Abstract
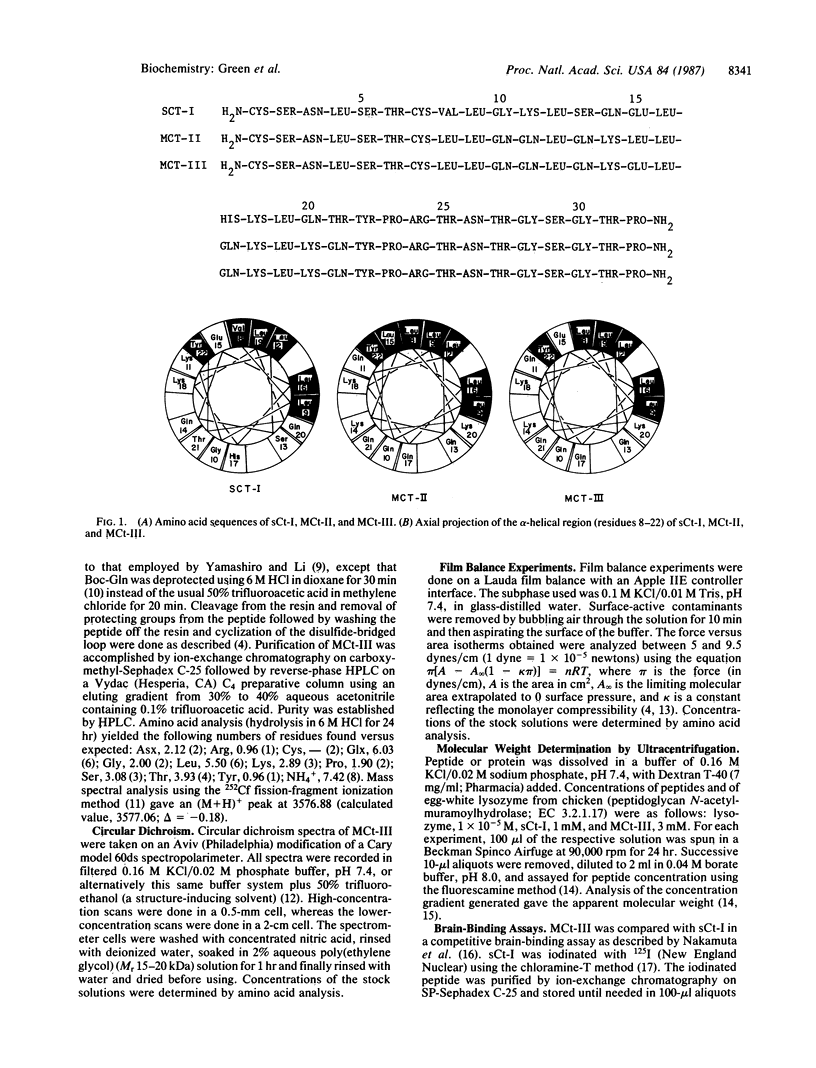
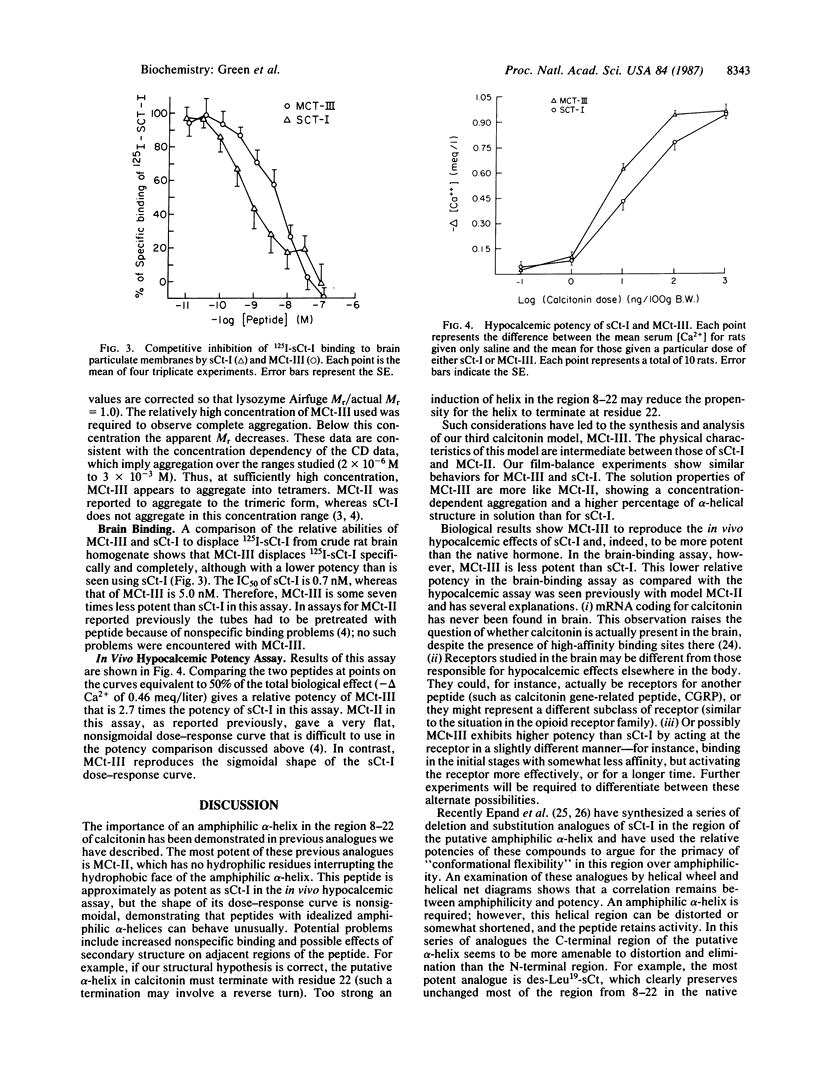
2A new calcitonin analogue, model calcitonin III (MCt-III), has been synthesized, and its biological and physical characteristics have been studied. This analogue has an idealized alpha-helix from residue 8-22 with glutamate at position 15 interrupting an otherwise continuous surface of aliphatic side chains (those of leucine residues) on the hydrophobic face of the helix. MCt-III differs from a previous model, MCt-II, only by the substitution Leu15----Glu and is here compared with salmon calcitonin I (sCt-I) and MCt-II to elucidate further the role of the putative amphiphilic alpha-helix in determining biological and physical properties of the hormone. MCt-III shows physical properties intermediate between those of sCt-I and MCt-II, demonstrating the influence of appropriately positioned single residues on properties of amphiphilic structures. In our two biological assays, a brain-binding assay and an in vivo hypocalcemic assay, MCt-III reproduces the sigmoidal dose-response curves of sCt-I; this contrasts with the behavior of MCt-II, which demonstrated unusual dose-response curves in these two assays. MCt-III is almost three times more potent than sCt-I in our hypocalcemic assay; this activity groups MCt-III among the most potent known analogues of sCt-I.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bothwell M. A., Howlett G. J., Schachman H. K. A sedimentation equilibrium method for determining molecular weights of proteins with a tabletop high speed air turbine centrifuge. J Biol Chem. 1978 Apr 10;253(7):2073–2077. [PubMed] [Google Scholar]
- Chang C. T., Wu C. S., Yang J. T. Circular dichroic analysis of protein conformation: inclusion of the beta-turns. Anal Biochem. 1978 Nov;91(1):13–31. doi: 10.1016/0003-2697(78)90812-6. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Weiss R. M., Terwilliger T. C. The hydrophobic moment detects periodicity in protein hydrophobicity. Proc Natl Acad Sci U S A. 1984 Jan;81(1):140–144. doi: 10.1073/pnas.81.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epand R. M., Epand R. F., Orlowski R. C., Seyler J. K., Colescott R. L. Conformational flexibility and biological activity of salmon calcitonin. Biochemistry. 1986 Apr 22;25(8):1964–1968. doi: 10.1021/bi00356a019. [DOI] [PubMed] [Google Scholar]
- Epand R. M., Seyler J. K., Orlowski R. C. The hydrophobic moment of the amphipathic helix of salmon calcitonin and biological potency. Eur J Biochem. 1986 Aug 15;159(1):125–127. doi: 10.1111/j.1432-1033.1986.tb09841.x. [DOI] [PubMed] [Google Scholar]
- Fukushima D., Yokoyama S., Kézdy F. J., Kaiser E. T. Binding of amphiphilic peptides to phospholipid/cholesterol unilamellar vesicles: a model for protein--cholesterol interaction. Proc Natl Acad Sci U S A. 1981 May;78(5):2732–2736. doi: 10.1073/pnas.78.5.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greff D., Toma F., Fermandjian S., Löw M., Kisfaludy L. Conformational studies of corticotropin1-32 and constitutive peptides by circular dichroism. Biochim Biophys Acta. 1976 Jul 19;439(1):219–231. doi: 10.1016/0005-2795(76)90177-x. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hennessey J. P., Jr, Johnson W. C., Jr Information content in the circular dichroism of proteins. Biochemistry. 1981 Mar 3;20(5):1085–1094. doi: 10.1021/bi00508a007. [DOI] [PubMed] [Google Scholar]
- Kaiser E. T., Kézdy F. J. Amphiphilic secondary structure: design of peptide hormones. Science. 1984 Jan 20;223(4633):249–255. doi: 10.1126/science.6322295. [DOI] [PubMed] [Google Scholar]
- Kaiser E. T., Kézdy F. J. Secondary structures of proteins and peptides in amphiphilic environments. (A review). Proc Natl Acad Sci U S A. 1983 Feb;80(4):1137–1143. doi: 10.1073/pnas.80.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe G. R., Kaiser E. T. Design, synthesis, and characterization of a model peptide having potent calcitonin-like biological activity: implications for calcitonin structure/activity. Biochemistry. 1985 Apr 9;24(8):1971–1976. doi: 10.1021/bi00329a026. [DOI] [PubMed] [Google Scholar]
- Nakamuta H., Furukawa S., Koida M., Yajima H., Orlowski R. C., Schlueter R. Specific binding of 125I-salmon calcitonin to rat brain: regional variation and calcitonin specificity. Jpn J Pharmacol. 1981 Feb;31(1):53–60. doi: 10.1254/jjp.31.53. [DOI] [PubMed] [Google Scholar]
- Pollet R. J., Haase B. A., Standaert M. L. Macromolecular characterization by sedimentation equilibrium in the preparative ultracentrifuge. J Biol Chem. 1979 Jan 10;254(1):30–33. [PubMed] [Google Scholar]
- Provencher S. W., Glöckner J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 1981 Jan 6;20(1):33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G., Mermod J. J., Amara S. G., Swanson L. W., Sawchenko P. E., Rivier J., Vale W. W., Evans R. M. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983 Jul 14;304(5922):129–135. doi: 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]



