Abstract
Objective:
To assess prospectively progression and relationship of hallucinations and sleep disorders over a 10-year longitudinal study of patients with Parkinson disease (PD).
Methods:
Eighty-nine patients with PD were recruited to fill cells of normal sleep without hallucinations (n = 20), sleep fragmentation only (n = 20), vivid dreams/nightmares (n = 20), hallucinations with insight (n = 20), and hallucinations without insight (n = 9). At baseline, 0.5, 1.5, 4, 6, and 10 years, sleep disorders and hallucinations were assessed by standardized scales with the longitudinal data analyzed by generalized estimating equations with assumptions of linearity in time.
Results:
At 10 years, we could account for all subjects (27 interviewed, 61 deceased, and 1 too ill for interview). Hallucination prevalence and severity increased over time (p < 0.0001, p = 0.0001). Acting out dreams also increased over time (p = 0.001). In contrast, presence of vivid dreams/nightmares or sleep fragmentation did not increase over time. For all visits, the prevalence of sleep fragmentation did not differ between subjects with vs without hallucinations (odds ratio [OR] = 1.50, p = 0.09). However, severe sleep fragmentation was associated with concurrent hallucinations (OR 2.01, p = 0.006). The presence of hallucinations was also highly associated with concurrent vivid dreams/nightmares (OR = 2.60, p < 0.0001) and with concurrent acting out dreams (OR = 2.38, p = 0.0004). Among the baseline nonhallucinators, no sleep abnormalities at study entry predicted future development of hallucinations.
Conclusions:
Hallucinations and sleep abnormalities follow very different patterns of progression in PD over 10 years. Whereas patients with hallucinations often have concurrent sleep aberrations, no sleep problem is predictive of future hallucinations.
GLOSSARY
- CI
= confidence interval;
- GEE
= generalized estimating equation;
- MMSE
= Mini-Mental State Examination;
- OR
= odds ratio;
- PD
= Parkinson disease;
- PSQI
= Pittsburgh Sleep Quality Index;
- UPDRS
= Unified Parkinson's Disease Rating Scale;
- UPDRSm
= motor section of the Unified Parkinson's Disease Rating Scale.

Podcast

e–Pub ahead of print
Sleep disorders and hallucinations are common complications of chronic Parkinson disease (PD) and its pharmacologic treatment.1 While their relationship is not fully understood, they both influence overall disability and quality of life.2-4 Our 6-year longitudinal study documented that sleep disorders and hallucinations often co-occur, but they follow different patterns of progression.5 Whereas hallucination prevalence and severity significantly increased over time, global sleep disorders fluctuated widely with no evidence of severity progression. The presence of vivid dreams/nightmares correlated with concurrent hallucinations, but when they occurred in nonhallucinators, they did not predict the future development of hallucinations. We have continued to follow this cohort over 10 years and report on the survivors to investigate the progressive pattern of sleep abnormalities and hallucinations and to assess their relationship.
METHODS
Study design.
This prospective, 10-year longitudinal study (1999–2009) examined a cohort of patients with PD at a university center and involved assessments of hallucinations, sleep, motor function, and cognition at baseline, 0.5, 1.5, 4, 6, and 10 years.
Participants.
Using computerized randomization methods based on outpatient appointment time, we screened patients with PD (defined by the presence of at least 3 of 4 cardinal features: tremor, bradykinesia, rigidity, postural reflexes) on levodopa treatment (Hoehn & Yahr stage 2 or 3 when ON) with a modified version of the thought disorder item from the Unified Parkinson's Disease Rating Scale (UPDRS).6 Patients without a 24-hour caregiver or with concomitant strokes, AD, delirium, delusions, or neuroleptic treatment were excluded. Over a 6-month period, screened subjects were categorized into 5 strata, based on their response to the thought disorder item of the UPDRS: 0 = normal behavior without sleep problems or hallucinations; 1 = sleep fragmentation only; 2 = altered dream phenomena, vivid dreams, or nightmares; 3 = hallucinations with retained insight; 4 = hallucinations without retained insight. We recruited up to 20 patient/caregiver couples for each baseline stratum to participate in a standardized, structured interview at baseline and sequentially thereafter.
Assessment tools.
Study outcomes in this study included the following: the Rush Hallucination Inventory7 for hallucinations; a modified version of the Pittsburgh Sleep Quality Index (PSQI) for sleep8; the motor section of the UPDRS (UPDRSm)6 for assessments of parkinsonism; and the Mini-Mental State Examination (MMSE) as a global cognitive assessment. Current medications were categorized as levodopa, agonists, anticholinergics, amantadine, sleep medications, and neuroleptics.
The Rush Hallucination Inventory7 examined the presence and frequency of hallucinations in visual, auditory, tactile, and olfactory domains. Whereas the interview tool separated illusions, defined as misinterpretations of existing stimuli, from hallucinations, defined as spontaneous misperceptions, we considered them together under the single designation of hallucinations and used the highest score from any sensory domain for analysis. At each interview, we assessed severity of hallucinations based on frequency in the past month (severity score: 0 = none, 1 = less than once weekly, 2 = once or twice weekly, 3 = at least 3 times weekly). Severe hallucinations were defined as a score of 3. Data were collected with both patient and caregiver present, and when discordant answers were obtained, the sole interviewer for each time point used best clinical judgment to record a single response.
The modified version of the PSQI used the original subjective sleep quality, sleep latency, sleep duration, habitual sleep deficiency, use of sleep medications, daytime sleepiness (scored 0–3), and acting out dreams. For monthly quantification of nocturnal awakenings as an index of sleep fragmentation, we used 0 = ≤30, 1 = 31–60, 2 = 61–90, and 3 = >90. A total modified PSQI was the sum of these items. Enthusiasm was not considered clinically relevant to PD sleep and was not used, and independent caregiver assessment was also not used because the methodology included both patient and caregiver participation in the interview. Because vivid dreams and nightmares are not covered on the PSQI, we assessed these behaviors with questions that assessed the presence of each and their severity based on frequency (0 = none, 1 = < once weekly, 2 = 1–2 times weekly, 3 = ≥3 times weekly). A single score, based on the highest score for vivid dreams or nightmares, was used as an index of vivid dreams/nightmares.
Longitudinal designs.
At each time point, a single interviewer conducted all evaluations, and interviewers were not aware of prior data. Patients enrolled in the program did not change their PD medication treatment during the first 6 months of the study, but thereafter, best medical management guided pharmacologic decisions.
Standard protocol approvals, registrations, and patient consents.
Patients and caregivers gave informed consent for the questionnaire study as approved by the Rush University Human Investigation Committee.7 The same committee approved the full protocol with regular renewals over the longitudinal study. The study began in 1999 and was not registered in a public trials registry.
Data analysis.
Summary statistics at each time point were expressed as percentages or mean ± SD, and compared hallucinators and nonhallucinators using Fisher exact test or Wilcoxon rank sum test. The association between the hallucinatory status at current visit vs death status at the next visit was compared using the Cochran-Mantel-Haenszel test and presented in odds ratios (OR) and 95% confidence intervals (CI), as was the association between the presence of sleep fragmentation or vivid dreams at current visit and hallucination at the next visit. Spearman rank correlation coefficients (r) evaluated the association between hallucination severity, PSQI, UPDRSm, and MMSE scores at baseline and 10 years.
To determine the longitudinal relationship between hallucination, sleep disorders, and other factors, we employed the method of generalized estimating equations (GEE) with assumptions of linearity in time. The GEE model allows for within-subject correlation and is suitable for the longitudinal analysis of binary, ordinal, and continuous outcomes.9,10 These models estimate ORs that indicate the relationship between the response variable and the risk factors. Putative risk factors tested included age, gender, PD duration, UPDRSm score, MMSE score, levodopa dose, exposures to agonists, anticholinergics, amantadine and selegiline, original UPDRS thought disorder score, follow-up time, and PSQI score at each visit. The GEE analyses were carried out using SAS PROC GENMOD without imputation for missing data.11 Significance was set at 0.05 (2-sided).
RESULTS
Patient sample.
Enrollment methodology has been previously reported.5,7 Eighty-nine subjects with PD enrolled in the study, 60 without hallucinations and 29 with hallucinations. The full complement of 20 patients for each UPDRS modified thought disorder stratum was identified for baseline categories 0 (normal behavior), 1 (sleep fragmentation), 2 (altered dream phenomena, vivid dreams, or nightmares), and 3 (hallucinations with retained insight). Nine patients had a baseline score of 4 (hallucinations without retained insight).
At 10 years, we could account for all patients (27 interviewed, 61 dead, 1 too ill to be interviewed). At all other visits missing data never exceeded 10% (table 1). Twenty-two of the 27 subjects interviewed at 10 years had completed all 6 assessments.
Table 1 Summary of interviews
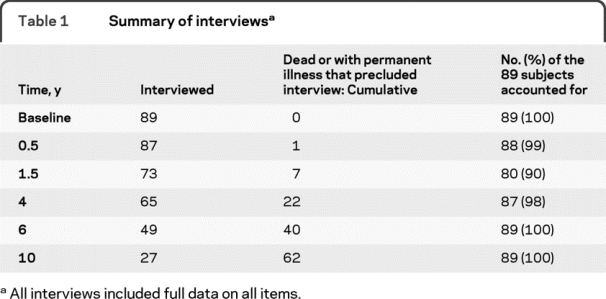
Clinical characteristics at baseline.
The baseline demographics were previously described.5,7 Briefly, at study enrollment, the subjects' mean age was 67.7 years (±9.5), with a mean PD duration of 10.3 years (±6.9). The gender distribution was 54% men and 46% women. The mean UPDRS ON motor score was 28.4 (±11.2) and the mean MMSE score was 26.9 (±3.2). All subjects received levodopa (entry criteria) at a mean daily dose of 494.7 mg (±369.6). Forty-two percent of the sample received agonists, but other antiparkinsonian drugs were used infrequently (anticholinergic drugs 16%, amantadine 18%).
At baseline, sleep fragmentation, vivid dreams/nightmares, and hallucination status were predetermined as part of the inclusion criteria. The mean PSQI score for each entry category was as follows: 1) normal behavior (n = 20), 3.2 (±2.3); 2) sleep fragmentation (n = 20), 7.2 (± 3.1); 3) vivid dream/nightmares (n = 20), 7.2 (±3.6); 4) hallucinations with retained insight (n = 20), 5.9 (±3.7); 5) hallucinations without retained insight (n = 9), 6.0 (±5.1). The baseline PSQI score was not correlated with the MMSE or UPDRSm score.
At baseline, subjects with hallucinations had higher mean UPDRS motor scores (p = 0.023, Wilcoxon rank sum test) and lower MMSE scores (p < 0.0001, Wilcoxon rank sum test) than those without hallucinations. Higher baseline hallucination severity scores were associated with both lower MMSE scores (r = −0.41, p < 0.0001) and higher UPDRS motor score (r = 0.22, p = 0.035). The hallucinators used agonists more frequently than nonhallucinators (agonists, 59% vs 33%, p = 0.038, Fisher exact test) at baseline, but there were no differences in levodopa daily dose or other parkinsonian medication usage (including sleep medications).
Clinical characteristics at 10 years.
Motor UPDRS increased (p < 0.0001, GEE model), and MMSE scores declined (p < 0.0001, GEE model) over 10 years. Among the clinical variables listed in table 2, none distinguished the patients with hallucinations from those without hallucinations at 10 years. At 10 years, 2 observed baseline correlations did not recur: hallucination severity score and MMSE (r = −0.08, p = 0.69) and hallucination severity score and UPDRS (r = 000.8, p = 0.97).
Table 2 Patient characteristics
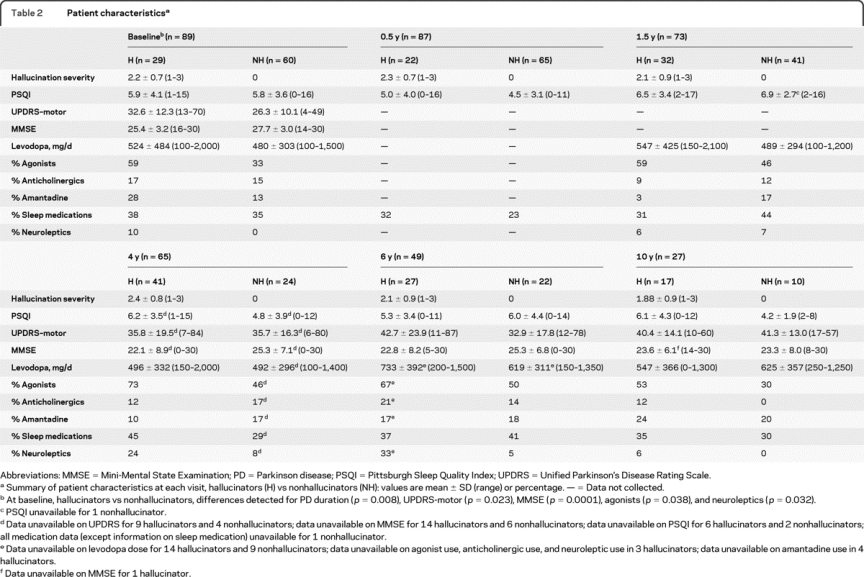
Longitudinal prevalence and severity progression of hallucinations over 10 years.
Over 10 years, the prevalence of hallucinators increased (33% at baseline, 25% at 0.5 years, 44% at 1.5 years, 63% at 4 years, 55% at 6 years, 63% at 10 years). The odds of having hallucinations increased annually by a factor of 1.23 (CI = 1.1–1.3, p < 0.0001, GEE model). Once a subject developed hallucinations, the likelihood of continuing with hallucinations on the next interview was high (OR = 5.6, CI = 3.2–9.8, p < 0.0001, Cochran-Mantel-Haenszel test). Although the baseline group was selected to have 60 nonhallucinators and 29 hallucinators, at the end of the study, only 4 surviving subjects still had never hallucinated.
Likewise, the severity of hallucinations increased over time. The odds of increasing 1 point annually on the Rush Hallucination Inventory severity score was 1.14 (CI = 1.1–1.2, p = 0.0001, GEE model). During the 10 years, 46% of the cohort had severe hallucinations on at least one interview. The odds of having severe hallucinations increased annually by a factor of 1.14 (CI = 1.1–1.2, p = 0.002, GEE model). The risk of developing severe hallucinations over time was associated with baseline hallucination status (hallucinations vs no hallucinations, p = 0.0006, GEE model) and baseline MMSE score (p = 0.02, GEE model).
Longitudinal prevalence and severity progression of sleep abnormalities over 10 years.
In contrast to hallucinations, sleep abnormalities varied in their progression over time. At baseline, 81% had at least 1 sleep abnormality (sleep fragmentation 58%, vivid dreams/nightmares 43%, daytime sleepiness 36%, and acting out dreams 12%). At the end of 10 years, for all living patients and for those who died (data from their preceding visit), 98% had experienced at least 1 sleep abnormality, but the only significant increase related to acting out dreams: 12% at baseline, 10% at 0.5 years, 26% at 1.5 years, 38% at 4 years, 25% at 6 years, and 33% at 10 years. The odds of acting out dreams increased annually by a factor of 1.16 (CI = 1.07–1.25, p = 0.0001, GEE model). In contrast, prevalence of sleep fragmentation (p = 0.09), prevalence of vivid dreams (p = 0.90), and daytime sleepiness (p = 0.15) did not increase (GEE model), and likewise, the total modified PSQI score showed no progression of global sleep abnormalities over 10 years (p = 0.89, GEE model). The presence of sleep symptoms did not increase the odds of death at a subsequent visit (p = 0.39, Cochran-Mantel-Haenszel test).
Longitudinal associations between sleep problems and hallucinations over 10 years.
Over the study period, the modified PSQI sleep score was similar between subjects with hallucinations (mean ± SEM = 5.9 ± 0.4) vs without hallucinations (5.5 ± 0.4, p = 0.34, GEE model). At 10 years, the mean PSQI score was similar between hallucinators and nonhallucinators (6.1 ± 4.3 vs 4.2 ± 1.9) and was not associated with the MMSE or UPDRS motor score. Throughout the study period, the presence of vivid dreams was associated with concurrent hallucinations (OR = 2.19, CI = 1.40–3.42, p = 0.0006, GEE model), and specifically with nighttime hallucinations (OR = 2.20, CI = 1.13–4.25, p = 0.02), not daytime hallucinations (p = 0.90). Acting out dreams was associated with concurrent hallucinations (OR = 2.38, CI = 1.47–3.86, p = 0.0004, GEE model), and specifically with both daytime hallucinations (OR = 5.57, CI = 2.06–15.07, p = 0.0007) and nighttime hallucinations (OR = 2.14, CI = 1.02–4.51, p = 0.045). In contrast, daytime sleepiness and sleep fragmentation were not associated with hallucinations, although severe sleep fragmentation was associated with concurrent hallucinations (OR = 2.01, CI = 1.23–3.31, p = 0.006, GEE model).
Relationship of sleep problems to the development of new hallucinations over 10 years.
To determine variables related to the new development of hallucinations, we applied a multivariate GEE approach to hallucination presence/absence over the 5 interviews covering 10 years on patients without hallucinations at baseline. Only time had an effect on the risk of hallucination, and adjusted odds of having hallucinations increased annually by a factor of 1.26 (CI = 1.12–1.41, p < 0.0001, GEE model). There were no effects due to age, PD duration, baseline UPDRS score, baseline MMSE score, gender, medications, baseline behavioral classification based on the modified thought disorder UPDRS score, or the global modified PSQI score. Specifically, none of the following baseline features had predictive power to identify the subsequent development of hallucinations (Cochran-Mantel-Haenszel tests): sleep fragmentation (p = 0.35), vivid dreams/nightmares (p = 0.38), daytime sleepiness (p = 0.51), and acting out dreams (p = 0.45).
Mortality and nursing home placement.
Over the 10-year follow-up period, 62 subjects died or became too ill to participate in the study, 26 of the original 29 hallucinators and 36 of the original 60 nonhallucinators. There was a trend for hallucinations at the previous visit to increase the odds of death at the subsequent visit (OR = 1.86, CI = 0.99–3.53, p = 0.06, Cochran-Mantel-Haenszel test). The proportion of subjects who died by 10 years was similar between the group who never hallucinated (80%) and those who hallucinated at any time point (67%, p = 0.25, χ2 test).
DISCUSSION
This clinical study extends our earlier 6-year longitudinal evaluation of hallucinations in PD and focuses specifically on issues of interactions between sleep problems and hallucinations over many years. The concept that hallucinations are dream-like phenomena related to REM and non-REM disruptions has focused anatomic attention on nuclei including the reticular activating system and the parapontine nucleus.12,13 Our data reinforce the association between hallucinations and the presence and severity of concurrent vivid dreams/nightmares and between hallucinations and concurrent acting out dreams, but this association did not extend to other sleep aberrations, and specifically the very common problem of sleep fragmentation in PD. Whereas severe sleep fragmentation was more common among hallucinators than nonhallucinators, the presence of sleep fragmentation did not differentiate the 2 groups. Because sleep fragmentation and overall sleep function did not correlate with the progressive and chronic nature of vivid dreams/nightmares and hallucinations, the mechanisms underlying the different behaviors are not likely shared. Based on these findings that sleep fragmentation or vivid dreams/nightmares have no predictive influence on the future development of hallucinations, clinicians should no longer consider them as being a minor form of hallucinations or being manifestation of the less severe form of the same pathophysiologic aberration. As a reflection of this evolution, unlike the original UPDRS, the Movement Disorder Society–sponsored revision considers sleep problems and hallucinations as completely separate issues.14
Our data demonstrate that hallucinations are chronic and progressive over time. Whereas we purposefully set the prevalence of hallucinations at the beginning of the study to have 60 nonhallucinators, by 10 years, 93% of these subjects had become hallucinators on at least 1 interview. Further, hallucinations, once developed, became chronic for most patients, and the likelihood of remaining a hallucinator was very high. At a practical level of clinical management, these data demonstrate that the traditional term, “benign hallucinations,” formerly used to evoke the concept of transient or inconsequential hallucinations must be abandoned.
A Norwegian group used a different methodology to examine psychotic symptoms longitudinally in PD.15 Their 12-year population-based study used only the UPDRS thought disorder item to examine hallucinations. At baseline, they did not preselect patients to cover the different strata of thought disorder ratings, so that the baseline demographics represent prevalence rates more representative of a general population of patients with PD than ours. Whereas we examined the presence of active hallucinations at each time point, their definition of PD psychosis included subjects with a thought disorder score ≥2 (benign hallucinations with insight retained) or use of antipsychotic medications for previously documented hallucinations. Like our study, their analysis used GEE modeling to investigate risk factors for the development of psychotic symptoms longitudinally. In spite of these methodologic differences, the results of the 2 studies are remarkably similar. First, mortality was high in both studies: in our study, at 10 years only 30% were alive or able to participate in our study; in the Norwegian cohort, only 22% survived at the same time point. Second, hallucination prevalence increased over time in both studies. In terms of baseline risk factors for eventual hallucinations, we found only time influenced hallucination development, whereas the Norwegians found baseline levodopa equivalent dose, age at PD onset, and probable REM sleep behavioral disorder all independently increased the risk of eventual hallucinations. This observation on the role of dopaminergic medications has not been reported in prior longitudinal assessments, and most cross-sectional studies have failed to document dopaminergic medication dose as a determinant of hallucinations.1,16 Our study did not find an association between levodopa drug dose and hallucination risk, although our definition of hallucinations did not include patients without hallucinations who were taking antipsychotics because of past hallucinations. It is possible that patients on antipsychotics tolerated larger doses of levodopa or other dopaminergic drugs, thereby accounting for this observation.
Although hallucinations were the likely outcome for patients with chronic PD, not all patients developed this complication. In spite of 10 years of follow-up, a long duration of both PD and PD treatment, 4 subjects never hallucinated. In our view, these patients, though uncommon, may hold the genetic, environmental, or metabolic clue to understanding protective factors against this nearly inevitable outcome. With collaborations at sites actively studying hallucinations in PD, a sufficiently robust sample size could be potentially identified for a detailed study of genetic, neuroimaging, and environmental characteristics of these resilient and persistent nonhallucinators. Because prior longitudinal studies have documented that the primary issues in late PD management are nonmotor complications,16,17 the identification of protective factors against hallucinations may offer clinicians and patients strategies to improve quality of life in longstanding PD.
ACKNOWLEDGMENT
The conduct of this longitudinal study involved several colleagues who actively participated in the collection or analysis of data at various phases, but were not involved in the current 10-year data collection or current analysis: Eric J. Pappert, Michael Pass, Rema Raman, Andrew Stemer, Linda Curgian, Joanne Wuu, and Sue Leurgans.
DISCLOSURE
Dr. Goetz serves on a scientific advisory board for the Parkinson's Disease Foundation; served as Editor-in-Chief of Movement Disorders; receives royalties from the publication of Textbook of Clinical Neurology, 3rd edition (Saunders, 2007) and Jean-Martin Charcot: Constructing Neurology (Oxford University Press, 1995); has served as a consultant for Asubio Pharmaceuticals, Inc., Boehringer Ingelheim, IMPAX Laboratories, Inc., i3 Research, Ingenix, Neurim Pharmaceuticals, Novartis, Osmotica Pharmaceutical Corp., Oxford BioMedica Plc, Solvay Pharmaceuticals, Inc., Solvay Pharmaceuticals, Inc., Teva Pharmaceutical Industries Ltd., United Biosource Corporation, and UCB; receives research support from Boehringer Ingelheim, Ceregene, Merck Serono, Santhera Pharmaceuticals, the NIH (U01NS043127-09 [consultant], U01NS043127-09 [consultant], 1K23 NS060949-02 [mentor], and 5K23 NS052487-05 [mentor]), and the Michael J. Fox Foundation; and directs the Rush Parkinson's Disease Research Center, which receives support from the Parkinson's Disease Foundation. Dr. Stebbins has served/serves on the editorial boards of Movement Disorders and the Journal of Clinical and Experimental Neuropsychology and receives research support from the NIH (NIA AG09466 [Co-PI] and NINDS N057514 [coinvestigator]), the American Cancer Society, the Michael J. Fox Foundation for Parkinson Research, and the Fragile-X Foundation. Dr. Ouyang and A. Negron report no disclosures.
Address correspondence and reprint requests to Dr. Christopher G. Goetz, Movement Disorders Section, Department of Neurological Sciences, Rush University Medical Center, 1725 W. Harrison St., Suite 1106, Chicago, IL 60612 cgoetz@rush.edu
Editorial, page 1762.
e-Pub ahead of print on October 20, 2010, at www.neurology.org.
Study funding: Supported by the Parkinson's Disease Foundation.
Disclosure: Author disclosures are provided at the end of the article.
Received March 15, 2010. Accepted in final form June 7, 2010.
REFERENCES
- 1.Fénelon G, Mahieux F, Huon R, Ziégler M. Hallucinations in Parkinson's disease: prevalence, phenomenology and risk factors. Brain 2000;123:733–745. [DOI] [PubMed] [Google Scholar]
- 2.Goetz CG, Stebbins GT. Risk factors for nursing home placement in advanced Parkinson's disease. Neurology 1993;43:2227–2229. [DOI] [PubMed] [Google Scholar]
- 3.Goetz CG, Stebbins GT. Mortality and hallucination in nursing home patients with advanced Parkinson's disease. Neurology 1995;45:669–671. [DOI] [PubMed] [Google Scholar]
- 4.Scaravilli T, Gasparoli E, Rinaldi F. Health-related quality of life and sleep disorders in Parkinson's disease. Neurol Sci 2003;24:209–210. [DOI] [PubMed] [Google Scholar]
- 5.Goetz CG, Wuu J, Curgian LM, Leurgans S. Hallucinations and sleep disorders in PD: six year prospective longitudinal study. Neurology 2005;64:81–86. [DOI] [PubMed] [Google Scholar]
- 6.Fahn S, Elton RL, UPDRS program members. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, eds. Recent Developments in Parkinson's Disease, vol. 2. Florham Park, NJ: Macmillan Healthcare Information; 1987:153–163. [Google Scholar]
- 7.Goetz CG, Leurgans S, Pappert EJ, Raman R, Stemer AB. Prospective longitudinal assessment of hallucinations in Parkinson's disease. Neurology 2001;57:2078–2082. [DOI] [PubMed] [Google Scholar]
- 8.Buysse DJ, Reynolds CF, Monk TH, Berman SR. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 9.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22. [Google Scholar]
- 10.Zeger SL, Liang K-Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986;42:121–130. [PubMed] [Google Scholar]
- 11.SAS/STAT User's Guide, version 8. Cary, NC: SAS Institute Inc.; 1999. [Google Scholar]
- 12.Comella CL, Tanner CM, Ristanovic RK. Polysomnographic sleep measures in Parkinson's disease in patients with treatment-induced hallucinations. Ann Neurol 1993;34:710–714. [DOI] [PubMed] [Google Scholar]
- 13.Arnulf I, Bonnet AM, Damier P, Bejjani BP. Hallucinations, REM sleep and Parkinson's disease: a medical hypothesis. Neurology 2000;55:281–288. [DOI] [PubMed] [Google Scholar]
- 14.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society UPDRS Revision Task Force. Movement Disorder Society–sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170. [DOI] [PubMed] [Google Scholar]
- 15.Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. A 12-year population-based study of psychosis in Parkinson disease. Arch Neurol 2010;67:996–1001. [DOI] [PubMed] [Google Scholar]
- 16.Holroyd S, Currie L, Wooten GF. Prospective study of hallucinations and delusions in Parkinson's disease. J Neurol Neurosurg Psychiatry 2001;70:734–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord 2008;23:837–844. [DOI] [PubMed] [Google Scholar]


