. J Burns Wounds. 2007;7:e7. [PMC free article] [PubMed] [Google Scholar]
- 6.Le JM, Weinstein D, Gubler U, Vilcek J. Induction of membrane-associated interleukin 1 by tumor necrosis factor in human fibroblasts. J Immunol. 1987;138(7):2137–2142. [PubMed] [Google Scholar]
- 7.Solis-Herruzo JA, Brenner DA, Chojkier M. Tumor necrosis factor alpha inhibits collagen gene transcription and collagen synthesis in cultured human fibroblasts. J Biol Chem. 1988;263(12):5841–5845. [PubMed] [Google Scholar]
- 8.Rayment EA, Upton Z. Finding the culprit: a review of the influences of proteases on the chronic wound environment. Int J Low Extrem Wounds. 2009;8(1):19–27. doi: 10.1177/1534734609331596. [DOI] [PubMed] [Google Scholar]
- 9.Trengove NJ, Stacey MC, MacAuley S, Bennett N, Gibson J, Burslem F, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen. 1999;7(6):442–452. doi: 10.1046/j.1524-475x.1999.00442.x. [DOI] [PubMed] [Google Scholar]
- 10.Anumolu SS, DeSantis AS, Menjoge AR, Hahn RA, Beloni JA, Gordon MK, et al. Doxycycline loaded poly(ethylene glycol) hydrogels for healing vesicant-induced ocular wounds. Biomaterials. 2010;31(5):964–974. doi: 10.1016/j.biomaterials.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milano S, Arcoleo F, D’Agostino P, Cillari E. Intraperitoneal injection of tetracyclines protects mice from lethal endotoxemia downregulating inducible nitric oxide synthase in various organs and cytokine and nitrate secretion in blood. Antimicrob Agents Chemother. 1997;41(1):117–121. doi: 10.1128/aac.41.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lalloo A, Chao P, Hu P, Stein S, Sinko PJ. Pharmacokinetic and pharmacodynamic evaluation of a novel in situ forming poly(ethylene glycol)-based hydrogel for the controlled delivery of the camptothecins. J Control Release. 2006;112(3):333–342. doi: 10.1016/j.jconrel.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Qiu B, Stefanos S, Ma J, Lalloo A, Perry BA, Leibowitz MJ, et al. A hydrogel prepared by in situ cross-linking of a thiol-containing poly(ethylene glycol)-based copolymer: a new biomaterial for protein drug delivery. Biomaterials. 2003;24(1):11–18. doi: 10.1016/s0142-9612(02)00227-2. [DOI] [PubMed] [Google Scholar]
- 14.Deshmukh M, Singh Y, Gunaseelan S, Gao D, Stein S, Sinko PJ. Biodegradable poly(ethylene glycol) hydrogels based on a self-elimination degradation mechanism. Biomaterials. 2010;31(26):6675–6684. doi: 10.1016/j.biomaterials.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollinworth H. Pain relief. Nurs Times. 2001;97(28):63–66. [PubMed] [Google Scholar]
- 16.Mi L, Gong W, Nelson P, Martin L, Sawyer TW. Hypothermia reduces sulphur mustard toxicity. Toxicol Appl Pharmacol. 2003;193(1):73–83. doi: 10.1016/s0041-008x(03)00352-1. [DOI] [PubMed] [Google Scholar]
- 17.Jones V, Milton T. When and how to use hydrocolloid dressings. Nurs Times. 2000;96(4 Suppl):5–7. [PubMed] [Google Scholar]
- 18.Thomas S, Hay P. Fluid handling properties of hydrogel dressings. Ostomy Wound Manage. 1995;41(3):54–56. 58–59. [PubMed] [Google Scholar]
- 19.Amadeu TP, Seabra AB, de Oliveira MG, Costa AM. S-nitrosoglutathione-containing hydrogel accelerates rat cutaneous wound repair. J Eur Acad Dermatol Venereol. 2007;21(5):629–637. doi: 10.1111/j.1468-3083.2006.02032.x. [DOI] [PubMed] [Google Scholar]
- 20.Eaglstein WH, Davis SC, Mehle AL, Mertz PM. Optimal use of an occlusive dressing to enhance healing. Effect of delayed application and early removal on wound healing. Arch Dermatol. 1988;124(3):392–395. [PubMed] [Google Scholar]
- 21.Ovington LG. The well-dressed wound: an overview of dressing types. Wounds. 1998;10(Suppl A):1A–11A. [Google Scholar]
- 22.Gates JL, Holloway GA. A comparison of wound environments. Ostomy Wound Manage. 1992;38(8):34–37. [PubMed] [Google Scholar]
- 23.Karacsonyi C, Shanmugam N, Kagan E. A clinically relevant in vitro model for evaluating the effects of aerosolized vesicants. Toxicol Lett. 2009;185(1):38–44. doi: 10.1016/j.toxlet.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Pino MA, Billack B. Reduction of vesicant toxicity by butylated hydroxyanisole in A-431 skin cells. Cutan Ocul Toxicol. 2008;27(3):161–172. doi: 10.1080/15569520802092070. [DOI] [PubMed] [Google Scholar]
- 25.Anumolu SS, Singh Y, Gao D, Stein S, Sinko PJ. Design and evaluation of novel fast forming pilocarpine-loaded ocular hydrogels for sustained pharmacological response. J Control Release. 2009;137(2):152–159. doi: 10.1016/j.jconrel.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta D, Tator CH, Shoichet MS. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials. 2006;27(11):2370–2379. doi: 10.1016/j.biomaterials.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Xu Z. Physical characterization of a chitosan-based hydrogel delivery system. J Pharm Sci. 2002;91(7):1669–1677. doi: 10.1002/jps.10157. [DOI] [PubMed] [Google Scholar]
- 28.Rudraraju VS, Wyandt CM. Rheology of microcrystalline cellulose and sodiumcarboxymethyl cellulose hydrogels using a controlled stress rheometer: part II. Int J Pharm. 2005;292(1–2):63–73. doi: 10.1016/j.ijpharm.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Qiao C, Jiang S, Dong D, Ji X, An L, Jiang B. The critical lowest molecular weight for PEG to crystallize in cross-linked networks. Macromol Rapid Commun. 2004;25:659–663. [Google Scholar]
- 30.de Souza Costa-Junior E, Pereira MM, Mansur HS. Properties and biocompatibility of chitosan films modified by blending with PVA and chemically crosslinked. J Mater Sci Mater Med. 2009;20(2):553–561. doi: 10.1007/s10856-008-3627-7. [DOI] [PubMed] [Google Scholar]
- 31.Park H, Park K, Kim D. Preparation and swelling behavior of chitosan-based superporous hydrogels for gastric retention application. J Biomed Mater Res A. 2006;76(1):144–150. doi: 10.1002/jbm.a.30533. [DOI] [PubMed] [Google Scholar]
- 32.Tan H, Chu CR, Payne KA, Marra KG. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30(13):2499–2506. doi: 10.1016/j.biomaterials.2008.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ash M, Ash I. Endicott. New York: Synapse Information Resources, Inc; 2004. Handbook of preservatives. [Google Scholar]
- 34.Uglea CV. Oligomer technology and applications. Boca Raton, FL: CRC press; 1998. [Google Scholar]
- 35.Jones DS, Irwin CR, Woolfson AD, Djokic J, Adams V. Physicochemical characterization and preliminary in vivo efficacy of bioadhesive, semisolid formulations containing flurbiprofen for the treatment of gingivitis. J Pharm Sci. 1999;88(6):592–598. doi: 10.1021/js9803095. [DOI] [PubMed] [Google Scholar]
- 36.Carlfors J, Edsman K, Petersson R, Jornving K. Rheological evaluation of Gelrite in situ gels for ophthalmic use. Eur J Pharm Sci. 1998;6(2):113–119. doi: 10.1016/s0928-0987(97)00074-2. [DOI] [PubMed] [Google Scholar]
- 37.Serra L, Domenech J, Peppas NA. Drug transport mechanisms and release kinetics from molecularly designed poly(acrylic acid-g-ethylene glycol) hydrogels. Biomaterials. 2006;27(31):5440–5451. doi: 10.1016/j.biomaterials.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Ferrero C, Munoz-Ruiz A, Jimenez-Castellanos MR. Fronts movement as a useful tool for hydrophilic matrix release mechanism elucidation. Int J Pharm. 2000;202(1–2):21–28. doi: 10.1016/s0378-5173(00)00407-5. [DOI] [PubMed] [Google Scholar]
- 39.Llabot JM, Manzo RH, Allemandi DA. Drug release from carbomer:carbomer sodium salt matrices with potential use as mucoadhesive drug delivery system. Int J Pharm. 2004;276(1–2):59–66. doi: 10.1016/j.ijpharm.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Tessmar JK, Gopferich AM. Customized PEG-derived copolymers for tissue-engineering applications. Macromol Biosci. 2007;7(1):23–39. doi: 10.1002/mabi.200600096. [DOI] [PubMed] [Google Scholar]
- 41.Crank J, Park G. Diffusion in polymers. London and New York: Academic Press; 1968. [Google Scholar]
- 42.Ritger PL, Peppas NA. A simple equation for description of solute release. II. Fickian and anomalous release from swellable devices. J Control Release. 1987;5:37–42. [PubMed] [Google Scholar]
- 43.Siepmann J, Lecomte F, Bodmeier R. Diffusion-controlled drug delivery systems: calculation of the required composition to achieve desired release profiles. J Control Release. 1999;60(2–3):379–389. doi: 10.1016/s0168-3659(99)00093-0. [DOI] [PubMed] [Google Scholar]
- 44.Korsmeyer RW, Gurney R, Doelker E, Buri P, Peppas NA. Mechanism of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35. doi: 10.1002/jps.2600721021. [DOI] [PubMed] [Google Scholar]
- 45.Trommer H, Neubert RH. Overcoming the stratum corneum: the modulation of skin penetration. A review. Skin Pharmacol Physiol. 2006;19(2):106–121. doi: 10.1159/000091978. [DOI] [PubMed] [Google Scholar]
- 46.Tewari-Singh N, Rana S, Gu M, Pal A, Orlicky DJ, White CW, et al. Inflammatory biomarkers of sulfur mustard analog 2-chloroethyl ethyl sulfide-induced skin injury in SKH-1 hairless mice. Toxicol Sci. 2009;108(1):194–206. doi: 10.1093/toxsci/kfn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kurokawa I, Mizutani H, Kusumoto K, Nishijima S, Tsujita-Kyutoku M, Shikata N, et al. Cytokeratin, filaggrin, and p63 expression in reepithelialization during human cutaneous wound healing. Wound Repair Regen. 2006;14(1):38–45. doi: 10.1111/j.1743-6109.2005.00086.x. [DOI] [PubMed] [Google Scholar]
- 48.Reynolds LE, Conti FJ, Silva R, Robinson SD, Iyer V, Rudling R, et al. alpha3beta1 integrin-controlled Smad7 regulates reepithelialization during wound healing in mice. J Clin Invest. 2008;118(3):965–974. doi: 10.1172/JCI33538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hambrook JL, Howells DJ, Schock C. Biological fate of sulphur mustard (1,1′-thiobis(2-chloroethane)): uptake, distribution and retention of 35S in skin and in blood after cutaneous application of 35S-sulphur mustard in rat and comparison with human blood in vitro. Xenobiotica. 1993;23(5):537–561. doi: 10.3109/00498259309059394. [DOI] [PubMed] [Google Scholar]
- 50.Popiel S, Witkiewicz Z, Chrzanowski M. Sulfur mustard destruction using ozone, UV, hydrogen peroxide and their combination. J Hazard Mater. 2008;153(1–2):37–43. doi: 10.1016/j.jhazmat.2007.08.041. [DOI] [PubMed] [Google Scholar]


