Abstract
Background:
Cellular and animal studies suggest that hypercholesterolemia contributes to Alzheimer disease (AD). However, the relationship between cholesterol and dementia at the population level is less clear and may vary over the lifespan.
Methods:
The Prospective Population Study of Women, consisting of 1,462 women without dementia aged 38–60 years, was initiated in 1968–1969 in Gothenburg, Sweden. Follow-ups were conducted in 1974–1975, 1980–1981, 1992–1993, and 2000–2001. All-cause dementia was diagnosed according to DSM-III-R criteria and AD according to National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association criteria. Cox proportional hazards regression examined baseline, time-dependent, and change in cholesterol levels in relation to incident dementia and AD among all participants. Analyses were repeated among participants who survived to the age of 70 years or older and participated in the 2000–2001 examination.
Results:
Higher cholesterol level in 1968 was not associated with an increased risk of AD (highest vs lowest quartile: hazard ratio [HR] 2.82, 95% confidence interval [CI] 0.94–8.43) among those who survived to and participated in the 2000–2001 examination. While there was no association between cholesterol level and dementia when considering all participants over 32 years, a time-dependent decrease in cholesterol over the follow-up was associated with an increased risk of dementia (HR 2.35, 95% CI 1.22–4.58).
Conclusion:
These data suggest that midlife cholesterol level is not associated with an increased risk of AD. However, there may be a slight risk among those surviving to an age at risk for dementia. Declining cholesterol levels from midlife to late life may better predict AD risk than levels obtained at one timepoint prior to dementia onset. Analytic strategies examining this and other risk factors across the lifespan may affect interpretation of results.
GLOSSARY
- AD
= Alzheimer disease;
- BMI
= body mass index;
- CI
= confidence interval;
- DBP
= diastolic blood pressure;
- DSM-III-R
= Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised;
- HR
= hazard ratio.
It is well-established in animal and cell culture studies that high cholesterol level is associated with amyloid-β deposition, one of the hallmark pathologies of Alzheimer disease (AD). Experimental studies have reported that cholesterol accelerates the production of amyloid-β by shifting amyloid precursor protein metabolism from α- to β-cleavage products, thus increasing the ratio of insoluble to soluble amyloid-β.1,2 Indeed, both rabbits and transgenic mice fed high-cholesterol diets have greater amyloid pathology relative to controls,3,4 and intake of cholesterol-lowering medications are associated with reduced pathology.5 In humans, high cholesterol level has been reported to be associated with the presence of early amyloid deposition in subjects aged 40–55 years, but less so in older subjects,6 suggesting the relationship changes with age.
Despite consistent associations at the cellular level and in animal models, the relationship between cholesterol level and dementia at the population level is less clear (see 7 for meta-analysis). When stratified by age at cholesterol measurement (midlife vs late life), however, patterns emerge. In support of the pathologic findings,6 epidemiologic data suggest that high cholesterol levels in midlife may increase risk for subsequent dementia and AD8–11; however, in late life, low cholesterol levels have been predictive of subsequent dementia12,13 or no association has been observed.14,15 Nevertheless, results are conflicting as some studies have not found high midlife cholesterol level to predict later dementia.16–18
Epidemiologic studies examining the cholesterol–dementia relationship include those with midlife or late-life approaches, as well as continuous, longitudinal approaches, depending on the availability of data. Differences in study designs, lengths of observational periods, analytical strategies, and the natural history of the disorder in relation to the timing of the occurrence of high cholesterol may influence observations. Given the importance of the timing of high cholesterol to the onset of dementia suggested above, it is necessary to study the relationship between cholesterol level and dementia over the lifespan.7,19 The present study examines the relationship between cholesterol level, measured from midlife to late life, and dementia in a population-based study of 5 birth cohorts of women followed for 32 years.
METHODS
Participants.
The Prospective Population Study of Women was initiated in 1968–1969 in Gothenburg, Sweden. A representative sample of 1,622 women living in Gothenburg, and born on specific dates of the years 1908, 1914, 1918, 1922, and 1930 (aged 38–60 years), were randomly selected from the Revenue Office Register. Women living both in the community and in institutions were included. There were 1,462 women who participated in the initial physical and mental health examination, resulting in a participation rate of 90%.20 Additional follow-ups were conducted in 1974–1975, 1980–1981, 1992–1993, and 2000–2001 (figure e-1 on the Neurology® Web site at www.neurology.org). Those who died or refused to take part were traced in records from hospitals and homes for the aged, inpatient and outpatient departments in psychiatric hospitals and clinics, municipal psychiatric outpatient departments in Gothenburg, the hospital-linkage system, and death certificates.21
Standard procedures and participant consents.
All participants (or their nearest relatives) gave their informed consent to participate in the study. The study, conducted in accordance with the provisions of the Helsinki Declaration, was approved by the Ethics Committee for Medical Research at Gothenburg University.
Examinations.
The detailed, longitudinal examinations of manifestations of aging and somatic and psychiatric disorders included a physical examination performed by a physician, electrocardiogram, chest X-ray, battery of blood tests, and neuropsychiatric examination. Participants were surveyed about a variety of potential risk factors for age-related diseases, such as smoking habits, alcohol intake, medication use, education, and medical history. Body weight was recorded to the nearest 0.1 kg, and body height was measured to the nearest centimeter. Body mass index (BMI) is a weight-per-height measurement and was calculated as kg/m2. Casual blood pressure was measured in the right arm in the seated position after 5 minutes' rest using a mercury manometer. Systolic and diastolic blood pressures were registered to the nearest 2 mm Hg. Diastolic blood pressure (DBP) was defined as Korotkoff phase 5. Diagnosis of myocardial infarction, stroke, other vascular diseases, diabetes, and cancer were based on self-reports and clinical examinations (including echocardiogram), case records, the hospital discharge registry, the national cancer registry, and the national stroke registry (from 1995) over the 32-year follow-up.
Assessments of dementia.
Neuropsychiatric examinations occurred over the entire 32 years of follow-up. More extensive neuropsychiatric examinations and close informant interviews to include dementia ascertainment began when participants were 70 years or older, and were performed by psychiatrists in 1992–1993 and experienced psychiatric nurses in 2000–2001.22,23 Medical records were collected from hospitals and outpatient departments in Gothenburg, and dementia diagnoses were based on consensus conferences by geriatric psychiatrists. The Swedish Hospital Discharge Registry provided medical diagnostic information for individuals discharged from hospitals since 1978. Thus, information regarding a dementia diagnosis was obtained for all study participants, since virtually all people in Sweden receive their health care from the Swedish health care system and all participants have an equal chance of having a case record.
Dementia was diagnosed according to DSM-III-R criteria.24 AD (probable and possible) was diagnosed according to the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association criteria.25 Year of dementia onset was estimated for each participant on the basis of neuropsychiatric status at examination, medical record review, and close informant interviews.
Serum cholesterol assessments.
Blood samples were drawn in the fasting state. At the conclusion of each examination, all chemical analyses were run in batch at the Department of Clinical Chemistry at the Sahlgrenska University Hospital in Gothenburg. Laboratory analyses were comparable between the examination years. Serum cholesterol was measured in g/L prior to 1980, then subsequently converted to mmol/L. Starting at the 1980–1981 examination, serum cholesterol was directly measured in mmol/L. At each examination, cholesterol levels beyond 3 standard deviations from the mean were excluded, thus 2 women were excluded in 1968–1969, 9 in 1974–1975, 17 in 1980–1981, 8 in 1992–1993, and 4 in 2000–2001.
Statistical analysis.
t Tests and χ2 tests, as appropriate, were used to assess differences in demographic and health-related characteristics at each examination by dementia diagnosis during the 32-year follow-up. No participants had dementia at baseline. Endpoints included all-cause dementia and AD without a history of stroke. Cox proportional hazards regression models were used to evaluate the relationship between cholesterol and dementia over the 32-year follow-up. Multiple models were run, including 1) considering different baseline cholesterol measurements defined by examination year and subsequent risk of dementia over 8–32 years; 2) examining baseline cholesterol in 1968–1969 and dementia only among those surviving to and participating in the 2000–2001 examination; 3) measuring cholesterol and covariates in a time-dependent manner at each examination in relationship to onset of dementia; and 4) examining time-dependent change in cholesterol levels between examinations and onset of dementia and AD. In the last analysis, we chose to examine time-dependent cholesterol change in relationship to dementia risk rather than change over 32 years, as only considering change in cholesterol between 1968 and 2000–2001 would restrict the sample to those surviving to 2001. Time at risk for these analyses was calculated to the end of the study period (i.e., 2000–2001 examination), diagnosis of dementia, or death. For all analyses, cholesterol was 1) considered as a continuous variable; 2) dichotomized at >6.5 mmol/L vs less, as other studies have done8,9; and 3) examined in quartiles (lowest quartile as reference). Cox proportional hazards models were also used to assess the association between baseline cholesterol in 1968–1969 and mortality over the 32-year follow-up.
Covariates were chosen based on those reported in the literature and factors that have been examined in other studies of cholesterol and dementia.8,10,12 Covariates were entered into regression models using a single step approach. Birth cohort (an indicator of age) was adjusted as a stratification variable. Additional covariates included DBP, BMI, cigarette smoking, and education. DBP and BMI were examined as continuous variables. Cigarette smoking was defined as ever vs never use in each examination year. Levels of education (completing ≤6 years vs >6 years compulsory education; 7 years for those born in 1930) were based on responses to the 1968–1969 survey. Covariates and cholesterol measurements were concurrent; time-dependent covariates were included in the time-dependent cholesterol analyses. The a priori p value was set at p < 0.05. Analyses were conducted using STATA version 10.0 (StataCorp, College Station, TX).
RESULTS
Participant characteristics.
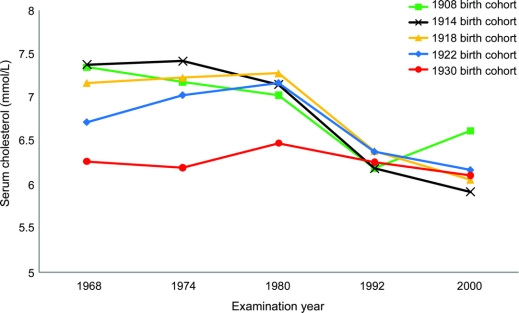
Over the 32-year follow-up, 161 (11.0%) of the 1,462 women developed dementia, including 80 participants who developed AD without a history of stroke. Total risk time evaluated was 41,219 risk-years. Number of dementia cases by birth cohort and characteristics of women at each examination are shown in table 1. Across all cohorts, participants who developed dementia over the follow-up had higher mean cholesterol levels (p < 0.01) in 1968–1969, 1974–1975, and 1980–1981, but not 1992–1993 or 2000–2001 compared to participants who did not develop dementia by the 2000–2001 examination. Mean BMI levels were also lower in 1992–1993 and 2000–2001 in women who developed dementia (p < 0.01) compared to women who did not. Mean cholesterol levels varied by birth cohort and over time (figure 1). In 1968–1969 and 1974–1975, the 1908 and 1914 birth cohorts had higher mean cholesterol levels (p < 0.05) compared to the 1930 birth cohort. There were no differences in mean cholesterol levels at other examinations, and mean levels decreased over the 32-year follow-up for all birth cohorts.
Table 1 Characteristics of PPSW participants by dementia status over 32 years (n = 1,462)
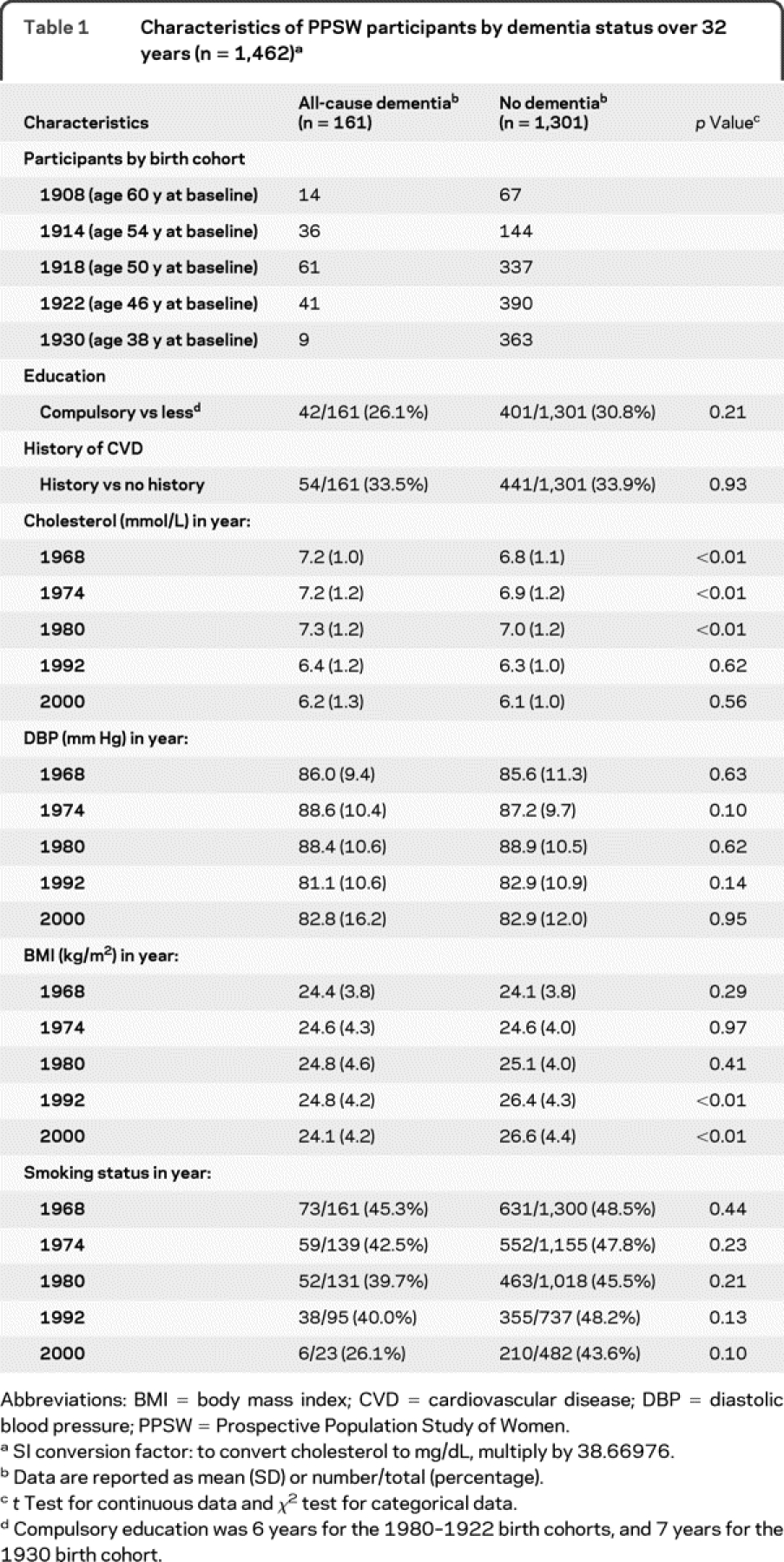
Figure 1 Mean cholesterol levels in the Prospective Population Study of Women by examination year and birth cohort
Serum cholesterol at each examination and risk of dementia (AD and all-cause dementia).
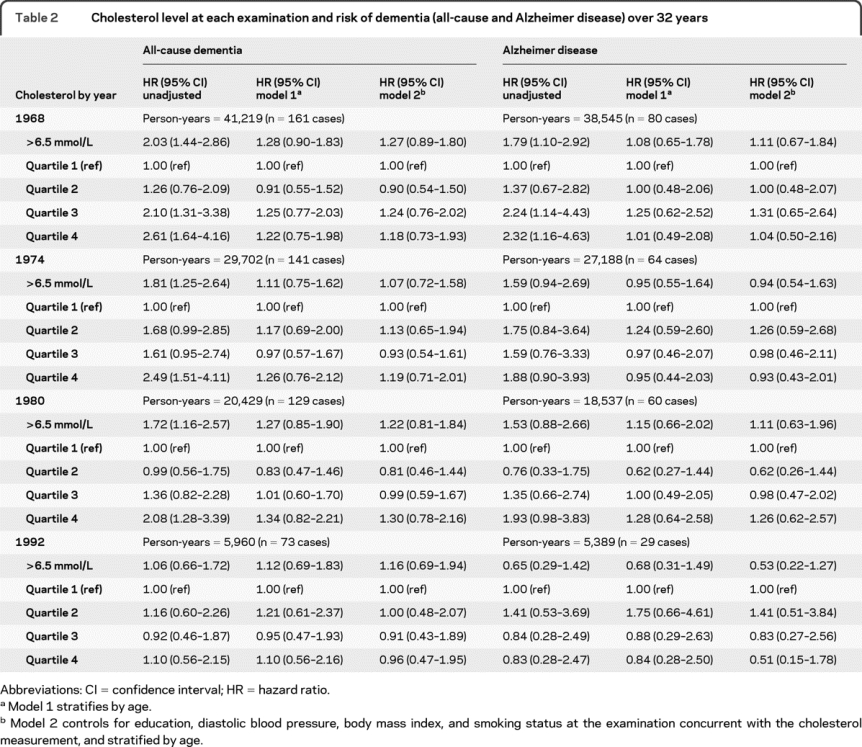
Evaluation of the cholesterol–dementia relationship using age-adjusted Cox proportional hazards models showed no associations from midlife to late life (table 2). There was also no association between cholesterol and risk of dementia when analyzing cholesterol as a time-dependent variable over the 5 examinations (data not shown).
Table 2 Cholesterol level at each examination and risk of dementia (all-cause and Alzheimer disease) over 32 years
Serum cholesterol in 1968 and risk of dementia (AD and all-cause) among those who survived to the 2000 examination.
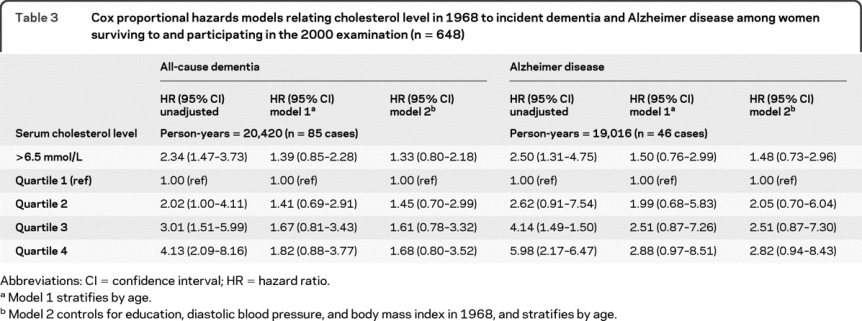
Some epidemiologic studies examining midlife cholesterol have information on dementia diagnosis only for those who survived to the follow-up examination,8,9,11 often decades after the midlife cholesterol measurement. In order to replicate these analyses and better determine the effects of survival on the results, the analysis was restricted to the 648 women who survived and participated in the 2000 examination (table 3). In univariate analyses, high cholesterol was associated with dementia such that the highest quartile, compared to the lowest, was associated with a fourfold increase in risk of all-cause dementia (hazard ratio [HR] = 4.13, 95% confidence interval [CI] 2.09–8.16) and almost a sixfold increase in risk of AD (HR = 5.98, 95% CI 2.17–16.47). However, the results were attenuated after age stratification and, while there was a trend for high cholesterol to increase risk, results were not significant at the p < 0.05 level.
Table 3 Cox proportional hazards models relating cholesterol level in 1968 to incident dementia and Alzheimer disease among women surviving to and participating in the 2000 examination (n = 648)
Change in cholesterol and risk of dementia (AD and all-cause).
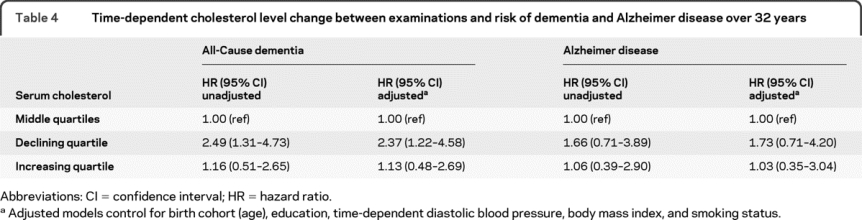
Finally, change in cholesterol between visits was examined as a predictor of subsequent dementia using Cox proportional hazards regression (table 4). Cholesterol change was analyzed by quartiles as a time-dependent variable at each examination. While the intervals between examinations varied over the follow-ups, the range for each quartile was relatively consistent. The middle 2 quartiles (change of ∼−0.50 to 0.75 mmol/L between examinations) was the reference group. The quartiles showing the greatest decline (reduction of cholesterol greater than 0.50 mmol/L) and the greatest increase (increase in cholesterol greater than 0.75 mmol/L) were examined in relationship to risk of dementia. Compared to the middle 2 quartiles, the quartile of greatest decrease in cholesterol was related to increased risk of dementia (multivariate adjusted HR = 2.37, 95% CI 1.22–4.58). While there was a similar trend for AD, results were not significant.
Table 4 Time-dependent cholesterol level change between examinations and risk of dementia and Alzheimer disease over 32 years
Serum cholesterol, lipid-lowering medications, and mortality.
Over the 32-year follow-up, 515 (35.2%) women died. Using age- and multivariate-adjusted Cox proportional hazards models, total cholesterol in 1968–1969 was not associated with a higher risk of mortality (table e-1). Information on lipid-lowering medication use was available at each examination. However, only 8 (0.5%) of 836 women present at the 1992–1993 examination and 40 (2.7%) of 660 women at the 2000–2001 examination were taking lipid-lowering medications. Neither excluding participants on lipid-lowering medications at these examinations nor controlling for medication use altered any results.
DISCUSSION
In this population-based study of 5 birth cohorts of women, age 38–60 at baseline and followed for up to 32 years, the relationship between cholesterol and dementia (all-cause and AD) was examined utilizing multiple statistical methodologies that have been previously published in the examination of this relationship. In summary: 1) high cholesterol at baseline in 1968–1969 was not associated with risk of all-cause dementia or AD once age was considered; 2) high cholesterol at baseline in 1968–1969 was more strongly associated (albeit not significant) with incident dementia (all-cause and AD) when only including those who survived to and participated in the 2000–2001 examination compared to the inclusion of all participants and their respective survival times; and 3) a decrease in cholesterol levels over the follow-up was associated with a modest increased risk of dementia.
There is increasing awareness that identification of risk factors for syndromes of late life, such as dementia, need to be considered using a life-course perspective. Throughout life, genetic and environmental (e.g., diet, physical activity, obesity) factors have interactive effects in predisposing a person to dementia. One impediment to the current lifespan approach evaluating the cholesterol–dementia relationship is that most longitudinal studies have only examined this relationship among people that survived to old age. In the present study, we used different methodologies to examine the cholesterol–dementia relationship. We examined the relationship continuously over the middle to late lifespan as well as only among those who survived to old age. A clear difference was demonstrated. When including all persons, there was no association between midlife cholesterol and risk of dementia or AD in multivariate models. In contrast, when only including those who survived to old age, there was a clear trend for high cholesterol to be associated with an increased risk of AD. This difference is likely due to a survival bias and competing mortality,23 and demonstrates that thoughtful consideration of this bias is needed when examining relationships between midlife risk factors and late-life outcomes only among survivors. Including survivors only may lead to an overestimation of association. As loss to follow-up is negligible in this study sample, this consideration is underscored.
Perhaps more importantly, the present study found that decreasing cholesterol between visits was associated with an increased risk of dementia, but not AD, similar to results found in other studies.9,17 Thus, unintended decreases in cholesterol levels (e.g., not via medications or cholesterol-lowering diet) greater than expected due to aging may be more indicative of dementia risk than midlife cholesterol levels and may reflect underlying dementia processes. This pattern is observed for other dementia risk factors, such as BMI23 and blood pressure.26 In these women, we observe declines in both BMI and blood cholesterol levels, yet these observations are statistically independent of each other indicating that decline in each parameter is important. Hypotheses related to observed declines may have to do with regions of the brain affected by amyloid deposition, such as the arcuate nucleus and in general, the hypothalamus, which are areas of homeostatic regulation.27 In addition, consequences of the dementia prodrome such as apathy or reduced olfactory function28,29 may lead to decreased energy intake, which may also affect blood cholesterol levels.
There are some limitations and methodologic factors that need to be addressed. First, it is often difficult to discriminate between AD and VAD. However, our criteria for AD are strict and we exclude all cases with stroke or infarcts on CT. Second, loss of participants due to death or refusal may have influenced the results, particularly in the oldest age groups. While we have information from examinations, close informants, case records, hospital registers, and death certificates, some of these secondary sources are known to underrate dementia. Thus, undiagnosed cases of dementia may be included in the no-dementia group, which would most likely diminish differences between the 2 groups, and lead to conservative estimates of effects. Third, APOE ε4 genotyping and low-density lipoprotein and high-density lipoprotein cholesterol were not available. However, a recent meta-analysis7 reported no interaction between the APOE ε4 allele and total cholesterol in predicting dementia risk or an association between high-density lipoprotein and dementia. Finally, the study is composed of women in Sweden and the results may not be generalizable to men or other ethnicities.
Despite these limitations, the present study has several notable strengths. First, among the strengths of this study are the 32 years of follow-up with multiple cholesterol measurements and health information, which has allowed for the lifespan examination of the relationship and temporality between cholesterol and dementia. Second, there was no loss to follow-up because information regarding a dementia diagnosis was obtained for all study participants. Participants who died or refused to take part in the study were traced through several registries and records from hospital systems and homes for the aged. Although case records may underdiagnose the number of dementia cases, this methodologic aspect has a distinct advantage over other longitudinal studies because persons lost to follow-up are not representative of the population in that they are more likely to be ill and/or cognitively impaired. Finally, the study timeframe was 1968–2000; only during the last few years of the study were statins and other lipid-lowering drugs available. Thus, without potential confounding with medications, a true relationship between cholesterol and dementia is observed.
On neither an individual nor population level can we determine whether a person will develop dementia in late life based on a midlife cholesterol level. However, in accordance with heart healthy guidelines, we suggest that midlife cholesterol levels be monitored and treated via diet, exercise, and medication as recommended or required. In addition, we suggest that declines in metabolic parameters, such as blood cholesterol levels, BMI, and blood pressure, be monitored with aging, and that there may be precedent for stabilization of these parameters in relation to lowering dementia risk.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by H. Shao, Dr. Zandi, and Dr. Mielke.
DISCLOSURE
Dr. Mielke receives research support from the NIH (R21 NS06027-01 [PI], R21 AG028754 [PI], and R01 AG21136 [Investigator]) and from the George and Cynthia Mitchell Foundation. Dr. Zandi receives research support from the NIH (NIMH K01 MH072866-01 [PI], NIMH R01 MH083738-01 [PI], NIA BJ18-JHU-ARRA [sub-contract PI], R01MH076953 [Coinvestigator], R37AA12303 [Coinvestigator], R01MH079240 [Coinvestigator], NIMH K99MH086049-01A1 [Co-mentor], R37DA011796 [Coinvestigator], and NIMH R01 MH 42243 [Coinvestigator]). Mr. Shao reports no disclosures. Dr. Waern receives research support from the Swedish Research Council and the Swedish Council for Working Life and Social Research. Dr. Östling receives research support from Stiftelsen Söderström-Königska Sjukhemmet. Dr. Guo reports no disclosures. Dr. Bjorkelund serves on scientific advisory boards for LifeGene Sweden and the Swedish Council on Health Technology Assessment; serves as an Associate Editor for the Scandinavian Journal of Primary Health Care; and receives research support from the Swedish Council for Working Life and Social Research. Dr. Lissner reports no disclosures. Dr. Skoog has served on scientific advisory boards for Pfizer Inc. and AstraZeneca; has served on an editorial advisory board for International Psychogeriatrics; receives royalties from publishing Alzheimers sjukdom och andra kognitiva sjukdomar (English title: Alzheimer's Disease and Other Cognitive Disorders [Liber, 2003]); serves on speakers' bureaus for Shire plc, Janssen-Cilag, Pfizer Inc., Novartis, and Esai Inc.; and has received research support from the Swedish Research Council, Swedish Council for Working Life and Social Research, the Alzheimer's Association, and the Bank of Sweden Tercentenary Foundation. Dr. Gustafson serves as a consultant for the Albuquerque Area Indian Health Board; has served on the speakers' bureau for Shire plc; and receives research support from the NIH (NIA 5R03AG026098 [PI]) and the Swedish Research Council.
Supplementary Material
Address correspondence and reprint requests to Dr. Michelle M. Mielke, Johns Hopkins University School of Medicine, Department of Psychiatry, Division of Geriatric Psychiatry and Behavioral Sciences, Bayview–Alpha Commons Building, 4th floor–Room 454, Baltimore, MD 21224 mmielke1@jhmi.edu
Editorial, page 1862
Supplemental data at www.neurology.org
e-Pub ahead of print on November 10, 2010, at www.neurology.org.
Study funding: Supported by the NIH (NIA 1R03AG026098-01A1, NIA 1R21AG028754, and NINDS R21NS060271-01), the Swedish Research Council (11267 and 2005-8460), the Swedish Brain Power Project, EU FP7 project LipiDiDiet (211696), Swedish Council for Working Life and Social Research (1154), FAS EpiLife (2006–1506), Swedish Alzheimer Association, Stiftelsen Söderström-Königska Sjukhemmet, Stiftelsen för Gamla Tjänarinnor, Hjalmar Svenssons Foundation, The Swedish Society of Medicine, The Göteborg Medical Society, the Lions Foundation, the Dr. Felix Neubergh Foundation, the Wilhelm and Martina Lundgren Foundation, the Elsa and Eivind Kison Sylvan Foundation, and the Alzheimer's Association Zenith Award (ZEN-01-3151).
Disclosure: Author disclosures are provided at the end of the article.
Received April 18, 2010. Accepted in final form July 20, 2010.
REFERENCES
- 1.Fassbender K, Simons M, Bergmann C, et al. Simvastatin strongly reduces levels of Alzheimer's disease beta-amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci USA 2001;98:5856–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc Natl Acad Sci USA 1998;95:6460–6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Refolo LM, Malester B, LaFrancois J, et al. Hypercholesterolemia accelerates the Alzheimer's amyloid pathology in a transgenic mouse model. Neurobiol Dis 2000;7:321–331. [DOI] [PubMed] [Google Scholar]
- 4.Sparks DL, Scheff SW, Hunsaker JC 3rd, Liu H, Landers T, Gross DR. Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp Neurol 1994;126:88–94. [DOI] [PubMed] [Google Scholar]
- 5.Refolo LM, Pappolla MA, LaFrancois J, et al. A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer's disease. Neurobiol Dis 2001;8:890–899. [DOI] [PubMed] [Google Scholar]
- 6.Pappolla MA, Bryant-Thomas TK, Herbert D, et al. Mild hypercholesterolemia is an early risk factor for the development of Alzheimer amyloid pathology. Neurology 2003;61:199–205. [DOI] [PubMed] [Google Scholar]
- 7.Anstey KJ, Lipnicki DM, Low LF. Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry 2008;16:343–354. [DOI] [PubMed] [Google Scholar]
- 8.Kivipelto M, Helkala EL, Laakso MP, et al. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med 2002;137:149–155. [DOI] [PubMed] [Google Scholar]
- 9.Notkola IL, Sulkava R, Pekkanen J, et al. Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer's disease. Neuroepidemiology 1998;17:14–20. [DOI] [PubMed] [Google Scholar]
- 10.Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer's and vascular dementia three decades later. Dement Geriatr Cogn Disord 2009;28:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 2005;64:277–281. [DOI] [PubMed] [Google Scholar]
- 12.Mielke MM, Zandi PP, Sjogren M, et al. High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology 2005;64:1689–1695. [DOI] [PubMed] [Google Scholar]
- 13.Reitz C, Tang MX, Luchsinger J, Mayeux R. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch Neurol 2004;61:705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li G, Shofer JB, Kukull WA, et al. Serum cholesterol and risk of Alzheimer disease: a community-based cohort study. Neurology 2005;65:1045–1050. [DOI] [PubMed] [Google Scholar]
- 15.Yoshitake T, Kiyohara Y, Kato I, et al. Incidence and risk factors of vascular dementia and Alzheimer's disease in a defined elderly Japanese population: the Hisayama Study. Neurology 1995;45:1161–1168. [DOI] [PubMed] [Google Scholar]
- 16.Kalmijn S, Foley D, White L, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men: The Honolulu-Asia Aging Study. Arterioscler Thromb Vasc Biol 2000;20:2255–2260. [DOI] [PubMed] [Google Scholar]
- 17.Stewart R, White LR, Xue QL, Launer LJ. Twenty-six-year change in total cholesterol levels and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol 2007;64:103–107. [DOI] [PubMed] [Google Scholar]
- 18.Tan ZS, Seshadri S, Beiser A, et al. Plasma total cholesterol level as a risk factor for Alzheimer disease: the Framingham Study. Arch Intern Med 2003;163:1053–1057. [DOI] [PubMed] [Google Scholar]
- 19.Launer LJ. The epidemiologic study of dementia: a life-long quest? Neurobiol Aging 2005;26:335–340. [DOI] [PubMed] [Google Scholar]
- 20.Bengtsson C, Blohme G, Hallberg L, et al. The study of women in Gothenburg 1968–1969: a population study: general design, purpose and sampling results. Acta Med Scand 1973;193:311–318. [DOI] [PubMed] [Google Scholar]
- 21.Skoog I, Nilsson L, Palmertz B, Andreasson LA, Svanborg A. A population-based study of dementia in 85-year-olds. N Engl J Med 1993;328:153–158. [DOI] [PubMed] [Google Scholar]
- 22.Guo X, Waern M, Sjogren K, et al. Midlife respiratory function and incidence of Alzheimer's disease: a 29-year longitudinal study in women. Neurobiol Aging 2007;28:343–350. [DOI] [PubMed] [Google Scholar]
- 23.Gustafson DR, Backman K, Waern M, et al. Adiposity indicators and dementia over 32 years in Sweden. Neurology 2009;73:1559–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-III-R. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 25.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 26.Stewart R, Xue QL, Masaki K, et al. Change in blood pressure and incident dementia: a 32-year prospective study. Hypertension 2009;54:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abizaid A, Horvath TL. Brain circuits regulating energy homeostasis. Regul Pept 2008;149:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geda YE, Roberts RO, Knopman DS, et al. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry 2008;65:1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA. Olfactory impairment in presymptomatic Alzheimer's disease. Ann NY Acad Sci 2009;1170:730–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.