Abstract
Among individuals with Rheumatoid Arthritis (RA), pain-associated stress can severely impact wellbeing. Psychological attributes, such as a sense of personal mastery, may attenuate the effects of chronic pain on life quality. We tested the hypothesis that a high sense of mastery would predict lower pain, perceived stress, fatigue, and mean arterial pressure (MAP) than would a low sense of mastery during an acute, interpersonal stressor.
Seventy-four individuals with RA completed a psychophysiological laboratory session involving MAP measurements, as well as self-ratings of stress, joint pain, and fatigue. Measurements were collected before, during, and after an interpersonal stressor. To assess personal mastery, exploratory and confirmatory factor analyses were conducted on the Pearlin Mastery Scale based on recommendations by Reich and Zautra (1991)
The Pearlin Mastery Scale yielded two distinct factors: fatalism and control. Both fatalism and control were significant predictors of the wellbeing variables. Individuals with a highly fatalistic style demonstrated higher general levels of mean arterial pressure (F(1) = 3.41, p<.1) and reported greater joint pain (F(1) = 4.72, p<.05) across all periods. Individuals with a high sense of control also evidenced lower MAP (F(1) = 3.73, p<.1) and reported less stress (F(1) = 7.44, p<.01) and fatigue (F(1) = 5.16, p<.05). Neither fatalism nor control were related to objective measures of disease severity (r's = −.10, p=NS and −.02, p=NS, respectively).
RA patients with a high level of personal mastery, as evidenced by scores on two distinct indices, experience lower MAP, and report less pain, stress and fatigue. Although fatalism and control were not related to objective disease state, they seem to play an important role in the experience of wellbeing for people with RA.
Keywords: arthritis, blood pressure, chronic pain, mastery, personal control, stress
There is little doubt that chronic pain can severely impact wellbeing, affecting all areas of life: physical, social, cognitive, and emotional (1-6). Equally clear, however, is the observation that pain intensity is not a reliable predictor of pain-induced disability (7-9), and substantial person-to-person variability exists in the degree to which chronic pain interferes with life. The identification of factors that accentuate or attenuate the impact of pain on wellbeing, therefore, remains a high priority. One of these psychological factors that may buffer against the negative consequences of chronic pain is a sense of personal control.
Control beliefs have been described as bridging the gap between psychological and physiological processes in healthy and ill individuals (10). Through a variety of mechanisms, personal control has been shown to affect both physiological (11-12) and psychological (13) aspects of chronic diseases. Not only does personal control seem to enhance health-seeking behavior (14), but it also has been shown to promote wellbeing (15). Individual differences in the capacity to sustain beliefs in personal control have been shown to impact disease progression (16) and play a strong role in attenuating the impact of stress on life satisfaction (17).
Control may be especially important in moderating health consequences of acute stressors. Major life events and daily, interpersonal stressors may exacerbate disease states in a variety of chronic illnesses (18-20). Importantly, personal control has been shown to reduce stress associated with environmental events in individuals with Rheumatoid Arthritis (RA; 10 & 21). In the present study, we sought to elucidate the role of personal mastery in the psychological and somatic experience of chronic pain. We focused on individuals with RA, a potentially debilitating disorder that affects approximately 3% of the older adult population (22).
RA is a systemic, autoimmune disorder that causes pain and physical disability primarily through inflammation of synovial joints (23). The impact of this disease on wellbeing may be moderated by psychological factors (24). A recent empirical study by Evers et al. (18) found that both coping and interpersonal variables predict long term RA disease activity at both a 3 and 5-year follow-up, suggesting psychological health can durably impact physiological health. Stress is an important element of RA, as the unpredictable nature of disease flare-ups can complicate the management of functional disability (25). Furthermore, perceptions of disease uncontrollability in RA patients have been shown to increase stress and psychopathological symptoms, such as depression and anxiety (26). Given the complexity of balancing the management of disease fluctuation with the course of daily life events, RA patients are especially vulnerable to the detrimental effects associated with a loss of control (25). For example, while RA is associated with a greater risk for depression (27), evidence suggests that the absence of personal mastery may confer a particular vulnerability in these individuals (28).
Control is used broadly in the literature and encompasses many different constructs and domains (29). In the current study, personal control was assessed with the Pearlin Mastery Scale (30). Mastery is a closely related concept to control and, with a few exceptions (13 & 24), the two terms are often used synonymously (31-33). While the Pearlin Mastery Scale is often considered to be a unifactorial scale, there is some evidence supporting a 2-factor structure (13). In a sample of bereaved and physically disabled older adults, fatalism and control emerged as separate and stable beliefs. Control was described as the belief that one can create or facilitate positive events. Fatalism was defined as the belief that one could not avoid negative events. This factor structure is similar to many other popular two-factor models of control: internal versus external (35), efficacy versus constraints (34), and competence versus contingency (29). These recurrent themes demonstrate the importance in distinguishing control over positive versus negative life events. It is unknown, however, whether or not these two elements of mastery are predictive of health.
This study was conducted with three aims: First, to assess the validity of the 2-factor model of personal mastery in RA patients. Second, to explore the relation of personal mastery to health and wellbeing in RA patients. Third, to test if control and/or fatalism are protective against the effects of an acute stressor. In order to test the above aims, a psychophysiological, interpersonal stress paradigm was used. Stress, blood pressure, fatigue, and pain were measured in RA patients before, during, and after an acute stressor. The use of a laboratory protocol allowed us to closely examine the potential stress-protective effects of personal mastery. Furthermore, the highly controlled environment enabled the gathering of physiological variables such as mean arterial pressure (MAP), which serves as a more objective marker of health and stress-reactivity. Four specific hypotheses were tested: 1) As predicted by Reich and Zautra (13), control and fatalism would emerge as distinct constructs with unique predictive attributes. 2) Control and fatalism would predict the dependent variables over and above the effects of neuroticism. Because neuroticism is related to preoccupation and anxiety over potential negative events, research has shown it prudent to control for its effects when examining sensitivity and reactivity to stressors (36-37). Previous research has suggested that personal control operates independently from neuroticism, but researchers have not examined the influence of this potential confound on different dimensions of personal mastery (38). 3) High control and low fatalism would be associated with lower overall levels of pain, stress, fatigue, and MAP. 4) High control and low fatalism would predict less pain, stress, fatigue, and MAP reactivity to the acute stressor.
Methods
Participants
Participants were 74 individuals, with a confirmed diagnosis of RA, who were randomly selected from a larger study (N=128) of psychological factors in disease progression. Participants were recruited from the Greater Phoenix metropolitan area of Arizona. Exclusionary criteria included a diagnosis of Lupus and use of cyclical estrogen replacement therapies. The study sample consisted of 27 males (37%) and 46 females (63%), with an age range of 23 to 81 years and mean age of 56 (s.d. = 13.2). The sample was predominantly Caucasian (94%). Incomplete data were obtained from one participant, who was excluded from all analyses.
Measures
Mastery
Personal mastery was measured with the Pearlin Mastery Scale (30). The 7-item scale is organized with a Likert-type response, ranging from 1 (strongly disagree) to 4 (strongly agree). Items include, “There is really no way I can solve some of the problems in my life,” and “I can do just about anything I set my mind to do.” The scale authors report good reliability, stabilility, and construct validity (39), and subsequent analyses have found good internal reliability (α = .69 - ω = .81, 40 & 41).
Neuroticism
Neuroticism was measured with the NEO Five-Factor Inventory (42). The 12-item scale was organized with a Likert-type response ranging from 1 (strongly disagree) to 5 (strongly agree). Items included “I often feel helpless and want someone else to solve my problems,” and “I often feel tense and jittery.” Neuroticism, as measured by the NEO Five-Factor Inventory, is known to be a stable personality trait over time (42). Good reliability was found in the present sample (α = .84).
Cardiovascular measures
Blood pressure measurements were collected with an Industrial and Biomedical Sensors Corporation (Waltham, MA) automated blood pressure monitor (IBS, Model SD-700A). MAP was used as the primary cardiovascular dependent measure and was calculated by: (2 * diastolic blood pressure + systolic blood pressure) / 3. Measurements were taken at 2-minute intervals and were averaged within periods of interest (i.e. baseline, stress 1, stress 2, recovery). MAP has been widely used as a measure of cardiovascular reactivity to stress (eg. Clark, Adams, & Clark, 2001).
Fatigue, stress, and pain
Fatigue was measured with a single item, which asked participants to rate their experience at that moment from 0 (no fatigue) to 100 (fatigue as bad as it can be). Stress was measured with a similar, single-item scale. Pain was measured using a list of joints from the Rapid Assessment of Disease Activity in Rheumatoid Arthritis (RADAR; 43). Participants were shown a body diagram with the highlighted joints and were instructed to rate their pain at each joint at that moment from 0 (no pain) to 3 (severe pain). Joint ratings were averaged to produce one joint pain score per measurement period. There was strong internal reliability for this measure (α = .92), supporting the use of an aggregate joint pain score.
Procedures
Participants were first consented through mail and completed a questionnaire packet containing the mastery and neuroticism measures at home. Upon completion of the initial measures, participants were then scheduled for a laboratory session that occurred an average of one month later.
All lab procedures were conducted at the Carl T. Hayden VA Medical Center in Phoenix, Arizona. Upon arrival, participants were again consented and were then assessed by one of the study Rheumatologists and scored on joint swelling and tenderness. After the physician assessment, the following protocol was carried out:
Baseline (10 minutes)
Participants were instructed to rest while listening to relaxing music. Experimenters left the immediate area during this stage of the protocol. Blood pressure measurements were taken at minutes 5, 7, and 9. Following the 10 minutes of relaxation, participants were administered scales of pain, fatigue, and stress.
Stressor 1 (15 minutes)
Immediately after completion of baseline measures, the first stressor was administered. Participants were informed they would be giving a 5-minute speech in front of 2 observers who would evaluate the speech for content, clarity, and style. Participants were also told that the interviews would be audiotaped and later evaluated by a team of psychologists. Participants were given 10 minutes to prepare their speech. During this preparation time, a research assistant sat in close proximity (approximately 3 feet) and watched the participant. Participants were not allowed to ask questions or otherwise engage the experimenter once the preparation period began. Blood pressure measurements were taken at 2-minute intervals while the participant prepared their speech. Following this initial period, a second experimenter came into the room. Both experimenters stood in close proximity while the participant recalled their speech. This period lasted for 5 minutes. Blood pressure measurements continued at 2-minute intervals. Participants were instructed to continue speaking if they stopped before the 5 minutes were concluded. Following the speech task, measures of pain, fatigue, and stress were again collected.
Stressor 2 (15 minutes)
Immediately after completion of stressor 1 measures, a second stressor was administered. Participants were asked to discuss a recent conflict with a close family member or friend. After the initial description, participants were asked a number of questions regarding specifics of the event. As with the other stages, blood pressure measurements were taken at 2-minute intervals and measures of pain, fatigue, and stress were collected immediately post-task. The procedure used was similar to those reported in other labs (e.g. 44−45) and was designed to evoke reactions similar to those in response to real-life interpersonal stress over and above the effects of speaking itself. Furthermore, the literature suggests that blood pressure reactivity is greater following interpersonal speech tasks than it is following other typical laboratory stressors such as the cold pressor or mental arithmetic tasks (44).
Recovery (20 minutes)
Immediately after completion of stressor 2 measures, participants were instructed to sit quietly while listening to relaxing music. In order to minimize ischemic pain resulting from inflation of the cuff, cardiovascular measurements were taken at minutes 15, 17, and 19 of the 20 minute period. Following the recovery period, assessments of pain, fatigue, and stress were collected. Height and weight measurements were then obtained and participants were debriefed and thanked.
Data analytic strategy
Analyses to examine the factor structure of the mastery measure were performed on the total sample (N=179). All hypotheses tests were performed on the group of 73 individuals who completed the stress laboratory. With the exception of blood pressure, complete data were obtained from all participants. Due to instrumentation and measurement error, three individuals did not yield complete cardiovascular data. These individuals were excluded from cardiovascular analyses.
Exploratory factor analysis (EFA) of the Pearlin Mastery Scale was conducted using Maximum Likelihood with Varimax rotation. Maximum Likelihood is recommended when data are relatively normally distributed (46). Confirmatory factory analysis (CFA) was performed with LISREL, version 8.7 (47). Both the single-factor and two-factor models were tested. A chi-square estimator divided by its degrees of freedom was used to determine fit. Acceptable values were considered to fall between 2 and 3 (48). Even though an EFA had been previously conducted by Reich and Zautra (1991), we determined it was necessary to conduct a new EFA in our study because more than 15 years had elapsed since the previous EFA and we were working with an entirely different sample (RA patients vs. bereaved adults).
Primary hypotheses tests were conducted with repeated-measures General Linear Models (GLMs). Separate GLMs were performed for pain, fatigue, stress, and MAP. Each of these dependent variables was treated as a repeated measure. Because measures obtained for stressor 1 and stressor 2 were highly correlated (ranging from .71 to .96), the two stress periods were averaged. Therefore, for all analyses, repeated measures were: baseline, stress, and recovery. All tests were conducted with continuous measures of predictor variables; however, median splits were used in figures for illustrative purposes.
Results
Factor analysis of mastery
In the EFA, fatalism, comprised of items 1, 2, 3, 5, and 7, made up the first component. The internal reliability of this factor was good (α = .79). Control was devised of items 4 and 6 and had an adequate alpha of .65. The two subscales were not correlated (r = −.16, p = .19). This factor structure is the same as that reported previously (13) although, in that study, the two factors were correlated (r = −.37, p<.05). Given this factor structure, all tests included both control and fatalism as independent predictors.
In the CFA, the χ2 statistic for the 2-factor model was 30.1 (df=13), p = .004, while the χ2 for the single-factor model was 73.1 (df=14), p <.0001. The 2-factor model demonstrated substantially improved predictive power over the single-factor model (difference in chi-squares = 43, p < .0001. Several additional fit measures recommended by the literature confirmed a good fit for the two-factor model. Using this method, an acceptable model of 2.4 is yielded by the 2-factor model. Root mean square error of approximation (RMSEA) was .08, which indicates a reasonable fit (49-50). The non-normed fit index (NNFI) was .93, falling between established ranges of acceptable and excellent fits (51 The single-factor model did not meet acceptable levels of fit on any of the three criteria (χ2 / df = 5.2, RMSEA = .15, NNFI = .79). The 2-factor model, therefore, emerged as the best structure model for personal mastery.
Covariates and confounds
Two demographic variables (age and gender) known to broadly affect psychological and biological variables were tested as predictors of fatalism and control. Age was not a predictor of control (r = −.14, p = .25) or fatalism (r = −.09, p = .19). Gender was related neither to control (t(71) = .51, p = .61) nor fatalism (t(71) = 1.39, p = .17). Age and gender were therefore not considered to be significant confounds with this sample.
The present study was designed to test how psychological styles predict wellbeing, in the context of rheumatic disease. It may be argued that fatalism and control beliefs are themselves products of disease severity. In order to examine this possibility, the correlation between an objective measure of disease severity (physician-rated joint swelling) and fatalism and control were calculated. Joint swelling was not correlated with control (r = −.10, p = .46) or fatalism (r = −.02, p = .87). Control and fatalism did not seem to be influenced by physiological disease severity.
Since neuroticism is often associated with generally high reports of negative psychological, somatic, and affective states, the relationships between neuroticism and the mastery factors were assessed. Neuroticism was significantly correlated with fatalism (r = .34, p = .004) but not control (r = −.08, p = .53). Neuroticism was therefore included as a covariate in all GLMs.
Primary hypotheses tests
Perceived Stress
Both control and neuroticism independently predicted levels of stress through the session (F(1) = 12.36, p = .001, F(1) = 7.44, p = .008, respectively). Neuroticism was associated with higher levels of stress while control was associated with lower levels of stress. No significant main effects emerged for fatalism. No independent variable predicted change in stress over time. Table 2 presents a summary of all main and repeated-measures effects.
Table 2.
F values (df = 1) for GLM main and repeated-measures effects.
| Control |
Fatalism |
Neuroticism |
||||
|---|---|---|---|---|---|---|
| Main | Repeated | Main | Repeated | Main | Repeated | |
| Stress | 7.44*** | .28 | .98 | 1.24 | 12.36*** | .85 |
| MAP | 3.73* | 3.73* | 3.41* | .05 | 1.13 | 5.62** |
| Joint Pain | .08 | 5.07** | 4.72** | .04 | 1.94 | .519 |
| Fatigue | 5.16** | 1.25 | .49 | 1.66 | 3.29* | 2.00 |
p < .1,
p < .05,
p < .01
Fatigue
Control significantly predicted overall levels of fatigue (F(1) = 5.16, p = .026), with those higher in control evidencing less fatigue. There were no significant effects for neuroticism or fatalism.
Mean Arterial Pressure
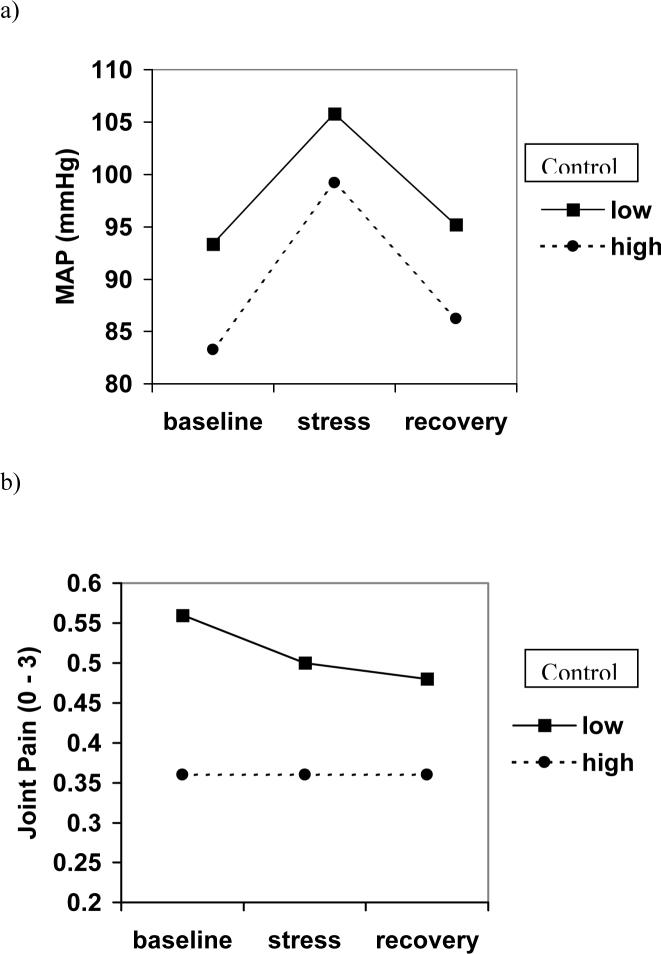
After controlling for age and bmi, both neuroticism (F(1) = 5.616, p = .021) and control (F(1) = 4.906, p = .031) were significant predictors of change in blood pressure over the lab session. Further, fatalism emerged as a marginally significant predictor of overall levels of blood pressure (F(1) = 3.724, p = .058). As seen in Figure 2a, individuals high in control appear to rise and recover slightly more sharply than low-control individuals.
Figure 2.
Repeated-measures effects of control on a) mean arterial pressure and b) joint pain.
Pain
A main effect for fatalism on joint pain emerged (F(1) = 4.72, p = .033). Higher levels of fatalism were associated with greater self-reports of joint pain throughout the protocol. Control did not predict general levels of pain (F(1) = .080, p = .778) but did predict change in pain over time (F(1) = 5.07, p = .028). Higher levels of control were associated with a lack of response to the stressor, evidenced by a flat line across time periods (Figure 2b). Individuals with low control showed a slight drop in joint pain during the stressor and into the recovery period. Neuroticism was not related to pain.
Discussion
This study represents the first attempt to measure the effects of mastery during an acute stressor. It was hypothesized that the two elements of mastery (control and fatalism) would predict both overall levels and acute changes to stress in ratings of stress, MAP, fatigue, and pain. Results supported the position that control and fatalism differentially predict general levels of stress, MAP, fatigue, and pain. Neither control nor fatalism predicted every laboratory outcome. In fact, control and fatalism operated independently from each other, and from neuroticism, to predict the outcome measures. These results support previous assertions that, given similar levels of chronic disease activity, psychological variables such as personal mastery can have a significant impact on health-related outcomes such as pain, fatigue, stress, and blood pressure in a pre/post laboratory stress task (52).
Some limitations in the generalizability of the findings should be mentioned. First, the control factor that emerged as a result of the CFA contained only two items. It is possible that the addition of at least one item resembling control in the model could have enhanced the reliability of the measure and strengthened its effects. Future research should consider this possibility in exploring the predictive utility of fatalism and control with other health variables. Second, the interpretation of the findings is limited in respect to the cross-sectional design. It is unknown whether or not acute laboratory stressors provide an accurate assessment of stress response in those with a fairly constant stressor of chronic disease. However, the repeated-measures nature of our laboratory experiment allows us to cautiously infer results as prospective. In measuring health-related variables among chronic pain populations, laboratory designs provide convenience, as well as the ability to measure certain variables, such as heart rate, that are difficult to assess in a real-life setting. Recent methodological advances, however, permit the ipsative-normative measurement of some health-related and psychological variables.
Although personal mastery is conventionally treated as a unifactorial trait, the present study identified two aspects of mastery: control and fatalism. Exploratory and confirmatory factor analyses supported the two-factor model, originally reported by Reich and Zautra (13). The independence of control and fatalism were also supported by a lack of correlation between the factors, and their differing relationship to other variables of interest. Fatalism was significantly correlated with neuroticism, suggesting that this aspect of personal mastery is part of a larger network of negative perceptions and experiences. Control, however, was not correlated with neuroticism, and thus may have unique clinical implications for those with chronic illness. These findings strongly support the independent nature of control and fatalism, as suggested in Hypothesis 1.
Control and fatalism operated in different ways to predict health and wellbeing. Fatalism predicted only main effects, suggesting that this cognitive attribute is a better predictor of general levels of coping, rather than reactivity to stress. Fatalism was a significant predictor of joint pain, and a marginally significant predictor of mean arterial pressure. Control, on the other hand, played a more dynamic role, predicting both main effects (stress, mean arterial pressure, and fatigue) and stress-reactivity effects (joint pain and mean arterial pressure). Individuals high in control showed no change in joint pain in reaction to the stress task. Interestingly, individuals low in control evidenced a drop in joint pain during the stressor and continuing into the recovery period. While this effect was not predicted, it is feasible that the stressor provided a distraction from the pain. It is important to note, however, that the reduction in pain was very slight and levels did not go as low as those experienced by the high control group. Both control and fatalism emerged as significant predictors after controlling for neuroticism, supporting Hypotheses 2 & 3. Taken together, the results suggest that a fatalistic disposition is important in every-day experiences, while control is important in both every-day experiences and, to a lesser degree, reactions to acute stressors (Hypothesis 4). These results are supported by recent evidence linking low personal mastery to high aortic calcification, a known risk factor for coronary heart disease (53). Given that hypertension is a predictor of aortic calcification (54), the present results suggest that high blood pressure could possibly mediate a relationship between low personal control and coronary heart disease.
While this study did not attempt to capture the phenomenological aspect of control and fatalism, it is not difficult to posit ways in which mastery can influence health. Laboratory measures of cardiovascular reactivity have been successfully correlated with ambulatory measures (Kamarck et al. 2003), so we have an empirical basis to believe that our results can be interpreted as suggestive of real-life experiences. For instance, individuals who can maintain a sense of control in the face of a daily stressor could interpret the experience as less threatening than if they failed to maintain a sense of control, preventing overactivation of the sympathetic nervous system and subsequent negative health effects (e.g., disease flare). Conversely, individuals with a highly fatalistic disposition might be seen as being more weary of their environment, fueling pain and other effects of chronic stress. Clinically, control and fatalism represent possible objectives for intervention with chronic pain patients. Even if the more general factor of neuroticism is addressed through therapy, control and fatalism may be more directly targeted by goal-setting and problem-solving strategies. To the extent that these techniques may increase control and decrease fatalism, they may also reduce physical consequences of stress, either from acute stressors or the chronic disease itself. It is also possible that empowering the patient (e.g., by doctors giving additional medical information and encouraging patients to give input into treatment approach) may itself be a useful adjunct to treatment. Certainly, a sense of control over one's health is an important psychological factor, as it is often cited as the primary reason why individuals choose complimentary and integrative medicine approaches over traditional methods (55). However, we are hesitant to fully endorse a psychological intervention aimed at modifying control behaviors in the context of chronic pain until more research is conducted.
The current findings suggest that facets of mastery, particularly control and fatalism, may be meaningfully related to important markers of physical health and emotional well-being in RA patients. Future studies are needed to determine a causal relation between mastery and health and wellbeing, hopefully by employing longitudinal methods that can detect how changes in one affect the other.
Figure 1.
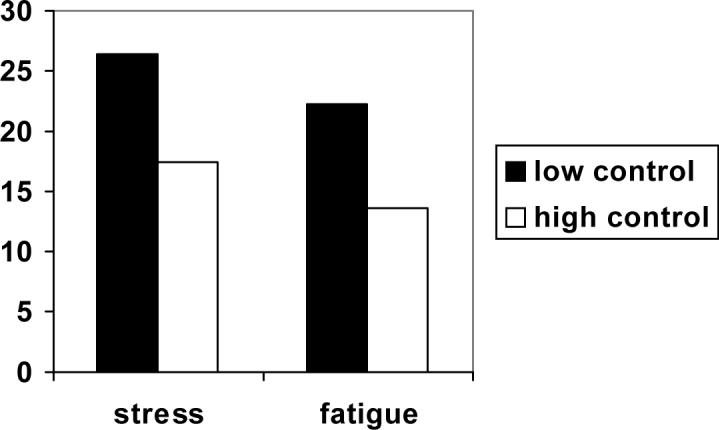
Comparison of low and high control on reports of stress and fatigue.
Appendix
Table 1.
Correlations between independent, dependent, and control variables at baseline.
| RA patients (n = 73) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 1. Control | −.16 | −.08 | −.14 | −.28* | −.10 | −.27* | −.31* | −.31** | −.15 | |
| 2. Fatalism | .34** | −.09 | −.09 | .02 | .14 | .22 | .21 | .31** | ||
| 3. Neuroticism | −.12 | .12 | .14 | .32** | .03 | .13 | .24* | |||
| 4. Age | −.06 | .07 | −.07 | .01 | −.18 | .01 | ||||
| 5. BMI | −.06 | .21 | .31* | .32** | .12 | |||||
| 6. Swelling | .08 | −.11 | .06 | .12 | ||||||
| 7. Stress | .32** | .49** | .46** | |||||||
| 8. MAP | .18 | .20 | ||||||||
| 9. Fatigue | .75** | |||||||||
| 10. Joint pain | ||||||||||
References
- 1.Dick B, Eccleston C, Crombez G. Attentional functioning in Fibromyalgia, rheumatoid arthritis, and musculoskeletal pain patients. Arthritis Care Res. 2002;47:639–44. doi: 10.1002/art.10800. [DOI] [PubMed] [Google Scholar]
- 2.Asmundson GJG, Kuperos JL, Norton GR. Do patients with chronic pain selectively attend to pain-related information? Preliminary evidence for the mediating role of fear. Pain. 1997;72:27–32. doi: 10.1016/s0304-3959(97)00010-9. [DOI] [PubMed] [Google Scholar]
- 3.Crombez G, Vervaet L, Lysens R, Baeyens F, Eelen P. Avoidance and confrontation of painful back straining movements in chronic back pain patients. Behav Modif. 1998;22:62–77. doi: 10.1177/01454455980221004. [DOI] [PubMed] [Google Scholar]
- 4.Vlaeyen JWS, Kole-Snijders AMJ, Boeren RGB, Van Eek H. Fear of movement (re)injury in chronic low back pain and its relation to behavioural performance. Pain. 1995;62:363–72. doi: 10.1016/0304-3959(94)00279-N. [DOI] [PubMed] [Google Scholar]
- 5.Asmundson GJG, Norton GR, Jacobson SJ. Social, blood/injury, and agoraphobic fears in patients with physically unexplained chronic pain – are they clinically significant? Anxiety. 1996;2:28–33. doi: 10.1002/(SICI)1522-7154(1996)2:1<28::AID-ANXI4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Turk DC, Okifuji A, Scharff L. Chronic pain and depression – role of perceived impact and perceived control in different age cohorts. Pain. 1995;61:93–101. doi: 10.1016/0304-3959(94)00167-D. [DOI] [PubMed] [Google Scholar]
- 7.Crombez G, Vlaeyen WS, Heuts PHTG, Lysens R. Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability. Pain. 1999;80:329–39. doi: 10.1016/s0304-3959(98)00229-2. [DOI] [PubMed] [Google Scholar]
- 8.Epping-Jordan JE, Wahlgren DR, Williams RA, et al. Transition to chronic pain in men with low back pain: predictive relationships among pain intensity, disability, and depressive symptoms. Health Psychol. 1998;17:421–7. doi: 10.1037//0278-6133.17.5.421. [DOI] [PubMed] [Google Scholar]
- 9.Kovacs FM, Abraira V, Zamora J, et al. Correlation between pain, disability, and quality of life in patients with common low back pain. Spine. 2004;29:206–10. doi: 10.1097/01.BRS.0000107235.47465.08. [DOI] [PubMed] [Google Scholar]
- 10.Haidt J, Rodin J. Control and efficacy as interdisciplinary bridges. Rev Gen Psychol. 1999;3:317–337. [Google Scholar]
- 11.Pham LB, Taylor SE, Seeman TE. Effects of environmental predictability and personal mastery on self-regulatory and physiological processes. Pers Soc Psychol Bull. 2001;27:611–20. [Google Scholar]
- 12.Gerin W, Litt MD, Deich J, Pickering TG. Self-efficacy as a moderator of perceived control effects on cardiovascular reactivity: is enhanced control always beneficial? Psychosom Med. 1995;57:390–7. doi: 10.1097/00006842-199507000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Reich JW, Zautra AJ. Experimental and measurement approaches to internal control in at risk older adults. J Soc Issues. 1991;47:143–58. [Google Scholar]
- 14.DiMatteo MR. Enhancing patient adherence to medical recommendations. JAMA. 1994;271:79–83. doi: 10.1001/jama.271.1.79. [DOI] [PubMed] [Google Scholar]
- 15.Lorig KR, Ritter P, Stewart AL, Sobel DS, Brown BW, Bandura A, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care. 2001;39:1217–23. doi: 10.1097/00005650-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Rodin J. Aging and health: effects of the sense of control. Science. 1986;233:1271–6. doi: 10.1126/science.3749877. [DOI] [PubMed] [Google Scholar]
- 17.Walker JG, Littlejohn GO, McMurray NE, Cutolo M. Stress system response and rheumatoid arthritis: a multilevel approach. Rheumatology. 1999;38:1050–7. doi: 10.1093/rheumatology/38.11.1050. [DOI] [PubMed] [Google Scholar]
- 18.Evers AWM, Kraaimaat FW, Geenen R, Jacobs JWG, Bijlsma JWJ. Stress-vulnerability factors as long-term predictors of disease activity in early rheumatoid arthritis. J Psychosom Res. 2003;55:293–302. doi: 10.1016/s0022-3999(02)00632-3. [DOI] [PubMed] [Google Scholar]
- 19.Zautra AJ, Hoffman J, Potter P, Matt KS, Yocum D, Castro L. Examination of changes in interpersonal stress as a factor in disease exacerbations among women with rheumatoid arthritis. Ann Behav Med. 1997;19:279–86. doi: 10.1007/BF02892292. [DOI] [PubMed] [Google Scholar]
- 20.Affleck G, Urrows S, Tennen H, Higgins P, Pav D, Aloisi R. A dual pathway model of daily stressor effects on rheumatoid arthritis. Ann Behav Med Medicine. 1997;19:161–70. doi: 10.1007/BF02883333. [DOI] [PubMed] [Google Scholar]
- 21.Tennen H, Affleck G, Urrows S, Higgins P, Mendola R. Perceiving control, construing benefits, and daily processes in rheumatoid arthritis. Can J Beh Sci. 1992;24:186–203. [Google Scholar]
- 22.Gabriel SE. The epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am. 2001;27:269–81. doi: 10.1016/s0889-857x(05)70201-5. [DOI] [PubMed] [Google Scholar]
- 23.Anderson KO, Bradley LA, Young LD, McDaniel LK, Wise CM. Rheumatoid arthritis: review of psychological factors related to etiology, effects, and treatment. Psychol Bull. 1985;98:358–87. [PubMed] [Google Scholar]
- 24.Younger JW, Zautra AJ. Arthritis – Psychological. In: Fick George., editor. The Encyclopedia of stress. 2nd edition. Academic Press; in press. [Google Scholar]
- 25.Affleck G, Tennen H, Pfeiffer C, Fifield J. Appraisals of control and predictability in adapting to a chronic disease. J Pers Soc Psychol. 1987;53:273–9. doi: 10.1037//0022-3514.53.2.273. [DOI] [PubMed] [Google Scholar]
- 26.Murphy H, Dickens C, Creed F, Bernstein R. Depression, illness perception, and coping in rheumatoid arthritis. J Psychosom Res. 1999;46:155–64. doi: 10.1016/s0022-3999(98)00073-7. [DOI] [PubMed] [Google Scholar]
- 27.Dickens C, McGowan L, Clark-Carter D, Creed F. Depression in rheumatoid arthritis: a systematic review of the literature with meta-analysis. Psychosom Med. 2002;64:52–60. doi: 10.1097/00006842-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Rudy TE, Kerns RD, Turk DC. Chronic pain and depression: toward a cognitive-behavioral mediation model. Pain. 1988;35:129–140. doi: 10.1016/0304-3959(88)90220-5. [DOI] [PubMed] [Google Scholar]
- 29.Skinner EA. A guide to constructs of control. J Pers Soc Psychol. 1996;71:549–70. doi: 10.1037//0022-3514.71.3.549. [DOI] [PubMed] [Google Scholar]
- 30.Pearlin LI, Schooler C. The structure of coping. J Health Soc Behav. 1978;22:337–56. [PubMed] [Google Scholar]
- 31.Gertrudis IJM, Kempen MJ, Johan O. Personality, chronic medical morbidity, and health-related life among older persons. Health Psychol. 1997;16:539–46. doi: 10.1037//0278-6133.16.6.539. [DOI] [PubMed] [Google Scholar]
- 32.Thoits PA. Stressors and problem-solving: the individual as psychological activist. J Health Soc Behav. 1994;35:143–60. [PubMed] [Google Scholar]
- 33.Ross CE, Broh BA. The roles of self-esteem and the sense of personal control in the academic achievement process. Sociol Educ. 2000;73:270–84. [Google Scholar]
- 34.Lachman ME, Weaver SL. The sense of control as a moderator of social class differences in health and well-being. J Pers Soc Psychol. 1998;74:763–73. doi: 10.1037//0022-3514.74.3.763. [DOI] [PubMed] [Google Scholar]
- 35.Rotter JB. Generalized expectancies for internal versus external control of reinforcement. Psychol Monogr. 1966;80:1–28. [PubMed] [Google Scholar]
- 36.Bolger N, Zuckerman A. A framework for studying personality in the stress process. J Pers Soc Psychol. 1995;69:890–902. doi: 10.1037//0022-3514.69.5.890. [DOI] [PubMed] [Google Scholar]
- 37.Smith B, Zautra A. Interpersonal sensitivity and reactivity to spousal conflict in healthy older women. Pers Individ Dif. 2001;31:915–23. [Google Scholar]
- 38.Kempen GIJM, Jelicic M, Ormel J. Personality, chronic medical morbidity, and health-related quality of life among older persons. Health Psychol. 1997;16:539–46. doi: 10.1037//0278-6133.16.6.539. [DOI] [PubMed] [Google Scholar]
- 39.Pearlin LI, et al. The stress process. J Health Soc Behav. 1981;22:337–356. [PubMed] [Google Scholar]
- 40.Stets JE, Burke PJ. Inconsistent Self-Views in the Control Identity Model. Soc Sci Res. 1994;23:236–62. [Google Scholar]
- 41.Seeman TE. Personal control and coronary artery disease: How generalized expectancies about control may influence disease risk. J Psychosom Res. 1991;35:661–9. doi: 10.1016/0022-3999(91)90116-6. [DOI] [PubMed] [Google Scholar]
- 42.Saucier G. Replicable item subcomponents in the NEO five-factor inventory. J Pers Assess. 1998;70:263–76. doi: 10.1207/s15327752jpa7002_6. [DOI] [PubMed] [Google Scholar]
- 43.Mason JH, Anderson JJ, Meenan RF, Haralson KM, Lewis-Stevens D, Kaine JL. The Rapid Assessment of Disease Activity in Rheumatology (RADAR) questionnaire: validity and sensitivity to change of a patient self-report measure of joint count and clinical status. Arthritis Rheum. 1992;35:156–62. doi: 10.1002/art.1780350206. [DOI] [PubMed] [Google Scholar]
- 44.Dimsdale JE, Stern MJ, Dillion E. The stress interview as a tool for examining physiological reactivitiy. Psychosom Med. 1998;50:64–71. doi: 10.1097/00006842-198801000-00008. [DOI] [PubMed] [Google Scholar]
- 45.Ewart CK, Kolodner KB. Predicting ambulatory blood pressure during school: Effectiveness of social and nonsocial reactivitiy tasks in black and white adolescents. Psychophysiology. 1993;30:30–8. doi: 10.1111/j.1469-8986.1993.tb03202.x. [DOI] [PubMed] [Google Scholar]
- 46.Fabrigar LR, Wegener DT, MacCullum RC, Strahan EJ. Evaluating the use of exploratory factor analysis in psychological research. Psychol Methods. 1999;4:272–99. [Google Scholar]
- 47.Jöreskog KG, Sörbom D. LISREL 8: Structural equation modeling with the SIMPLIS command languge. Chicago: 1993. Scientific Software International. [Google Scholar]
- 48.Carmines EG, Mciver JP. Analyzing models with unobserved variables: Analysis of covariance structures. In: Bohrnstedt GW, Borgatta EF, editors. Social Measures. Sage; Beverly Hills: 1981. pp. 65–115. [Google Scholar]
- 49.Marsh HW, Balla JR, Hau KT. An evaluation of incremental fit indices: A clarification of mathematical and empirical processes. In: Marcoulides GA, Schumacker RE, editors. Advanced structural equation modeling techniques. Erlbaum; Hillsdale, New Jersey: 1996. pp. 315–53. [Google Scholar]
- 50.Schumacker RE, Lomax RG. A beginner's guide to structural equation modeling. Erlbaum; Mahwah, NJ: 1996. [Google Scholar]
- 51.McDonald RP, Marsh HW. Choosing a multivariate model: Noncentrality and goodness-of-fit. Psychol Bull. 1990;107:247–55. [Google Scholar]
- 52.Zautra AJ. Emotions, stress and health. Oxford University Press; New York: 2003. [Google Scholar]
- 53.Mathhews KA, Owens JF, Edmundowicz D, Lee L, Kuller LH. Positive and negative attributes and risk for coronary and aortic calcification in healthy women. Psychosom Med. 2006;68:355–61. doi: 10.1097/01.psy.0000221274.21709.d0. [DOI] [PubMed] [Google Scholar]
- 54.Iribarren C, Sidney S, Sternfeld B, Browner WS. Risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283:2810–5. doi: 10.1001/jama.283.21.2810. [DOI] [PubMed] [Google Scholar]
- 55.Vincent C, Furnham A. Why do patients turn to complementary medicine? An empirical study. Br J Clin Psychol. 1996;35:37–48. doi: 10.1111/j.2044-8260.1996.tb01160.x. [DOI] [PubMed] [Google Scholar]
- Clark R, Adams JH, Clark VR. Effects of john henryism and anger-coping on mean arterial pressure changes in african american women. Int J Behav Med. 2001;8:270–281. [Google Scholar]