Abstract
The Argonaute proteins play essential roles in development and cellular metabolism in many organisms, including plants, flies, worms, and mammals. Whereas in organisms such as Caenorhabditis elegans and Arabidopsis thaliana, creation of Argonaute mutant strains allowed the study of their biological functions, in mammals the application of this approach is limited by its difficulty and in the specific case of Ago2 gene, by the lethality of such mutation. Hence, in human cells, functional studies of Ago proteins relied on phenotypic suppression using small interfering RNA (siRNA) which involves Ago proteins and the RNA interference mechanism. This bears the danger of undesired or unknown interference effects which may lead to misleading results. Thus, alternative methods acting by different regulatory mechanisms would be advantageous in order to exclude unspecific effects. The knockdown may be achieved by using specific antisense oligonucleotides (asONs) which act via an RNase H-dependent mechanism, not thought to interfere with processes in which Agos are involved. Different functional observations in the use of siRNA versus asONs indicate the relevance of this assumption. We developed asONs specific for the four human Agos (hAgos) and compared their activities with those obtained by siRNA. We confirm that hAgo2 is involved in microRNA (miRNA)- and in siRNA-mediated silencing pathways, while the other hAgos play a role only in miRNA-based gene regulation. Using combinations of asONs we found that the simultaneous down-regulation of hAgo1, hAgo2, and hAgo4 led to the strongest decrease in miRNA activity, indicating a main role of these proteins.
Keywords: human Ago proteins, antisense oligonucleotide, RNAi, miRNA, siRNA
INTRODUCTION
Argonaute proteins play important regulatory roles in the metabolism of cells and are involved in RNA interference (RNAi) mechanisms (Peters and Meister 2007; Höck and Meister 2008; Hutvagner and Simard 2008). They are basic proteins, able to bind short double-stranded RNA (Liu et al. 2004; Meister et al. 2004; Farazi et al. 2008).
The members of the Argonaute family are highly conserved among different organisms, from the fly to human. Two subfamilies are currently distinguished: the PIWI (P-element-induced wimpy testis) proteins and the Ago proteins. The PIWI-subfamily members are expressed only in germ-line cells and interact with piRNA, but their cellular functions are not yet clear (Sasaki et al. 2003; Meister et al. 2004; Aravin and Bourc'his 2008; Klattenhoff and Theurkauf 2008). In contrast, the Ago-subfamily is ubiquitously expressed and its members play key roles in the regulation of gene expression. They bind small interfering RNA (siRNA) and micro RNA (miRNA), leading to gene silencing of specific transcripts via inducing cleavage of the target mRNA or inhibition of its translation (Meister 2007; Eulalio et al. 2008; Filipowicz et al. 2008).
Human cells express four Ago proteins (hAgo1–hAgo4), and four PIWI proteins, called HIWI1, HIWI2, HIWI3, and HILI. Among all four Ago proteins, only Ago2 shows slicing activity and, as the major component of the RNA-induced silencing complex (RISC), it catalyzes the siRNA-induced cleavage of target mRNA (Liu et al. 2004; Meister et al. 2004). Interestingly, hAgo3 also carries a catalytic triad (DDH) such as hAgo2, but no catalytic activity was observed in vitro (Liu et al. 2004; Meister et al. 2004).
In organisms such as Drosophila melanogaster or Caenorhabditis elegans Ago proteins are functionally distinct (Hutvagner et al. 2004; Okamura et al. 2004; Vaucheret et al. 2004; Förstemann et al. 2007). Conversely, in mammalian cells the roles and the functional discrimination of the different Ago proteins are not well understood. Recently, Su et al. (2009) showed that knockout of mouse Ago1–Ago4 was related to the inefficiency of cells to perform miRNA-mediated gene silencing and stimulated their programmed death, while the reintroduction of any single Ago variant into these cells was able to rescue the wild-type phenotype. This indicated that all four Agos are involved in miRNA-mediated silencing and possess overlapping roles in this process in mice.
Although to date no structural data are available for the human Ago proteins, homology-based modeling with the structures of the Ago proteins of Thermus thermophilus (Wang et al. 2008, 2009), Aquifex aeolicus (Yuan et al. 2005, 2006; Rashid et al. 2007), and Pyrococcus furiosus (Song et al. 2004) provided new insights into the structure–function relationship of these proteins. These studies, in combination with biochemical approaches, allowed the identification of functional key residues on Ago proteins (Jinek and Doudna 2009) and of post-translational modification sites such as phosphorylation at S387, important for localization of hAgo2 to processing bodies (P-bodies) (Zeng et al. 2008) or prolyl 4-hydroxylation at P700, which is involved in the regulation of hAgo2 stability (Qi et al. 2008).
Further information on hAgos was derived from immunoprecipitation assays that allowed the detection by mass spectrometry analysis of several hAgo-associated proteins including Dicer and Decapping enzymes (DCP1 and DCP2) (Meister et al. 2005; Peters and Meister 2007) by microarray approaches of bound mRNA species (Landthaler et al. 2008) and by extensive sequencing of Ago-associated miRNA (Azuma-Mukai et al. 2008). These studies showed that hAgo complexes share a very similar proteomic composition, similar mRNA and miRNA-binding profiles, further demonstrating functional overlap among the different human Agos. These data, while providing important insights about hAgo-binding partners, also highlight the complexity of RNAi and other cellular processes in which these proteins are involved. Nevertheless, in vitro studies showed that a minimal RISC, composed only of hAgo2 and siRNA, is sufficient to induce cleavage of the substrate mRNA (Rivas et al. 2005), while for its loading with siRNA, two additional proteins are essential, i.e., Dicer and TRBP (MacRae et al. 2008).
So far, functional analysis of human Ago proteins made use of RNAi-based tools for their down-regulation or recombinant tagged-Agos for their overexpression and for tethering-based studies (Meister et al. 2004; Schmitter et al. 2006; Diederichs et al. 2008; Wu et al. 2008). Both approaches gave new insights into the involvement of these proteins in the RNAi pathway, though they are limited by several factors. The first strategy is based on siRNA, which are binding partners of these proteins and involved in the RNA-silencing pathway. By suppressing the expression of hAgos using these tools, the obvious consequence is a dying out of the silencing effect, which could lead to interference between observed functional phenotypes and the mechanism of inhibition. Restrictions of the second method include changes in protein expression, interactions and functionality due to tags, limited availability of cellular Ago cofactors, and interacting proteins, which could cause saturation of endogenous pathways and shifting of cellular equilibriums toward artificial systems.
Here we describe the design, characterization, and application of antisense oligonucleotides (asONs) as non-RNAi-based tools for the knockdown of human Ago expression on the mRNA and protein levels, and compared their activity with an RNAi-based approach. AsONs make use of RNaseH-based degradation of target mRNA (Dias and Stein 2002), which is distinct from the RNAi pathway and does not interfere with RNAi or its constituents. Using combinations of specific asONs we investigated functional relations among the hAgos in the miRNA- and siRNA-induced RNAi pathways.
RESULTS
AsONs down-regulate human Ago proteins with high efficiency and selectivity
For the identification of asONs that specifically target hAgo gene expression, we designed 31 sequences that were tested in cell culture for efficacy, half maximal inhibitory concentrations (IC50), and selectivity (Supplemental Table S1). Four asONs directed against hAgo1, hAgo2, hAgo3, or hAgo4 were identified (Supplemental Table S2) and chosen for further investigations.
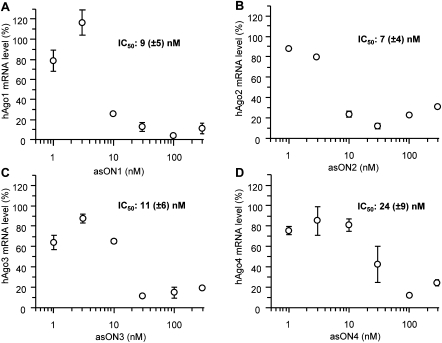
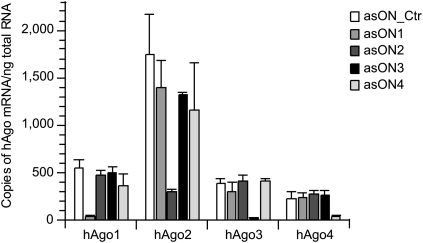
First, we analyzed the efficiency of asONs in suppressing their cognate target RNA (Fig. 1). Human endothelial cells (ECV304) were transfected with increasing amounts of asON, 24 h later total RNA was extracted, and the quantity of hAgo mRNA determined via reverse transcription–quantitative PCR (RT–qPCR) with target-specific primers. As control, an asON (asON_Ctr) was used that had no endogenous target and which was modified by phosphorothioate like the tested asONs. Dose-response curves showed that all asONs suppressed their target mRNA with high efficiency (Fig. 1A–D), whereas the asON_Ctr had no effect. The IC50 values of the four different specific asONs were in the low nanomolar range, demonstrating a similar efficacy of all asONs. Cytotoxicity tests have revealed that up to 1000 nM asON no-toxic side effects were seen (Supplemental Fig. 1). Therefore, in further experiments a concentration of 100 nM was used for each asON. The absolute quantification of hAgo transcripts in ECV304 cells was achieved by using standard curves and showed that the four hAgos are accumulated at different levels (Fig. 2), with the hAgo2 transcript being the most abundant, followed by hAgo1 and hAgo3 mRNAs that have similar levels, whereas hAgo4 mRNA showed the lowest amount. There was no unspecific knockdown observed for any other hAgo mRNA, proving the selectivity of all hAgo-directed asONs for their specific target mRNA (Fig. 2). For comparison, we tested hAgos-specific siRNA (siAgo1-4) designed by Meister et al. (2004), which showed similar efficiency in down-regulating their target mRNA (Supplemental Fig. 2).
FIGURE 1.
The selected asONs show a strong inhibitory effect on hAgo mRNA expression. Dose-response curves of asON1, which is specific for hAgo1 mRNA (A), asON2, which is directed against hAgo2 mRNA (B), asON3, which was designed for hAgo3 mRNA (C), and asON4, which was selected for hAgo4 mRNA (D). The IC50 determination was performed transfecting ECV304 cells with increasing amounts of asON (0–300 nM). Cells were collected 24 h after transfection and the total RNA was isolated and reverse transcribed. The cDNA was quantified by RT–qPCR and a housekeeping gene, β glucuronidase, was used as internal control. Indicated are the hAgo mRNA levels and the IC50 values, which were calculated by using GraFit5 program. As an additional control, cells were transfected with an asON (asON_Ctr) that has no endogenous target, and the hAgo mRNA level was set at 100%. Indicated are mean values and standard deviations.
FIGURE 2.
The asONs show a high selectivity toward their target mRNA. ECV304 cells were transfected with 100 nM asON, then 24 h after transfection, cells were collected and the total RNA was isolated and reverse transcribed. The cDNA was quantified by RT–qPCR and hAgo-specific standard curves were used for absolute quantification of hAgo mRNA. Indicated are copies of hAgo mRNA per nanogram total RNA. As control, asON_Ctr was used, which has no endogenous target, and a fully modified phosphorothioate backbone. Indicated are mean values and standard deviations.
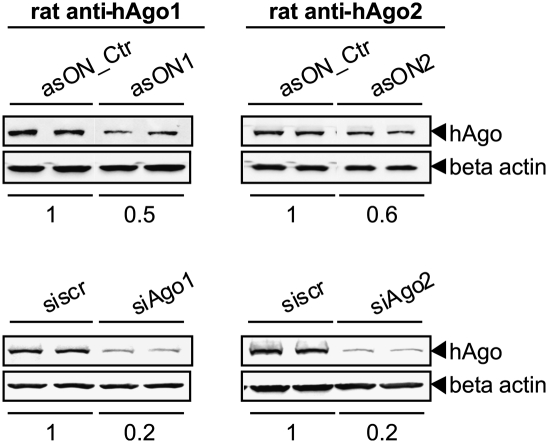
For functional studies and phenotype-based assays, gene expression needs to be regulated not only at the transcript but also at the protein level. We therefore investigated the effect of asONs and siAgos on the cellular amounts of hAgo1 and hAgo2 proteins by Western blot analysis of cell lysates obtained 48 h after transfection (Fig. 3). A depletion of human Ago proteins up to 50%–60% by the asONs and up to 20% by the siRNA was observed. The very low cellular concentration of hAgo3 and the lack of specific antibodies against hAgo4 did not permit this analysis for these proteins.
FIGURE 3.
Down-regulation of hAgos at the protein level by specific asONs or siAgos. ECV304 cells were transfected with 100 nM asON or siAgo (specific for hAgo1, [left] or for hAgo2, [right]). As controls, 100 nM asON_Ctr or siscr were used. After 48 h, the cells were collected, lysed, and a Western blot analysis was performed using rat-anti human Ago1 and Ago2 antibodies. The hAgo proteins were seen as bands with a molecular weight of ∼100 kDa. As loading control, β actin was detected, which is shown at the bottom. The ratio between hAgo protein and β actin was normalized to asON_Ctr or siscr, and the resulting values are indicated at the bottom of the blots.
Time-course experiments were also performed in order to determine the time point at which the protein expression reached its minimum (Supplemental Fig. 3). The asON1 and asON2 showed a slow, but measurable suppression of hAgo1 and hAgo2, respectively, reaching its maximal effect 48 h after transfection.
In summary, our results showed that the developed asONs were specific for their individual target sequences, and upon transfection led to a decrease of mRNA transcripts and protein levels, allowing functional analysis of hAgos.
Human Ago proteins play important roles in the miRNA-mediated silencing pathway
We investigated the effect of hAgo down-regulation by using asONs and siAgos on miRNA-regulated gene silencing. For this purpose we selected a miRNA-based system in which suppression of the target gene could be measured at the protein level by an enzymatic assay. The target gene chosen was Renilla luciferase carrying a mutated 3′UTR of the high-mobility group AT-hook 2 gene (RL-Hmga2m7) (Mayr et al. 2007). This construct contains seven binding sites for a miRNA “mutated let-7a” (mlet-7a), which does not occur naturally but behaves like its wild-type counterpart, allowing an unbiased analysis of the miRNA-induced phenotype.
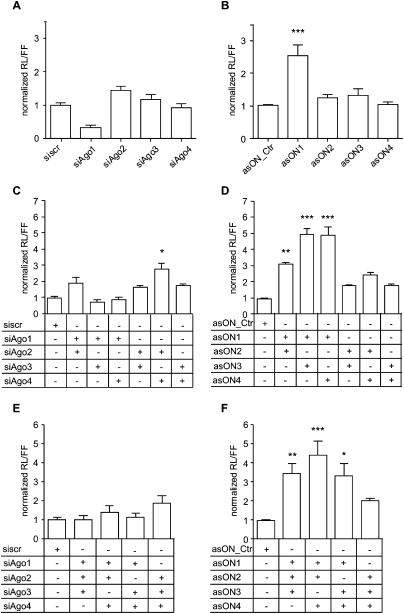
ECV304 cells were transfected with hAgo-specific asONs or siAgos, and after 24 h from the first transfection with mlet-7a and its target RL-Hmga2m7 (Fig. 4). The down-regulation of hAgo2 and hAgo3 using single siAgos (Fig. 4A) led, in each case, to derepression of the reporter gene. On the contrary, for hAgo1, the treatment with siRNA showed an increased miRNA-based activity. After transfection with asONs (Fig. 4B), this effect was not seen for hAgo1. Instead, a 2.5-fold release of the miRNA effect in asON1-treated cells indicated a main role in this pathway for hAgo1.
FIGURE 4.
Human Ago proteins play important roles in miRNA-mediated gene silencing. ECV304 cells were transfected with 100 nM asON or siAgo, then 24 h later with 100 nM mlet-7a, RL-Hmga2m7, and pGL3-control. The day after cells were collected, the Renilla luciferase (RL) and firefly luciferase (FF) activities were measured, and the ratio RL/FF was built. Every experiment was performed in triplicate and values were normalized to asON_Ctr or siscr, which were set at 1. Data were then compared using Friedman test and the Program Prism 4 (GraphPad Software) (n = 9–15). Indicated are mean values ±SEM; *P < 0.05, **P < 0.01, ***P < 0.001. (A,B) Effect of single hAgo depletion via siAgo (A) or asON (B) on the miRNA-mediated silencing pathway. (C,D) Effect of cosuppression of two hAgos on the miRNA-mediated silencing pathway. ECV304 cells were transfected with 100 nM each siAgos (C) or asON (D) (asON_Ctr or siscr in a concentration of 200 nM), then 24 h later with mlet-7a, RL-Hmga2m7, and pGL3-control as described. (E,F) Effect of down-regulation of three hAgos on the miRNA-mediated silencing pathway. ECV304 cells were transfected with 100 nM each siAgo (E) or asON (F) (asON_Ctr or siscr in a concentration of 300 nM).
Next, we investigated the effect of simultaneous cosuppression of two (Fig. 4C,D) and three (Fig. 4E,F) different hAgo species on the RNAi-based gene-silencing processes. The efficiency of asONs or siAgos combinations in suppressing hAgos was tested at the mRNA (Supplemental Fig. 4) as well as at the protein levels (Supplemental Fig. 5).
For siAgos, the strongest derepression of the target gene was observed after codown-regulation of hAgo2 and hAgo4 (threefold), whereas only a minor effect was observed for the other combinations (Fig. 4C). A different result was obtained using asONs, of which all combinations resulted in a release (two- to sixfold) of the miRNA inhibition (Fig. 4D). The strongest effects were observed when hAgo1 and hAgo3 or hAgo4 were cosuppressed, followed by hAgo2 in combination with hAgo1.
Finally, after cotransfection with different combinations of three siAgos (Fig. 4E), the extent of target derepression was lower than using asONs and comparable to the effect seen with a single siAgo. On the contrary, in cells treated with asONs we observed for all combinations an increase in target gene expression, especially upon cosuppression of hAgo1, hAgo2, and hAgo4 (Fig. 4F).
The fact that the absolute values for the control asON_Ctr and the siscr were very similar in every experiment, allowed a direct comparison of the results. For increasing numbers of hAgos suppressed by asONs we have observed an additive effect, whereas this was not the case for siAgos, thus indicating that asONs can be used in combinations for studying functional relations among hAgos.
Human Ago2 is the key protein in the siRNA-mediated gene silencing
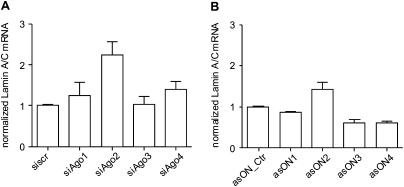
To study the effect of hAgo knockdown on the siRNA-mediated pathway, we used the siRNA siLam that targets the endogenously transcribed Lamin A/C transcript (Elbashir et al. 2001) and measured suppression of the target gene at the mRNA level via RT–qPCR (Fig. 5). The ECV304 cells transfected first with hAgo-specific asONs or siAgos and with siLam the following day were collected for total RNA extraction after 48 h from the first transfection.
FIGURE 5.
Effect of the down-regulation of hAgos on siRNA-based gene regulation. A day after the transfection of ECV304 cells with 100 nM siAgo (A) or asON (B), a second transfection was performed with 100 pM siLam. Then, 48 h after the first transfection, cells were collected, the total RNA was isolated, reverse transcribed, and the cDNA was quantified by RT–qPCR. Values of the housekeeping gene β glucuronidase were used for the normalization of the data. As controls, asON_Ctr and siscr were used and set at 1. Indicated are mean values ±SEM (n = 4-6).
Down-regulation by siAgos or asONs showed that only hAgo2 is involved in siRNA-mediated gene suppression (Fig. 5). This result was predictable, since hAgo2 is the only one carrying a slicing activity (Liu et al. 2004; Meister et al. 2004). Accordingly, the codown-regulation of hAgos showed that the siRNA pathway is highly affected by hAgo2 down-regulation, regardless of the cosuppressed hAgo species (data not shown).
Taken together, our test systems showed that only hAgo2 is involved in the siRNA-mediated pathway, while the miRNA-based silencing is a more complex process that also requires the intervention of other noncatalytically active Ago proteins, clearly highlighting major differences between the miRNA and siRNA pathways.
On the technical level, this study shows that the asON designed here are suited for functional studies of human Agos.
DISCUSSION
Strategies currently used for studying the role of individual genes and gene products involved in the RNAi process are based on gain-of-function by overexpressing the protein of interest (Meister et al. 2004; Diederichs et al. 2008), or on loss-of-function via siRNA-mediated knockdown, or by knocking out of entire genes when possible (Meister et al. 2004; Lykke-Andersen et al. 2008; Su et al. 2009). In the human system, the established methods might be of limited use due to several observations, including saturation of endogenous pathways, functional hindrance due to tags, or fading silencing effects.
Here, we demonstrated that an alternative and more unbiased asON-based approach allows one to study the biology of the different hAgo species. We showed that the antisense sequences designed and used in this study have similar IC50 values, which are on the order of a few nanomolar (Fig. 1). Here, a first-generation chemistry was used, i.e., all-phosphorothioate-modified oligonucleotide; thus, it seems to be warranted to speculate on an even increased asON potency in the use of more advanced designs and chemistries for asON including, for example, a gapmer design (Grünweller and Hartmann 2007) or the LNA- and 2′-O-alkyl modifications (Kurreck 2003; Jepsen and Wengel 2004; Wilson and Keefe 2006).
Despite the high-sequence homology among all four hAgos, the combination of sequence alignment and predictions of mRNA secondary structure allowed us to generate tools that are selective for their target transcript (Fig. 2).
The mechanism of action of the asONs used here is thought to be based on their specific hybridization with matching transcripts, followed by recognition of the DNA–RNA duplex by the cellular RNaseH enzyme and cleavage of the RNA (Dias and Stein 2002).
Comparison between asONs and siAgos showed that for hAgo1 and hAgo2 the same extent of transcript inhibition led to different repression at the protein levels (Fig. 3), 50% and 20%, respectively. However, results of the functional assays demonstrated that an inhibition level of 50% was correlated with significant changes in the phenotype. Effects on hAgo3 and hAgo4 could be tested only at the mRNA level due to low cellular expression and lack of suited antibodies, respectively. Though, based on the phenotype observed in functional assays and on the level of inhibition of the target transcripts, we assume that upon specific asON or siAgo treatment, hAgo3 and hAgo4 protein levels would also be reduced to the same extent as we have observed for the others.
The efficiency and selectivity of these asONs toward their targets and their RNAi-independent inhibition mechanism make them suited and useful tools for the functional analysis of hAgo proteins.
Studies in mice provided important information on the four Ago proteins and suggested essential and overlapping roles of these proteins in regulating gene expression and in development (Lykke-Andersen et al. 2008; Su et al. 2009). In humans, immunoprecipitation assays showed that the four hAgo proteins associate with siRNA and miRNA independently of their sequence (Meister et al. 2004; Azuma-Mukai et al. 2008). Down-regulation of hAgo2 and hAgo3 using single siAgos suggested a role of these proteins in the miRNA-mediated pathway, while inhibition of hAgo1 was shown to enhance the miRNA activity (Fig. 4A). Hence, using asONs, we found that a key role in this pathway is played by hAgo1 (Fig. 4B). Its suppression leads to the strongest release of the miRNA-based silencing effect when compared with the other hAgos. Since the asONs tools are not thought to interfere with the natural hAgo pathways, discrepancies between the effects of asON1 or siAgo1 on miRNA function may be due to interference between the siRNA inhibitor and the investigated pathway. This highlights the complexity of the RNAi pathway and suggests a potential for each hAgo to functionally substitute each other.
Upon cosuppression by asONs of hAgo1 with every other hAgo, ECV304 cells showed a reduced miRNA-mediated silencing of the reporter gene (Fig. 4D,F). These results suggest that hAgos work in a concerted way to achieve gene silencing, and that their functional separation, which initially appeared to be partially lost in mammals (Meister et al. 2005; Peters and Meister 2007; Azuma-Mukai et al. 2008; Landthaler et al. 2008; Su et al. 2009), might indeed still be present. Down-regulation of hAgos by siAgos showed instead that hAgo2 in combination with hAgo3 or hAgo4 seems to be relevant in the miRNA-mediated gene silencing (Fig. 4C,E).
Notably, asONs can be used in combination allowing the study of functional relations among hAgos, while combining siAgos decreased the amplitude of the observed phenotypes.
Among the four hAgos only hAgo2 shows in vitro slicing activity by which target transcripts are cleaved (Liu et al. 2004; Meister et al. 2004). This can be achieved only when hAgo2 is loaded with a short RNA that is fully complementary to mRNA. Naturally, in mammals, this phenomenon does not occur very frequently and only a few miRNA were described to match completely to their targets inducing their cleavage, such as miR-196, which is involved in homeobox gene regulation (Yekta et al. 2004). Upon asON- and siAgo-based down-regulation of hAgos, we observe release of the siRNA-mediated gene silencing only in asON2- or siAgo2-treated cells, indicating that only hAgo2 can induce cleavage of the target mRNA (Fig. 5) and confirming the previous data obtained in vitro (Liu et al. 2004; Meister et al. 2004). Interestingly, reduction of hAgo1, hAgo3, and hAgo4 enhance siRNA efficiency upon asON-based knockdown (Fig. 5B), which might be due competition between these hAgos and hAgo2. This could be explained by the affinity of hAgos for short RNA, which may lead to binding and trapping of the siRNA, with consequent reduction of their cellular availability.
In summary, the asONs developed in this study allow the selective study of individual hAgo species without alteration of the others and do not introduce bias into the functional analysis of these proteins, such as the tested siAgos. Further experiments might increase the understanding of these proteins and their biological roles. Beside their functions in the RNAi process, hAgos were shown to play a role in transcriptional silencing (Janowski et al. 2006; Kim et al. 2006, 2008) and in miRNA maturation (Diederichs and Haber 2007). Additionally, hAgo2 was shown to shuttle between nucleus and cytoplasm (Ohrt et al. 2008) and new interaction partners for hAgos are increasingly identified (Lazzaretti et al. 2009; Takimoto et al. 2009; Weinmann et al. 2009), highlighting the complexity and the multiplicity of the processes in which these proteins are involved.
MATERIALS AND METHODS
Design of asONs
The asONs were designed using an established methodology that contains a systematic computational analysis of local structures of target RNA (NM_012199.2, NM_012154.2, NM_024852.2, NM_017629.2) and considers rules for the positioning of the asON along the identified favorable local target motifs (Patzel et al. 1999; Kretschmer-Kazemi Far et al. 2001).
Oligonucleotides and plasmids used in this study
The antisense sequences designed and used in this study have a fully modified phosphorothioate-backbone (asON_Ctrl 5′-TACCGCTCTTTTGACTTTTA; asON1 5′-GATCTTAGGGATGTCCACCTC; asON2 5′-GTGTTTTGTGTTGCTTTCACTCTC; asON3 5′-AGAAAAATGAACGCCCCACA; asON4 5′-CAGGCAAAGATTGGAAAGGG). The primers used for RT–qPCR experiments are: Fwd-hAgo1 5′-GACCTCCGCACGGGTATATG and Rev-hAgo1 5′-GGTTTCCCCACAGTGCCAAT; Fwd-hAgo2 5′-TGGTTTGGCTTCCATCAGTCC and Rev-hAgo2 5′-CCTTGTAAAACGCTGTTGCTGAC; Fwd-hAgo3 5′-ACCTGTGGGGCGTTCATTT and Rev-hAgo3 5′-GCCGAACAGACTGATGGAAT; Fwd-hAgo4 5′-CTCACCTCAAACCCTTTCCAATCTT and Rev-hAgo4 5′-AGGCTGCTGGAACACCGAG; Fwd-Lamin A/C 5′-AATGATCGCTTGGCGGTCTA and Rev-Lamin A/C 5′-GCCCTGCGTTCTCCGTTT and Fwd-β glucuronidase 5′-TTTGGAATTTTGCCGATTTCAT and Rev-β glucuronidase 5′-GCCGAGTGAAGATCCCTTT). Primers and asONs used in this study were purchased from Biomers with HPLC grade purification.
The RNA sequences used for experiments in cell culture are siLam (antisense: 5′-UGUUCUUCUGGAAGUCCAGtt; sense 5′-CUGGACUUCCAGAAGAACAtt), siscr (5′-CGAACUCACUGGUCUGACCtt; 5-GGUCAGACCAGUGAGUUCGtt), mlet-7a (antisense: 5′-UGCGUUAGUAGGUUGUAUAGUUU; sense: 5′-ACUAUACAAUCUACUGGCGUUCC), and the siRNA directed against hAgos were described previously (Meister et al. 2004) (siAgo1 antisense: 5′-GAGAAGAGGUGCUCAAGAAUU, sense: 5′-UUCUUGAGCACCUCUUCUCUU; siAgo2 antisense: 5′-GCACGGAAGUCCAUCUGAAUU, sense: 5′-UUCAGAUGGACUUCCGUGCUU; siAgo3 antisense: 5′-GAAAUUAGCAGAUUGGUAAUU, sense: 5′-UUACCAAUCUGCUAAUUUCUU; siAgo4 antisense: 5′-GGCCAGAACUAAUAGCAAUUU, sense: 5′-AUUGCUAUUAGUUCUGGCCUU). The siRNA were purchased from IBA with HPLC grade purification.
The Renilla luciferase reporter used in this study (RL-Hmga2m7) was previously described (Mayr et al. 2007) and carries seven binding sites for the mlet-7a. Transfection efficiency was determined by cotransfecting the reporter RL gene with a control plasmid pGL3-Control encoding the firefly luciferase (Promega).
Cell culture and transfection
ECV304 cells were transfected using 5 μg/mL of Lipofectamine2000 (Invitrogen) in 12-well plates (150,000 cells/well) with 0–300 nM asON or siAgo (400 μL final volume of the transfection solution) following the manufacturer's instructions. Cells were collected after 24 or 48 h and lysed with lysis buffer (1xPBS containing 1% Nonidet P-40). Cell lysates were stored at −80°C for Western blot analysis or total RNA extraction. For functional assay of hAgo proteins, 24 h after transfection with asONs or siAgo as described above, cells were again transfected with 100 ng of RL-Hmga2m7, 10 ng of pGL3-Control, 100 nM mlet-7a for the miRNA-regulated system, or only with 100 pM siLam for the siRNA-regulated pathway as previously described (Detzer et al. 2009). The day after, cells were collected and washed twice with 1xPBS. Cell pellets were frozen in liquid nitrogen and then stored at −80°C.
Luciferase assay and Western blot analysis
Cell pellets were resuspended in lysis buffer (Promega) and firefly, and Renilla luciferase activities were measured with the Dual-luciferase assay (Promega) following the manufacturer's instructions. To control for transfection efficiency, the ratio between the Renilla activity and the firefly activity was calculated. Then, values obtained from cells treated with asON1-4 or siAgo1-4 were normalized to those of the reporter obtained from cells transfected with asON_Ctrl or siscr.
For Western blot analysis, total protein content in cell lysates was quantified by noninterfering protein assay (Cell Concepts), separated by 10% SDS-PAGE, and then transferred onto PVDF membrane (Millipore). Membranes were blocked for 1 h in 5% [w/v] skim milk (Roth) in TBS-T (10 mM Tris-HCl at pH 7.6; 150 mM NaCl; 0.25% [v/v] Tween-20) and then incubated overnight at 4°C with primary antibody diluted in 3% [w/v] skim milk in TBS-T. Blots were washed with TBS-T and incubated with the HRP-conjugated secondary antibody for 1 h at room temperature. The antibody solution was removed, the membranes washed with TBS-T, and then incubated with ECL substrate (Pierce). Chemiluminescent signals were detected by FUSION-SL camera (Vilber Lourmat). The rat-anti hAgo1 used in this study, which was kindly provided by G. Meister (Rüdel et al. 2008), and rat-anti hAgo2 (Ascenion) were diluted 1:10 [v/v]. Commercial antibodies additionally used include rabbit-anti β actin antibody purchased from Abcam (dilution 1:10,000 [v/v]), goat-anti rat IgG from Jackson Immuno Research (dilution 1:5,000 [v/v]) and goat-anti rabbit IgG from Dako (dilution 1:2,000[v/v]).
RNA extraction and RT–qPCR
For total RNA extraction, cells were harvested, washed twice with 1xPBS, and lysed with 1xPBS containing 1% Nonidet P-40. The RNA was extracted using phenol/chloroform and incubated with Turbo DNase I (Ambion) for 1 h at 37°C. Total RNA was again purified by phenol/chloroform extraction, followed by ethanol precipitation, and quantified by Nanodrop spectrophotometer (Thermo Scientific). Reverse transcription was performed using a cDNA first-strand synthesis kit from Fermentas according to the manufacturer's instructions. RT–qPCR was performed using the Platinum SYBR Green qPCR Supermix (Invitrogen) on the GeneAmp 5700 thermal cycler (Applied Biosystems). Absolute quantification of hAgo mRNA was determined by using standard curves (copy numbers between 5 x 101 and 5 x 106), in which sequences were the same as the amplified sequences. Values obtained were divided by the nanograms of total RNA used in the reaction. Relative quantification was obtained using the δ-Ct method, by which the ratio between hAgo mRNA and a housekeeping gene (β glucuronidase) for each individual sample was calculated using the formula (2-ΔCt). Values obtained were normalized to the values of asON_Ctr- or siscr-treated groups, which were set at 100%. Each experiment was repeated independently three times.
SUPPLEMENTAL MATERIAL
Supplemental material can be found at http://www.rnajournal.org.
ACKNOWLEDGMENTS
We thank Gunter Meister (MPI of Biochemistry, Martinsried, Germany) for providing hAgo1-directed antibody, Andreas Dendorfer (Institute of Experimental and Clinical Pharmacology and Toxicology, University of Lübeck, Germany) for his help with the statistical analysis of the data, Alev Erogullari and Nora Hennies for their experimental support, and Sandra D. Laufer for her critical reading of the manuscript. This work was financially supported by a grant from the Medizinische Fakultät, Lübeck (FKZ: E23:2009).
Footnotes
Abbreviations: asON, antisense oligonucleotide; FF, firefly luciferase; IC50, half maximal inhibitory concentration; hAgo, human Ago protein; miRNA, micro RNA; P-bodies, processing bodies; RT–qPCR, reverse transcription–quantitative PCR ;RL, Renilla luciferase; RISC, RNA-induced silencing complex; RNAi, RNA interference; siRNA, small interfering RNA.
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.2204610.
REFERENCES
- Aravin AA, Bourc'his D 2008. Small RNA guides for de novo DNA methylation in mammalian germ cells. Genes Dev 22: 970–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma-Mukai A, Oguri H, Mituyama T, Qian ZR, Asai K, Siomi H, Siomi MC 2008. Characterization of endogenous human Argonautes and their miRNA partners in RNA silencing. Proc Natl Acad Sci 105: 7964–7969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detzer A, Overhoff M, Wünsche W, Rompf M, Turner JJ, Ivanova GD, Gait MJ, Sczakiel G 2009. Increased RNAi is related to intracellular release of siRNA via a covalently attached signal peptide. RNA 15: 627–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias N, Stein CA 2002. Antisense oligonucleotides: Basic concepts and mechanisms. Mol Cancer Ther 1: 347–355 [PubMed] [Google Scholar]
- Diederichs S, Haber DA 2007. Dual role for argonautes in MicroRNA processing and Posttranscriptional regulation of MicroRNA expression. Cell 131: 1097–1108 [DOI] [PubMed] [Google Scholar]
- Diederichs S, Jung S, Rothenberg SM, Smolen GA, Mlody BG, Habert DA 2008. Coexpression of Argonaute-2 enhances RNA interference toward perfect match binding sites. Proc Natl Acad Sci 105: 9284–9289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411: 494–498 [DOI] [PubMed] [Google Scholar]
- Eulalio A, Huntzinger E, Izaurralde E 2008. Getting to the root of miRNA-mediated gene silencing. Cell 132: 9–14 [DOI] [PubMed] [Google Scholar]
- Farazi TA, Juranek SA, Tuschl T 2008. The growing catalog of small RNAs and their association with distinct Argonaute/Piwi family members. Development 135: 1201–1214 [DOI] [PubMed] [Google Scholar]
- Filipowicz W, Bhattacharyya SN, Sonenberg N 2008. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat Rev Genet 9: 102–114 [DOI] [PubMed] [Google Scholar]
- Förstemann K, Horwich MD, Wee L, Tomari Y, Zamore PD 2007. Drosophila microRNAs are sorted into functionally distinct argonaute complexes after production by Dicer-1. Cell 130: 287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünweller A, Hartmann RK 2007. Locked nucleic acid oligonucleotides: The next generation of antisense agents? BioDrugs 21: 235–243 [DOI] [PubMed] [Google Scholar]
- Höck J, Meister G 2008. The Argonaute protein family. Genome Biol 9: 210–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutvagner G, Simard MJ 2008. Argonaute proteins: Key players in RNA silencing. Nat Rev Mol Cell Biol 9: 22–32 [DOI] [PubMed] [Google Scholar]
- Hutvagner G, Simard MJ, Mello CC, Zamore PD 2004. Sequence-specific inhibition of small RNA function. PLoS Biol 2: 465–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowski BA, Huffman KE, Schwartz JC, Ram R, Nordsell R, Shames DS, Minna JD, Corey DR 2006. Involvement of AGO1 and AGO2 in mammalian transcriptional silencing. Nat Struct Mol Biol 13: 787–792 [DOI] [PubMed] [Google Scholar]
- Jepsen JS, Wengel J 2004. LNA-antisense rivals siRNA for gene silencing. Curr Opin Drug Discov Devel 7: 188–194 [PubMed] [Google Scholar]
- Jinek M, Doudna JA 2009. A three-dimensional view of the molecular machinery of RNA interference. Nature 457: 405–412 [DOI] [PubMed] [Google Scholar]
- Kim DH, Villeneuve LM, Morris KV, Rossi JJ 2006. Argonaute-1 directs siRNA-mediated transcriptional gene silencing in human cells. Nat Struct Mol Biol 13: 793–797 [DOI] [PubMed] [Google Scholar]
- Kim DH, Saetrom P, Snove O, Rossi JJ 2008. MicroRNA-directed transcriptional gene silencing in mammalian cells. Proc Natl Acad Sci 105: 16230–16235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klattenhoff C, Theurkauf W 2008. Biogenesis and germline functions of piRNAs. Development 135: 3–9 [DOI] [PubMed] [Google Scholar]
- Kretschmer-Kazemi Far R, Nedbal W, Sczakiel G 2001. Concepts to automate the theoretical design of effective antisense oligonucleotides. Bioinformatics 17: 1058–1061 [DOI] [PubMed] [Google Scholar]
- Kurreck J 2003. Antisense technologies. Improvement through novel chemical modifications. Eur J Biochem 270: 1628–1644 [DOI] [PubMed] [Google Scholar]
- Landthaler M, Gaidatzis D, Rothballer A, Chen PY, Soll SJ, Dinic L, Ojo T, Hafner M, Zavolan M, Tuschl T 2008. Molecular characterization of human Argonaute-containing ribonucleoprotein complexes and their bound target mRNAs. RNA 14: 2580–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaretti D, Tournier I, Izaurralde E 2009. The C-terminal domains of human TNRC6A, TNRC6B, and TNRC6C silence bound transcripts independently of Argonaute proteins. RNA 15: 1059–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JD, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ 2004. Argonaute2 is the catalytic engine of mammalian RNAi. Science 305: 1437–1441 [DOI] [PubMed] [Google Scholar]
- Lykke-Andersen K, Gilchrist MJ, Grabarek JB, Das P, Miska E, Zernicka-Goetz M 2008. Maternal Argonaute 2 is essential for early mouse development at the maternal-zygotic transition. Mol Biol Cell 19: 4383–4392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae IJ, Ma E, Zhou M, Robinson CV, Doudna JA 2008. In vitro reconstitution of the human RISC-loading complex. Proc Natl Acad Sci 105: 512–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr C, Hemann MT, Bartel DP 2007. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science 315: 1576–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G 2007. miRNAs get an early start on translational silencing. Cell 131: 25–28 [DOI] [PubMed] [Google Scholar]
- Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T 2004. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell 15: 185–197 [DOI] [PubMed] [Google Scholar]
- Meister G, Landthaler M, Peters L, Chen PY, Urlaub H, Luhrmann R, Tuschl T 2005. Identification of novel argonaute-associated proteins. Curr Biol 15: 2149–2155 [DOI] [PubMed] [Google Scholar]
- Ohrt T, Muetze J, Staroske W, Weinmann L, Hock J, Crell K, Meister G, Schwille P 2008. Fluorescence correlation spectroscopy and fluorescence cross-correlation spectroscopy reveal the cytoplasmic origination of loaded nuclear RISC in vivo in human cells. Nucleic Acids Res 36: 6439–6449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura K, Ishizuka A, Siomi H, Siomi MC 2004. Distinct roles for argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev 18: 1655–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzel V, Steidl U, Kronenwett R, Haas R, Sczakiel G 1999. A theoretical approach to select effective antisense oligodeoxyribonucleotides at high statistical probability. Nucleic Acids Res 27: 4328–4334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters L, Meister G 2007. Argonaute proteins: Mediators of RNA silencing. Mol Cell 26: 611–623 [DOI] [PubMed] [Google Scholar]
- Qi HH, Ongusaha PP, Myllyharju J, Cheng DM, Pakkanen O, Shi YJ, Lee SW, Peng JM, Shi Y 2008. Prolyl 4-hydroxylation regulates Argonaute 2 stability. Nature 455: 421–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid UJ, Paterok D, Koglin A, Gohlke H, Piehler J, Chen JCH 2007. Structure of Aquifex aeolicus Argonaute highlights conformational flexibility of the PAZ domain as a potential regulator of RNA-induced silencing complex function. J Biol Chem 282: 13824–13832 [DOI] [PubMed] [Google Scholar]
- Rivas FV, Tolia NH, Song JJ, Aragon JP, Liu JD, Hannon GJ, Joshua-Tor L 2005. Purified Argonaute2 and an siRNA form recombinant human RISC. Nat Struct Mol Biol 12: 340–349 [DOI] [PubMed] [Google Scholar]
- Rüdel S, Flatley A, Weinmann L, Kremmer E, Meister G 2008. A multifunctional human Argonaute2-specific monoclonal antibody. RNA 14: 1244–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Shiohama A, Minoshima S, Shimizu N 2003. Identification of eight members of the Argonaute family in the human genome. Genomics 82: 323–330 [DOI] [PubMed] [Google Scholar]
- Schmitter D, Filkowski J, Sewer A, Pillai RS, Oakeley EJ, Zavolan M, Svoboda P, Filipowicz W 2006. Effects of Dicer and Argonaute down-regulation on mRNA levels in human HEK293 cells. Nucleic Acids Res 34: 4801–4815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JJ, Smith SK, Hannon GJ, Joshua-Tor L 2004. Crystal structure of argonaute and its implications for RISC slicer activity. Science 305: 1434–1437 [DOI] [PubMed] [Google Scholar]
- Su H, Trombly MI, Chen J, Wang XZ 2009. Essential and overlapping functions for mammalian Argonautes in microRNA silencing. Genes Dev 23: 304–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto K, Wakiyama M, Yokoyama S 2009. Mammalian GW182 contains multiple Argonaute-binding sites and functions in microRNA-mediated translational repression. RNA 15: 1078–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaucheret H, Vazquez F, Crete P, Bartel DP 2004. The action of ARGONAUTE1 in the miRNA pathway and its regulation by the miRNA pathway are crucial for plant development. Genes Dev 18: 1187–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YL, Sheng G, Juranek S, Tuschl T, Patel DJ 2008. Structure of the guide-strand-containing argonaute silencing complex. Nature 456: 209–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YL, Juranek S, Li HT, Sheng G, Wardle GS, Tuschl T, Patel DJ 2009. Nucleation, propagation and cleavage of target RNAs in Ago silencing complexes. Nature 461: 754–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann L, Hock J, Ivacevic T, Ohrt T, Mutze J, Schwille P, Kremmer E, Benes V, Urlaub H, Meister G 2009. Importin 8 is a gene silencing factor that targets argonaute proteins to distinct mRNAs. Cell 136: 496–507 [DOI] [PubMed] [Google Scholar]
- Wilson C, Keefe AD 2006. Building oligonucleotide therapeutics using non-natural chemistries. Curr Opin Chem Biol 10: 607–614 [DOI] [PubMed] [Google Scholar]
- Wu LG, Fan JH, Belasco JG 2008. Importance of translation and nonnucleolytic ago proteins for on-target RNA interference. Curr Biol 18: 1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekta S, Shih IH, Bartel DP 2004. MicroRNA-directed cleavage of HOXB8 mRNA. Science 304: 594–596 [DOI] [PubMed] [Google Scholar]
- Yuan YR, Pei Y, Ma JB, Kuryavyi V, Zhadina M, Meister G, Chen HY, Dauter Z, Tuschl T, Patel DJ 2005. Crystal structure of A-aeolicus Argonaute, a site-specific DNA-guided endoribonuclease, provides insights into RISC-mediated mRNA cleavage. Mol Cell 19: 405–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan YR, Pei Y, Chen HY, Tuschl T, Patel DJ 2006. A potential protein-RNA recognition event along the RISC-loading pathway from the structure of A-aeolicus argonaute with externally bound siRNA. Structure 14: 1557–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Sankala H, Zhang XX, Graves PR 2008. Phosphorylation of Argonaute 2 at serine-387 facilitates its localization to processing bodies. Biochem J 413: 429–436 [DOI] [PubMed] [Google Scholar]