Abstract
Objective:
Dysphagia is the main cause of aspiration pneumonia and death in Parkinson disease (PD) with no established restorative behavioral treatment to date. Reduced swallow safety may be related to decreased elevation and excursion of the hyolaryngeal complex. Increased submental muscle force generation has been associated with expiratory muscle strength training (EMST) and subsequent increases in hyolaryngeal complex movement provide a strong rationale for its use as a dysphagia treatment. The current study's objective was to test the treatment outcome of a 4-week device-driven EMST program on swallow safety and define the physiologic mechanisms through measures of swallow timing and hyoid displacement.
Methods:
This was a randomized, blinded, sham-controlled EMST trial performed at an academic center. Sixty participants with PD completed EMST, 4 weeks, 5 days per week, for 20 minutes per day, using a calibrated or sham, handheld device. Measures of swallow function including judgments of swallow safety (penetration–aspiration [PA] scale scores), swallow timing, and hyoid movement were made from videofluoroscopic images.
Results:
No pretreatment group differences existed. The active treatment (EMST) group demonstrated improved swallow safety compared to the sham group as evidenced by improved PA scores. The EMST group demonstrated improvement of hyolaryngeal function during swallowing, findings not evident for the sham group.
Conclusions:
EMST may be a restorative treatment for dysphagia in those with PD. The mechanism may be explained by improved hyolaryngeal complex movement.
Classification of evidence:
This intervention study provides Class I evidence that swallow safety as defined by PA score improved post EMST.
GLOSSARY
- CI
= confidence interval;
- EMST
= expiratory muscle strength training;
- MEP
= maximum expiratory pressure;
- PA
= penetration–aspiration;
- PD
= Parkinson disease;
- SWAL-QOL
= Swallowing Quality of Life Questionnaire;
- UES
= upper esophageal sphincter;
- UF
= University of Florida Movement Disorders Center;
- VA
= Veterans Affairs.

LOE Classification
Swallowing is a patterned sensorimotor process within a complex neural network involving automatic and volitional systems1–4 amenable to modification and adaptation. Accordingly, swallowing rehabilitation techniques aimed at restoration of function are gaining increased interest, particularly in populations like Parkinson disease (PD), where aspiration pneumonia is the leading cause of death.5–10 This article presents results from the first blinded, randomized placebo-controlled clinical trial, testing the effects of a restorative treatment called expiratory muscle strength training (EMST150, Aspire Products)11–17 for swallowing dysfunction in PD. EMST's mechanistic underpinning is its ability to generate increased submental musculature force activation.15
Decreased elevation and excursion of the hyolaryngeal complex is considered one cause of penetration and aspiration.18–21 Submental muscle contraction elevates the hyolaryngeal complex. EMST treatment produces significantly longer durations, higher peak amplitudes, and greater average amplitudes of the submental muscles' EMG signal when compared to dry and wet swallows.15 These findings are likely related to increased motor unit discharge rates, increased motor unit recruitment, and prolonged stimulation of the peripheral nerves.
We anticipated that 4 weeks of EMST would cause changes to swallowing safety as measured by the primary outcome variable of penetration–aspiration (PA) score, a validated and clinically utilized 8-point scale.22 We predicted that EMST would increase displacement and temporal measures of hyoid movement during swallowing. Additionally, we expected that these functional and physiologic changes would result in improvements of swallowing-related quality of life.23–25
METHODS
Participants.
Seventy-two participants with idiopathic PD were screened and recruited for study from the University of Florida Movement Disorders Center (UF) and the Malcom Randall Veterans Affairs (VA) Medical Center Movement Disorders Clinic, with 60 participants completing the protocol. Based on paired t test for alternatives with Gaussian distributions, a total of 30 participants per group would yield 80% power to detect a pre and post PA scale difference of effect size 1.14. Dropout rate was calculated at 10% attrition. A UF Movement Disorders fellowship trained neurologist completed a clinical assessment of each individual's PD disease severity (including blinded Unified Parkinson's Disease Rating Scale ratings). All participants with PD had to 1) meet the diagnostic criteria of the UK Brain Bank26; 2) report some degree of swallowing disturbance (i.e., reports of coughing with meals, increased eating duration); and 3) remain on the same PD medications throughout the study. Other inclusion criteria included 1) age between 55 and 85 years; 2) moderate clinical disability level (Hoehn & Yahr stages II–IV)7; and 3) score of at least 24 on the Mini-Mental State Examination.27 Exclusion criteria assessed by the recruiting neurologist prior to enrollment included 1) other neurologic disorders; 2) gastrointestinal disease; 3) gastroesophageal surgery; 4) head and neck cancer; 5) history of breathing disorders or diseases; 6) untreated hypertension; 7) heart disease; 8) history of smoking in the last 5 years; and 9) difficulty complying due to neuropsychological dysfunction (e.g., severe depression, psychosis). Another exclusion criterion was failure of a screening test of pulmonary function which was completed at baseline.
Design.
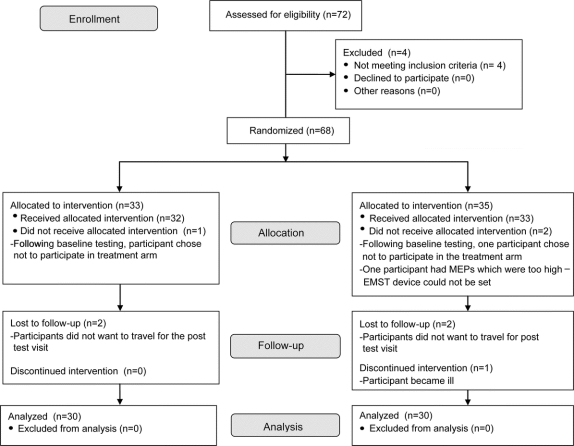
The design is detailed in figure 1. As this was a prospective, blinded, randomized, sham-controlled, clinical trial, all participants were randomly assigned to the active or sham treatment group. All participants took part in a baseline swallowing assessment followed by 4 weeks of active or sham treatment. Following completion of either treatment arm, participants returned for a post-treatment assessment.
Figure 1 Study participation and follow-up flow chart
EMST = expiratory muscle strength training; MEP = maximum expiratory pressure.
EMST/SHAM training.
For a depiction of the EMST device, see figure 2. The EMST treatment program uses a calibrated, one-way, spring-loaded valve to mechanically overload the expiratory and submental muscles.11–14,16–17,28 The adjustable spring allows for discrete and calibrated changes to the valve blocking the flow of air until sufficient expiratory pressure is produced. Once opened, air flows through the device. The physiologic load on the targeted muscles can be increased or decreased by varying the device setting.
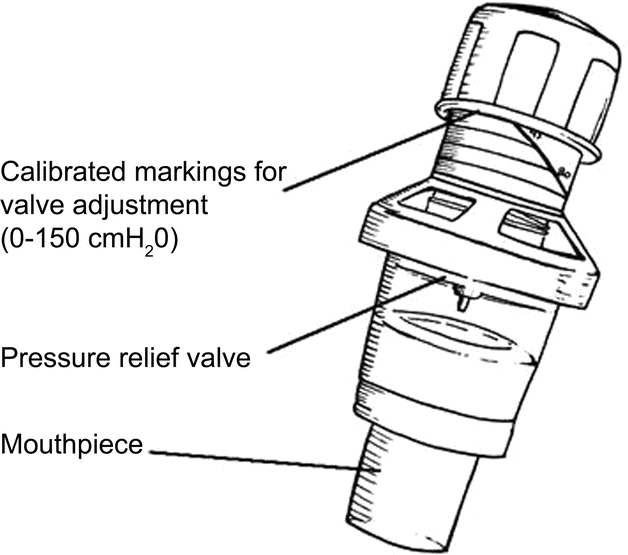
Figure 2 Expiratory muscle strength training device
Drawing reprinted with permission from Chest (Pitts et al. 2009;135:1301–1308).33
For the active treatment group, the EMST device was set weekly to 75% of the participant's average maximum expiratory pressure (MEP; see procedures below). Participants were visited weekly (during the 4-week training phase) by a clinician, blinded to treatment randomization. The sham device was identical to the EMST device except the pressure release valve was made to be nonfunctional by removing the spring. For the purpose of the sham treatment group, the device was also set to 75% of the participants' average MEP using the adjustable cap, therefore appearing as though the device was being manipulated, although it was providing little to no physiologic load to the targeted muscles. Given that the EMST and sham devices looked the same, both clinician and participant were blinded to treatment randomization.
During weekly visits by the clinician, participants were reminded how to properly use their device to facilitate independent daily treatment trials. Participants were instructed to wear nose clips, take a deep breath, hold their cheeks lightly (to reduce labial leakage), blow as hard as they could into the device, and identify that air was flowing freely through the device (once they reached threshold pressure). During the initial home training visit, participants were encouraged to identify whether they were completing the task appropriately. Feedback was provided to ensure accuracy of initial training. Once participants were able to identify accurate task completion, clinician-based feedback was eliminated. Each participant trained at home (independent of the clinician) completing 5 sets of 5 repetitions 5 days out of the week.11–14,16,17,28 Compliance with the training was tracked using a form provided by the clinician.
Baseline/post-training visits.
Although participants were assessed during 2 baseline measurement sessions, videofluoroscopic assessment of swallowing was only completed at the second baseline visit in order to limit radiation exposure (approximately 350 mrem). The same assessment protocol was completed following training. Participants were tested 1 hour following intake of their dopaminergic medications to ensure they were in a practically defined “on” state. Detailed description of the procedures used at assessment visits is provided below.
Maximum expiratory pressure.
Using a standardized protocol at each time point for assessment, participants were instructed to stand and occlude the nose with the nose clips. MEP measurements were completed using a pressure manometer (FLUKE 713–30G) coupled to a mouthpiece via 50 cm, and 2-mm inner diameter tubing, with an air leak created by a 14-gauge needle. With the device mouthpiece placed between the lips and behind the teeth, participants were instructed to inhale as deeply as possible and blow into the manometer tube quickly and forcefully. Solely verbal encouragement was provided to the participants. Three values within 5% of each other were required to achieve an average for the participants' individualized MEP score and this score set the EMST device for the subsequent training.
Videofluoroscopy.
Videofluoroscopy, commonly utilized in the clinical setting, was selected for examination of swallowing function. Participants sat upright and their swallowing function was recorded in the lateral viewing plane using a properly collimated Phillips radiographic/fluoroscopic unit (63-kV, 1.2-mA output, full field of view mode). The Kay Elemetrics Swallowing Signals Lab unit (Kay Elemetrics, Lincoln Park, NJ) digitally recorded the fluoroscopic images at 29.97 frames per second using a scan converter.
Participants completed ten 5-mL trials of thin liquid (Liquid E-Z Paque Barium Sulfate Suspension; 60% w/v, 41% w/w; from E-Z-EM) by cup and also a trial of one 3-oz sequential swallow of thin liquid by cup. Trials were presented in random order. During the swallowing examinations, all patients self-fed in order to approximate real-world feeding conditions. Participants were given the spoon or cup and prompted by the investigator to “place the liquid in your mouth and swallow when ready.”
Data analysis.
Licensed and certified speech pathologists with clinical expertise evaluating patients with PD analyzed the barium swallow studies and were blinded to the participants' identity and treatment randomization.
Primary outcome: Swallow safety (PA scores).
The PA scale is a clinically relevant, validated, and ordinal measure, where 1 indicates the safest swallow (no penetration or aspiration) and 8 indicates the least safe swallow, or silent aspiration.22 The scale measures whether or not material entered the airway and if it entered the airway, whether the residue remained or was expelled. The PA scale score served as the primary outcome variable calculated as the group comparison between baseline and posttreatment assessments during the swallowing of the 3-oz sequential bolus.
Secondary outcomes: Physiologic measures of swallow mechanism.
Duration of hyoid movement was completed by analyzing the digital recordings of the ten 5-mL thin swallow trials frame by frame or in slow motion using the Digital Swallowing Workstation. The examiner placed measurement tags at 1) the initiation of hyoid movement which resulted in the swallow and 2) the point when the hyoid returned to rest following the completion of the swallow. These tags were then used to calculate the duration of hyoid movement.
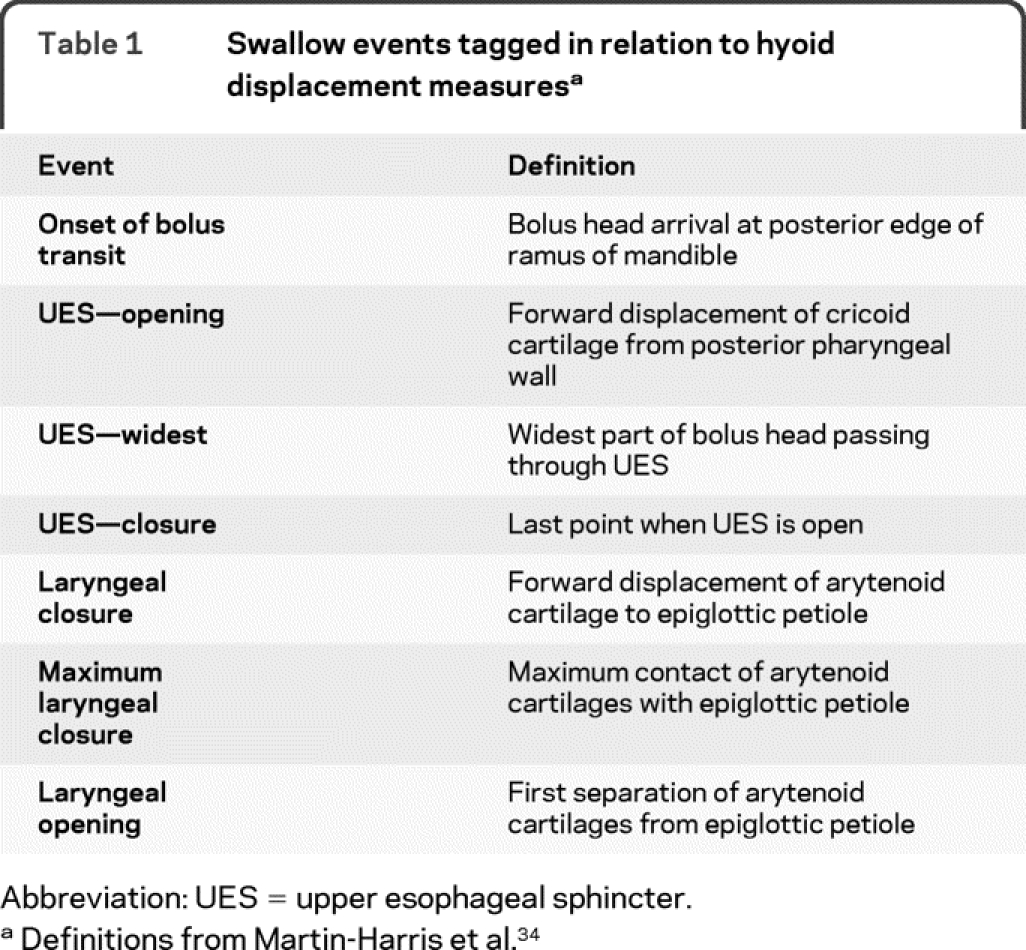
Using a MATLAB routine developed in preliminary studies by our laboratory, hyoid movement during swallowing was quantified.29 The program measured hyoid displacement for each frame of the swallow using the third cervical vertebrae (C3) as a stable physiologic reference point.29 The program automatically randomized all frames collected and as each frame was individually presented, the measurer placed a cursor to mark the most anterior and inferior points on the hyoid bone and the C3 vertebrae. The frames were then presented in sequential order, and the measurer tagged selected swallow events (table 1). The MATLAB routine calculated the hyoid bone displacement from C3 for each frame of the swallow. Displacement measures were then normalized to the first frame of that individual swallow. The program then provided hyoid displacements at each of the swallowing events.
Table 1 Swallow events tagged in relation to hyoid displacement measures
Swallowing quality of life measure.
The Swallowing Quality of Life Questionnaire (SWAL-QOL) was used to evaluate the participants' quality of life as related to swallowing function.23–25 This tool includes questions regarding both the oral and pharyngeal phases of swallowing as well as appetite, eating duration, and other factors affecting swallowing function.
Rater reliability.
To test for inter-rater and intrarater reliability for the outcomes of PA score and hyoid displacement, 25% of the total dataset was reanalyzed.
Standard protocol approvals, registrations, and patient consents.
This project was approved by the UF and VA Institutional Review Boards (154–2003 and 195-2005). Written consent was obtained from all participants. This study is registered in clinicaltrials.gov (identifier NCT00843739).
Statistical methods.
Descriptive statistics described the demographics of each treatment group. Treatment effect was analyzed utilizing a repeated-measures analysis of covariance, with time (2 levels: pre and post) as the within-subjects variable and group (EMST and sham) as the between-subjects variable. The intent-to-treat analysis was conducted for the primary outcome variable of PA scores, where the missing data were imputed by predicted values from a regression model including age, gender, disease severity, and pretreatment PA scores. Absolute risk reduction, the number needed to treat to gain one additional improvement, and the number needed to treat to gain one additional benefit (improvements gained + deteriorations prevented) were examined. Secondary outcome variables included hyoid duration, hyoid displacement measures, and SWAL-QOL scores.
RESULTS
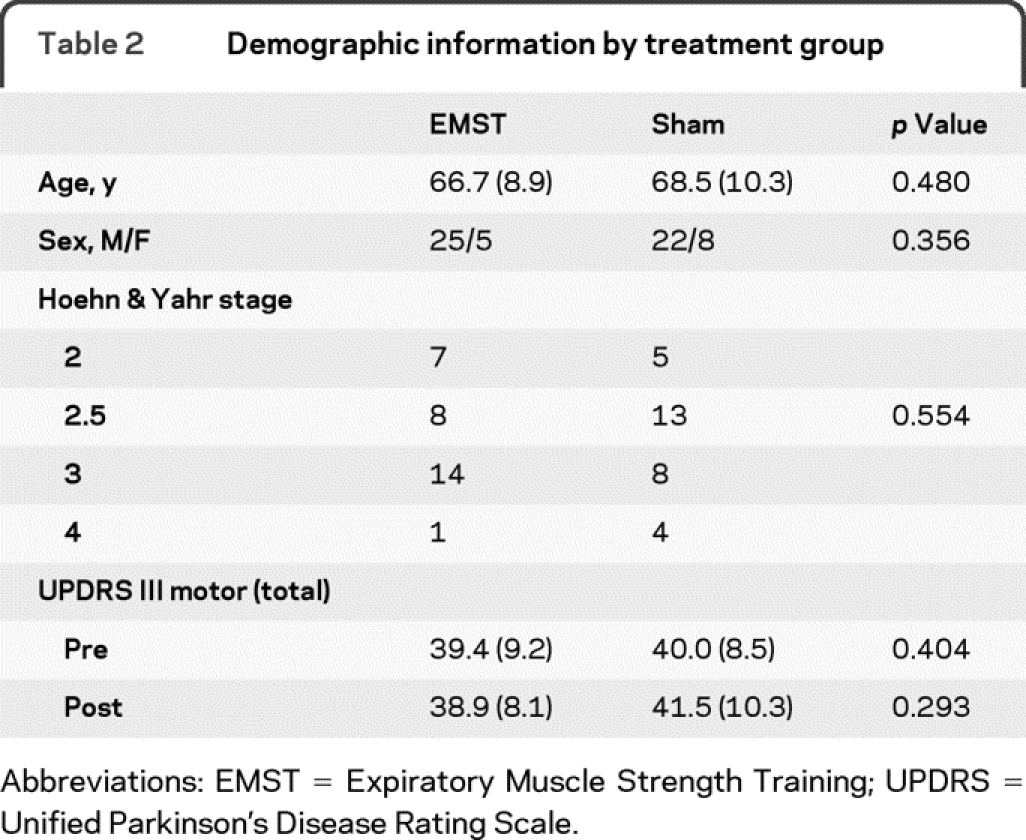
Seventy-two participants were recruited and screened for the study. Four participants did not meet eligibility criteria, with 1 failing pulmonary function screening, 2 having Mini-Mental State Examination scores below the cutoff, and 1 reporting smoking within 6 months from recruitment. A total of 33 participants were allocated to the EMST group and 35 to the sham group. A total of 30 participants completed the study in each group. Reasons for withdrawal from the study are shown in figure 1. Table 2 contains the descriptive statistics for the 60 participants who completed the trial. Figure 1 depicts the flow of participants through each stage of the clinical trial.
Table 2 Demographic information by treatment group
Reliability.
Inter-rater reliability was excellent, yielding intraclass coefficients of 0.98 (95% confidence interval [CI] 0.93 to 0.99). For hyoid displacement measures, both inter-rater and intrarater reliability were also excellent, with intraclass coefficients of 0.90 (95% CI 0.87 to 0.92) and 0.89 (95% CI 0.81 to 0.93).
Primary outcome: Swallow safety (PA scores).
There was no difference in the baseline characteristics of the EMST group compared to the sham treatment group (p = 0.881) (table 3). An interaction between time and group was identified with the use of repeated-measures analysis of covariance test (F = 10.87, p = 0.001). Mean PA scores improved for the EMST group (0.61 ± 1.43; 95% CI 0.10 to 1.11), but not the sham group (0.43 ± 1.14; 95% CI −0.82 to −0.04). Age (F = 0.64, p = 0.426), sex (F = 0.02, p = 0.894), and disease severity (F = 1.73, p = 0.193) had no significant influence on treatment effects. Cohen d = 0.55, indicating a moderate effect size when comparing experimental and sham groups post-treatment.
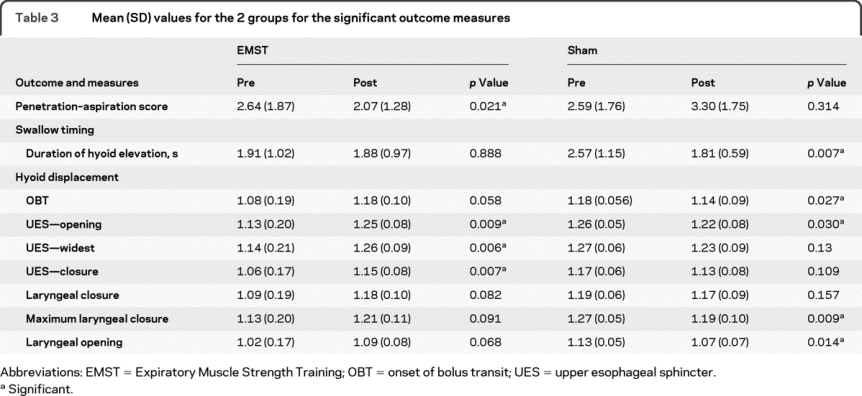
Table 3 Mean (SD) values for the 2 groups for the significant outcome measures
Eleven patients (33%) had improved PA scores following EMST as compared to 5 (14%) in the sham group; i.e., 19% of patients will have improved PA scores under EMST that will not occur under sham. Equivalently the number needed to treat to gain one additional improvement is 5.3. Considering that 3 patients (9%) had deteriorated PA scores following EMST as compared to 16 (46%) in the sham group, the number needed to treat to gain one additional benefit (improvements gained + deteriorations prevented) is 1.8 (95% CI 1.2 to 3.4).30,31
Secondary outcomes: Physiologic measures of swallow mechanism.
Table 3 shows that there was no statistical change in duration of hyoid movement over time in the EMST group but decreased significantly in the sham group post-treatment. There was no significant main effect of time. There was a time by treatment group interaction for hyoid movement duration, F = 5.388, p = 0.029 (table 3).
Time by treatment group interactions were significant for hyoid displacement at several swallow-specific events (table 3): onset of bolus transit, upper esophageal sphincter (UES) opening, UES at its widest opening, UES closure, laryngeal closure, maximum laryngeal closure, and laryngeal opening. Displacements increased (however not always to significant levels) for all events within the EMST group, but decreased (not always to significant levels) for all events in the sham group.
Swallowing quality of life measure.
There was improvement in swallowing-related quality of life secondary to treatment independent of the treatment group membership (F = 3.007, p = 0.007).
DISCUSSION
The current randomized clinical trial tested the effects of a 4-week, hypothesis-driven novel restorative treatment (EMST) for dysphagia in persons with PD. The data strongly supported the proposed hypotheses that the group receiving EMST performed superiorly compared to the sham group in both functional and physiologic measures of swallowing. An important and clinically relevant finding of this study was the significant reduction in the primary outcome variable of PA score pre- to post-EMST treatment. The presence and absence of penetration and aspiration is one of the most essential factors influencing recommendations made by speech language pathologists (e.g., diet modification, qualification for dysphagia therapy, ability to tolerate PO intake) for persons with dysphagia and has potentially critical implications for aspiration risk, which is the leading cause of death in PD.5–10
The mechanisms contributing to the improvement in PA score following EMST may be explained by further examining hyolaryngeal function. Duration of hyoid movement significantly shortened in the sham group but remained stable in the EMST group. While decreases in swallow durations have been considered desirable for overall functionality and swallow safety, researchers have recently revised their notion on swallow durations and now consider that faster swallowing is not necessarily more functional or safe.18,21,32 Rather, it may be reasonable to propose that there exists a minimum time requirement for coordination of airway protection and bolus propulsion and flow through the oropharynx and laryngopharynx.
Furthermore, the significant interactions between treatment group (EMST vs sham) and hyoid displacement at key swallowing events are enlightening when coupled with the changes found in swallow safety (i.e., PA score) after EMST. All hyoid displacement measures increased with EMST, particularly at UES-related swallowing events. Larger hyoid displacements during UES-related swallowing events should enable bolus material to flow into the upper esophagus in a relatively unobstructed manner by enabling the UES to open wider, and longer, thus resulting in better clearance of bolus material from the laryngopharynx. Reducing the presence of residue in the laryngopharynx in turn decreases aspiration risk. In the sham group, hyoid displacement decreased for all swallowing events. These results are consistent with the decrease in duration of hyoid movement observed in the sham group. The findings related to hyoid movement support the idea that improved hyolaryngeal movement should be targeted for improved swallow safety (e.g., reductions in PA).
Although participants in the EMST and sham groups showed significant differences in functional and physiologic measures of swallowing after treatment, both groups reported improvements in swallowing-related quality of life following treatment. This suggests that in addition to being an effective treatment for dysphagia, EMST is not burdensome for patients and can result in improvements to quality of life. These results together with previous reports of improvements in cough (i.e., airway clearance) with EMST in patients with multiple sclerosis, sedentary elderly, and patients with PD11–17,33 strengthen the rationale for use of this training program to induce reductions in aspiration risk.
This prospective, randomized, and blinded study of EMST on swallowing function has revealed a potentially straightforward and cost-effective therapy for reducing PA in PD. The generalization of these results is limited by the fact that overall the participants had only mild to moderately impaired swallowing. Studying the effects of EMST in a more impaired dysphagic population is of importance for assessing clinical utility of this treatment. Additionally, future studies should control patient-specific (e.g., disease severity) and dysphagia-specific (e.g., presence/absence of PA, baseline hyolaryngeal movement) domains in order to better examine the patient profiles for which EMST is most effective. For example, segregation of early vs late-stage PD may reveal the role of EMST as a preventative intervention for dysphagia. Longitudinal treatment designs may help define the durability of the treatment effect and any need for retraining or maintenance therapy, which is likely inevitable. Finally, it is essential that empirical study be completed to assess whether EMST treatment effects generalize to reductions in aspiration pneumonia in PD, where pulmonary sequelae is considered the leading cause of death.5–10
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Troche, Dr. Wheeler-Hegland, and Dr. Sapienza.
ACKNOWLEDGMENT
The authors thank the National Parkinson Foundation Center of Excellence for their support to the UF Movement Disorders Centers and the patients who took part in this study.
DISCLOSURE
Dr. Troche reports no disclosures. Dr. Okun serves on scientific advisory boards for the Dystonia Medical Research Foundation and the National Parkinson Foundation and also the Medical Advisory Board for the Tourette Syndrome Association; has received funding for travel and speaker honoraria from Medtronic, Inc. prior to 2010; has served/serves on the editorial boards of Neurology and Parkinsonism and Related Disorders; is a founder of the COMPRESS software used for deep brain stimulation (DBS) screening and has filed patents regarding double lead DBS, DBS targeting, and COMPRESS; receives royalties from the publication of Ultimate Neurology Review (DEMOS, 2007), Parkinson's Disease (Manson, 2009), and Deep Brain Stimulation for Neurological and Psychiatric Diseases (Humana Press, 2009); serves as Medical Director of the National Parkinson Foundation and as a member of the Ask the Expert Forum; and has received research support from Medtronic, Inc. (devices and training fellowship grants), the NIH (PI R21NS072897, PI R34MH080764), the University of Florida Foundation, the Parkinson Alliance, the Michael J. Fox Foundation, and the National Parkinson Foundation. Dr. Rosenbek serves on the editorial board of the Journal of Medical Speech Language Pathology; and receives royalties from the publication of Dysphagia and Movement Disorders (Plural Publishing, 2009) and Encyclopedia of Rare Dysphagias (Plural Publishing, 2010). Ms. Musson reports no disclosures. Dr. Fernandez serves on scientific advisory boards for EMD Serono, Inc. and Biogen Idec; serves as Medical Editor of the Movement Disorders Society Web Site; is a founder of the COMPRESS software used for deep brain stimulation (DBS) screening and has filed patents regarding double lead DBS, DBS targeting, and COMPRESS; has received royalties from the publication of Ultimate Review for the Neurology Boards (Demos Publishing, 2006 and 2010), A Practical Approach to Movement Disorders (Demos Publishing, 2007), Clinician's Desk Reference on Parkinson Disease (Manson Publishing, 2009), and Parkinson's Disease: A Guide to Patient Care (Springer Publishing, 2009); served as a member of the Ask The Doctor Forum; has received research support from AstraZeneca, Forest Laboratories, Inc., Novartis, Solvay Pharmaceuticals, Inc., Teva Pharmaceutical Industries Ltd., the NIH/NINDS (U10 NS53381-01 [PI]), the Huntington Study Group, the Movement Disorders Society, the Parkinson Study Group, the Society of Progressive Supranuclear Palsy, the Michael J. Fox Foundation, and the National Parkinson Foundation. Dr. Rodriguez has served on speakers' bureaus for Allergan, Inc., Valeant Pharmaceuticals International, Solstice Neurosciences, Inc., Teva Pharmaceutical Industries Ltd., Boehringer Ingelheim, and SCHWARZ PHARMA; and receives research support from Merz Pharmaceuticals, LLC and Ipsen. Ms. Romrell, Ms. Pitts, and Dr. Wheeler-Hegland report no disclosures. Dr. Sapienza serves on a scientific advisory board for and has potential royalty interest in Aspire Products, LLC; has received speaker honoraria from the American Speech-Language-Hearing Association; serves as an Associate Editor of Logopedics and Phoniatrics; receives royalties from the publication of Voice Disorders (Plural Publishing, Inc., 2009); and receives research support from the US Veterans Administraion, the NIH (NIDCHD HD046903-01A112/0 [PI]), CurePSP, and the Michael J. Fox Foundation.
DISCLAIMER
The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government. This material is based on work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development Service.
Address correspondence and reprint requests to Dr. Michelle S. Troche, PO Box 117420, University of Florida, Gainesville, FL 32611 michi81@ufl.edu
Study funding: Supported by the VA Rehab R&D (B3721R to C.S.) and the Michael J. Fox Foundation (00056150).
Disclosure: Author disclosures are provided at the end of the article.
Received February 15, 2010. Accepted in final form August 9, 2010.
REFERENCES
- 1.Hamdy S, Aziz Q, Rothwell J, et al. The cortical topography of human swallowing musculature in health and disease. Nat Med 1996;2:1217–1224. [DOI] [PubMed] [Google Scholar]
- 2.Martin RE, Sessle BJ. The role of the cerebral cortex in swallowing. Dysphagia 1993;8:195–202. [DOI] [PubMed] [Google Scholar]
- 3.Mosier K, Patel R, Liu W, Kalnin A, Maldjian J, Baredes S. Cortical representation of swallowing in normal adults: functional implications. Laryngoscope 1999;109:1417–1423. [DOI] [PubMed] [Google Scholar]
- 4.Zald DH, Pardo JV. The functional neuroanatomy of voluntary swallowing. Ann Neurol 1999;46:281–286. [PubMed] [Google Scholar]
- 5.Fernandez HH, Lapane LK. Predictors of mortality among nursing home residents with a diagnosis of Parkinson's disease. Med Sci Monit 2002;8:CR241–246. [PubMed] [Google Scholar]
- 6.Gorell JM, Johnson CC, Rybibki BA. Parkinson's disease and its comorbid disorders: an analysis of Michigan mortality data, 1970 to 1990. Neurology 1994;44:1865–1868. [DOI] [PubMed] [Google Scholar]
- 7.Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology 1967;17:427–442. [DOI] [PubMed] [Google Scholar]
- 8.Schiermeier S, Schafer T, Greulich W, Schlafke ME. Breathing and locomotion in patients with Parkinson's disease. Pflugers Arch 2001;443:67–71. [DOI] [PubMed] [Google Scholar]
- 9.Shill H, Stacy M. Respiratory function in Parkinson's disease. Clin Neurosci 1998;5:131–135. [PubMed] [Google Scholar]
- 10.Singer RB. Mortality in patients with Parkinson's disease treated with dopa. J Insur Med 1992;24:126–127. [PubMed] [Google Scholar]
- 11.Baker S, Davenport P, Sapienza C. Examination of strength training and detraining effects in expiratory muscles. J Speech Lang Hear Res 2005;48:1325–1333. [DOI] [PubMed] [Google Scholar]
- 12.Chiara T, Martin D, Davenport P, Bolser D. Expiratory muscle strength training in persons with multiple sclerosis having mild to moderate disability: effect on maximal expiratory pressure, pulmonary function, and maximal voluntary cough. Arch Phys Med Rehabil 2006;87:468–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiara T, Martin D, Sapienza C. Expiratory muscle strength training: speech production outcomes in patients with multiple sclerosis. Neurorehabil Neural Repair 2007;21:239–249. [DOI] [PubMed] [Google Scholar]
- 14.Saleem AF, Sapienza CM, Okun MS. Respiratory muscle strength training: treatment and response duration in a patient with early idiopathic Parkinson's disease. Neurorehabilitation 2005;20:323–333. [PubMed] [Google Scholar]
- 15.Wheeler KM, Chiara T, Sapienza CM. Surface electromyographic activity of the submental muscles during swallow and expiratory pressure threshold training tasks. Dysphagia 2007;22:108–116. [DOI] [PubMed] [Google Scholar]
- 16.Sapienza CM, Davenport PW, Martin D. Expiratory muscle training increases pressure support in high school band students. J Voice 2002;16:495–501. [DOI] [PubMed] [Google Scholar]
- 17.Wingate JM, Brown W, Shrivastav R, Davenport P, Sapienza C. Treatment outcomes for professional voice users. J Voice 2007;21:433–449. [DOI] [PubMed] [Google Scholar]
- 18.Schultz JL, Perlman AL, VanDaele DJ. Laryngeal movement, oropharyngeal pressure, and submental muscle contraction during swallowing. Arch Phys Med Rehabil 1994;75:183–188. [PubMed] [Google Scholar]
- 19.Kendall KA, Leonard RJ. Pharyngeal constriction in elderly dysphagic patients compared with young and elderly nondysphagic controls. Dysphagia 2001;16:272–278. [DOI] [PubMed] [Google Scholar]
- 20.Kendall KA, Leonard RJ. Hyoid movement during swallowing in older patients with dysphagia. Arch Otolaryngol Head Neck Surg 2001;127:1224–1229. [DOI] [PubMed] [Google Scholar]
- 21.Kendall KA, Leonard RJ. Bolus transit and airway protection coordination in older dysphagic patients. Laryngoscope 2001;111:2017–2021. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbek JC, Robbins J, Roecker EV, Coyle JL, Woods JL. A penetration-aspiration scale. Dysphagia 1996;11:93–98. [DOI] [PubMed] [Google Scholar]
- 23.McHorney CA, Bricker DE, Kramer AE, et al. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: I: conceptual foundation and item development. Dysphagia 2000;15:115–121. [DOI] [PubMed] [Google Scholar]
- 24.McHorney CA, Martin-Harris B, Robbins J, Rosenbek J. Clinical validity of the SWAL-QOL and SWAL-CARE outcome tools with respect to bolus flow measures. Dysphagia 2006;21:141–148. [DOI] [PubMed] [Google Scholar]
- 25.McHorney CA, Robbins J, Womax K, et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III: documentation of reliability and validity. Dysphagia 2002;17:97–114. [DOI] [PubMed] [Google Scholar]
- 26.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Davenport P, Sapienza CM. Effect of expiratory muscle strength training on elderly cough function. Arch Gerontol Geriatr 2008;48:361–366. [DOI] [PubMed] [Google Scholar]
- 29.Wheeler KM, Shrivastav R, Sapienza CM. An innovative method of measuring hyoid movement. Presented at the Dysphagia Research Symposium; March 2006; Scottsdale, AZ.
- 30.Bender R. Calculating confidence intervals for the number needed to treat. Control Clin Trials 2001;22:102–110. [DOI] [PubMed] [Google Scholar]
- 31.Froud R, Eldridge S, Lall R, Underwood M. Estimating number needed to treat from continuous outcomes in randomized controlled trials: methodological challenges and worked example using data from the UK back pain exercise and manipulation (BEAM) trial. BMC Med Res Meth 2009;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kendall KA. Oropharyngeal swallowing variability. Laryngoscope 2002;112:547–551. [DOI] [PubMed] [Google Scholar]
- 33.Pitts T, Bolser D, Rosenbek JC, Troche M, Okun MS, Sapienza C. Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest 2009;135:1301–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin-Harris B, Brodsky MB, Michel Y, Ford CL, Walters B, Heffner J. Breathing and swallowing dynamics across the adult lifespan. Arch Otolaryngol Head Neck Surg 2005;131:762–770. [DOI] [PubMed] [Google Scholar]