Abstract
The bacterial Ras-like protein Era has been reported previously to bind 16S rRNA within the 30S ribosomal subunit and to play a crucial role in ribosome assembly. An orthologue of this essential GTPase ERAL1 (Era G-protein-like 1) exists in higher eukaryotes and although its exact molecular function and cellular localization is unknown, its absence has been linked to apoptosis. In the present study we show that human ERAL1 is a mitochondrial protein important for the formation of the 28S small mitoribosomal subunit. We also show that ERAL1 binds in vivo to the rRNA component of the small subunit [12S mt (mitochondrial)-rRNA]. Bacterial Era associates with a 3′ unstructured nonanucleotide immediately downstream of the terminal stem–loop (helix 45) of 16S rRNA. This site contains an AUCA sequence highly conserved across all domains of life, immediately upstream of the anti-Shine–Dalgarno sequence, which is conserved in bacteria. Strikingly, this entire region is absent from 12S mt-rRNA. We have mapped the ERAL1-binding site to a 33 nucleotide section delineating the 3′ terminal stem–loop region of 12S mt-rRNA. This loop contains two adenine residues that are reported to be dimethylated on mitoribosome maturation. Furthermore, and also in contrast with the bacterial orthologue, loss of ERAL1 leads to rapid decay of nascent 12S mt-rRNA, consistent with a role as a mitochondrial RNA chaperone. Finally, whereas depletion of ERAL1 leads to apoptosis, cell death occurs prior to any appreciable loss of mitochondrial protein synthesis or reduction in the stability of mitochondrial mRNA.
Keywords: Era G-protein-like 1 (ERAL1), mitoribosome, ribosome assembly, rRNA, translation
Abbreviations: CLIP, cytoplasmic linker protein; COX, cyclo-oxygenase; DAP3, death-associated protein 3; ERAL1, Era G-protein-like 1; FBS, fetal bovine serum; GDH, glutamate dehydrogenase; HEK-293T, HEK (human embryonic kidney)-293 cells expressing the large T-antigen of SV40 (simian virus 40); HSP70, heat-shock protein 70; ICT1, immature colon carcinoma transcript 1; IP, immunoprecipitation; KH, K homology; LSU, large subunit; MEM, minimal essential medium; MGC, mammalian gene collection; MRP, mammalian ribosomal protein; mt, mitochondrial; MTND, mitochondrially encoded NADH dehydrogenase; NDUFA9, NADH dehydrogenase (ubiquinone) 1 α subcomplex 9; NDUFB8, NADH dehydrogenase (ubiquinone) 1 β subcomplex 8; NEAA, non-essential amino acids; NT, non-targeting; RNP, ribonucleoprotein; ROS, reactive oxygen species; SD, Shine–Dalgarno; siRNA, small interfering RNA; SSU, small subunit; TFB1M, transcription factor B1, mitochondrial; UTR, untranslated region
INTRODUCTION
Mammalian mitochondria possess their own genome, mtDNA (mitochondrial DNA), which encodes 13 proteins and all of the 24 RNA components necessary to drive intramitochondrial protein synthesis [1]. The remaining proteins required for this process, including all the protein components of the mitoribosome, are nuclear encoded, translated in the cytosol and imported into the organelle. Correct mtDNA expression is essential for all obligate aerobes, as these polypeptides constitute vital members of the protein complexes that couple oxidative phosphorylation. Protein synthesis is facilitated by the mitoribosome (i.e. the mitochondrial ribosome), whose assembly and molecular mechanisms are still largely uncharacterized. The mammalian 55S mitoribosome differs substantially from its 70S and 80S counterparts found in prokarya and the eukaryotic cytosol respectively [2,3]. Whereas it has minimized its rRNA components, it has extended its polypeptide constituents with the result that, although its overall mass is slightly greater than that of the bacterial ribosome (approx. 2.7 MDa), it has a decreased density. Thus the mt (mitochondrial)-SSU (small subunit) and mt-LSU (large subunit) are designated 28S and 39S respectively, generating a complete monosome of 55S. A highly purified bovine 55S mitoribosomal particle has been resolved to 13.5 Å (1 Å=0.1 nm) by cryoelectron microscopy. This indicates the positions of the shorter 12S and 16S mt-rRNA constituents relative to the 29 and 48 polypeptides within the mt-SSU and mt-LSU respectively [2]. It is possible, however, that a small number of less tightly associated bona fide members may have been lost during purification. Indeed, there is still some discussion as to the possible mitoribosomal association of a 5S rRNA species, which has been shown to be imported into mammalian mitochondria [4,5].
The assembly pathway of the mammalian mitoribosome is uncharacterized. It is believed that as many as 200 proteins are required to assemble the 80S ribosome in the yeast cytosol [6]. Bioinformatic analyses, however, suggest that mitoribosomal assembly may require only a minimal number of factors, more related to the situation in bacteria [7]. In an attempt to identify factors loosely associated with mitoribosomes, we recently performed a proteomic analysis of particles immunoprecipitated by the mtRRF (mitochondrial ribosome recycling factor) [8]. In addition to almost an entire set of mitoribosomal proteins, we identified nucleoid components and other proteins of unknown or diverse function. One of these proteins ERAL1 (Era G-protein-like 1), is the human orthologue of the Escherichia coli Ras-like protein Era [9]. In bacteria, this GTPase has been reported to be essential for cell division [10]. More recently, Era has been shown to bind via its C-terminal KH (K homology) domain to the 30S small ribosomal subunit near to the 3′ terminus of the 16S rRNA [11,12]. These elegant X-ray diffraction and cryoelectron microscopy studies show Era bound either to an oligoribonucleotide or to the entire 30S subunit lacking ribosomal protein S1. Era both occludes the binding site for S1 and inhibits the interaction of the SD (Shine–Dalgarno) sequence of mRNAs with the anti-SD sequence at the 3′ terminus of the mature 16S rRNA, each of which are required for translation initiation. Taken together, these results predict that Era plays an essential role in ribosome maturation and quality control. The few reports on the mammalian orthologue ERAL1, have suggested that it is a membrane-bound protein possibly associated with the endoplasmic reticulum [13] and that depletion led to growth arrest and apoptosis [14]. These latter functions were attributable to the conserved RNA-binding KH domain.
In the present study we show that ERAL1 is an essential mitochondrial protein. In a similar fashion to the bacterial orthologue, it associates mainly with the 28S mt-SSU where it acts as a chaperone for the 12S mt-rRNA, binding at the 3′ terminal stem–loop region. Depletion of ERAL1 leads to 12S instability and a consequent loss of newly synthesized 28S subunits. We were able to confirm apoptosis, but intriguingly this occurred prior to any appreciable effect on mitochondrial protein synthesis or on the steady-state level of most mt-mRNAs.
EXPERIMENTAL
Cell culture
Human HeLa cells were propagated in Eagle's MEM (minimal essential medium; Sigma–Aldrich), supplemented with 1× NEAA (non-essential amino acids), 10% (v/v) FBS (fetal bovine serum) and 2 mM L-glutamine, at 37 °C under a 5% CO2 humidified atmosphere. Osteosarcoma cells (143B.206 rho0) were provided by Professor R. Wiesner, Center for Molecular Medicine, University of Cologne, Germany, and were cultured in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% (v/v) FBS, 50 μg/ml uridine and 1× NEAA. Flp-In™T-Rex™-293 cells {HEK-293T [HEK (human embryonic kidney)-293 cells expressing the large T-antigen of SV40 (simian virus 40)]; Invitrogen} were grown in identical medium supplemented with 10 μg/ml Blasticidin S (Invitrogen). Post-transfection selection was effected with Hygromycin B (100 μg/ml). Growth curve analyses were performed in glucose medium.
Cell lysate, mitochondrial preparation and fractionation
Production of mitochondria and cell lysates were essentially as described in [15]. HEK-293T cells were homogenized on ice. Aggregates were removed by centrifugation at 400 g for 10 min at 4 °C. Mitochondria were precipitated via centrifugation at 11000 g for 10 min at 4 °C. The post-mitochondrial supernatant was used for further analysis. Proteinase K treatment was carried out in a 50 μl volume of isolation buffer (10 mM Tris/HCl, pH 7.4, 0.6 M mannitol, 1 mM EGTA and 0.1% BSA), lacking BSA, containing 30 μg of freshly isolated mitochondria and the appropriate amount of proteinase K. Reactions were incubated on ice for 30 min, stopped by the addition of 2 mM PMSF, and mitochondria were then washed twice in 1 ml of the isolation buffer (without BSA) and re-suspended in the desired buffer. Where necessary, mitochondria were solubilized by the addition of 1% (v/v) Triton X-100.
Production of FLAG-tagged constructs, transfection and expression
The original human ERAL1 clone was obtained from the MGC (mammalian gene collection; clone 4893068; accession number BC019094). All constructs were prepared by PCR using the MGC clone as a template. Constructs to facilitate inducible expression of C-terminally FLAG-tagged ERAL1 were prepared by generating an amplicon using the following primers: forward 5′-ctctctggatccatggctgcccccagctg-3′ and reverse 5′- ctctccggatccctacttatcgtcgtcatccttgtaatccttgaggagcttcacagag-3′ (the ATG start codon is in bold; the BamH1 restriction site is in italics). The original human MRPS26 (mammalian ribosomal protein S26) clone was obtained from MGC (clone 3051126; accession number BC013018). All constructs were prepared by PCR using the MGC clone as a template. Constructs to facilitate inducible expression of C-terminally FLAG-tagged MRPS26 were prepared by generating an amplicon using the following primers: forward 5′-tactatggatccaccatgctacgcgcgctgag-3′ and reverse 5′-atactactcgagctacttatcgtcgtcatccttgtaatcggagtccctgcgttgtgg-3′ (the ATG start codon is in bold; BamH1 and Xho1 restriction sites are in italics). Amplicons and pcDNA5/FRT/TO (Invitrogen) were digested with appropriate restriction enzymes and ligated. Fidelity and orientation were confirmed by sequence analysis. The human HEK-293T cells were transfected at ~50% confluency with the vectors pOG44 and pcDNA5/FRT/TO containing sequences of the genes to be expressed [FLAG-tagged ERAL1, MRPS27, ICT1 (immature colon carcinoma transcript 1)] as described previously [16].
Affinity purification and elution of FLAG-tagged polypeptides
For affinity purification either cell lysates or mitochondria from HEK-293T cells expressing FLAG-tagged derivatives were used. Cell lysates were prepared in lysis buffer [50 mM Tris/HCl, pH 7.4, 150 mM NaCl, 10 mM MgCl2, 1 mM EDTA, 1% (v/v) Triton X-100, 1× protease inhibitor cocktail (Roche) and 1 mM PMSF], and mitochondrial preparations were as described above. Mitochondrial preparations were treated with DNase I (0.5 units/mg of mitochondria) for 15 min at room temperature (22 °C), and proteinase K (5 μg/mg of mitochondria) for 30 min at 4 °C, reaction were then stopped by the addition by 1 mM PMSF. Pelleted mitochondria were washed (in homogenization buffer), digitonin-treated to remove the outer membrane (400 μg/mg of mitochondria), washed and finally resuspended in lysis buffer. Immunoprecipitation was effected with anti-FLAG M2-agarose affinity gel as recommended by the manufacturer (Sigma–Aldrich), followed by specific elution using 5 μg of 3× FLAG peptide per 100 μl of elution buffer.
Isokinetic sucrose gradient analysis
Total cell lysates (0.5–0.7 mg in lysis buffer) or eluted IPs (immunoprecipitations) were loaded on to a linear sucrose gradient [1 ml, 10–30% (v/v)] in 50 mM Tris/HCl, pH 7.2, 10 mM magnesium acetate, 40 mM NH4Cl, 0.1 M KCl, 1 mM PMSF and 50 μg/ml chloramphenicol, and with a Beckman OptimaTLX bench ultracentrifuge, using a TLS55 rotor at 39000 rev./min for 135 min at 4 °C. Fractions (100 μl) were collected and analysed by Western blot or by silver staining. For Western blot analysis, proteins from cell lysate, following sucrose-gradient separation, or after immunoprecipitation and elution, were separated by SDS/PAGE and transferred on to PVDF membranes as described in [8]. After binding of primary antibodies, visualization of specific proteins was facilitated with horseradish-peroxidase-conjugated secondary goat or mouse IgG (both Dako), using ECL (enhanced chemiluminescence) Plus reagents (GE Healthcare) using a Storm 860 (GE Healthcare) PhosphorImager or by exposure to standard autoradiographic film. The following rabbit polyclonal antibodies were used: anti-ERAL1, -MRPS25, -MRPS18B (Protein Tech Group, catalogue numbers: 11478, 15277, 16139), anti-GDH (glutamate dehydrogenase) (in-house production), anti-MRPL3 (Abcam; catalogue number AB39268). Mouse monoclonal antibodies against: DAP3 (death-associated protein 3), HSP70 (heat-shock protein 70), MRPL12 (Abcam; catalogue numbers AB11928, AB2799, AB58334), β-actin (Sigma–Aldrich; catalogue number A1978), porin, COX2 (cyclo-oxygenase 2) (Invitrogen, catalogue numbers A33319A, A6404), NDUFA9 [NADH dehydrogenase (ubiquinone) 1 α subcomplex 9] and NDUFB8 [NADH dehydrogenase (ubiquinone) 1 β subcomplex 8] (Mitosciences U.S.A.; catalogue numbers MS111, MS105) were used. For silver staining polyacrylamide gels were fixed in 50% methanol for 1 h, followed by a 15 min incubation in staining solution [0.8% AgNO3, 1.4% (v/v) NH4OH and 0.075% NaOH], the washes of 5 min in nanopure distilled water, and then developed in 0.055% formaldehyde/0.005% citric acid and fixed in 45% methanol/10% acetic acid.
siRNA (small interfering RNA) constructs and transfection
Three sequences targeting ERAL1 were tested for efficiency of protein depletion: siORF1 (+702) 5′-GUGUCCUGGUCAUGAACAA; siORF2 (+1096), 5′-GGAGGUGCCUUACAAUGUA; siUTR (+1698), in the 3′-UTR (untranslated region), 5′-CCUUGAACUUGGAUAAGAA (starting nucleotide positions are relative to sequence NM_005702; sense sequence only given). Transfections were performed on 20% confluent HeLa cells with Oligofectamine (Invitrogen) in Opti-MEM-I medium (Gibco) with 0.2 μM siRNA. Reverse transfections were performed as described in [17] on approx. 12000 HEK-293T cells per cm2 with Lipofectamine™ RNAiMAX (Invitrogen) in Opti-MEM-I medium (10–33 nM siRNA). Custom and control non-targeting (NT; OR-0030-neg05) duplex siRNAs were purchased pre-annealed from Eurogentec.
Northern analysis
RNA was isolated with TRIzol® as recommended by the supplier (Invitrogen). Northern blots were performed as described in [18]. Briefly, aliquots of RNA (4 μg) were electrophoresed through 1.2% (w/v) agarose under denaturing conditions and transferred on to GenescreenPlus membrane (NEN duPont) following the manufacturer's protocol. Radiolabelled probes were generated using random hexamers on PCR-generated templates corresponding to internal regions of the relevant genes.
Identification of oligoribonucleotides bound in vivo [CLIP (cytoplasmic linker protein) assays]
Assays were performed essentially as described in [19]. Briefly, cells expressing ERAL1 were grown to ~ 80% confluency in four 15 cm2 plates, washed in PBS and UV-irradiated at 400 mJ/cm2 in a Stratalinker (Stratagene). To ensure only short protected RNA species remained, cells were lysed, the bound RNP (ribonucleoprotein) was treated with RNase T1 and ERAL1 RNP was immunoprecipitated as above. Bound RNA was dephosphorylated and ligated to the 3′ linker as described in [19]. To visualize the complex, the 5′ termini were incubated with [γ-32P]ATP (3000 Ci/mol, PerkinElmer) and PNK (T4 polynucleotide kinase; New England Biolabs), separated by SDS/PAGE (10% Novex precast gels), transferred on to nitrocellulose (BA-85 Whatman) and subjected to autoradiography. For RNA isolation, bound RNP was cut from the nitrocellulose, protein was degraded with proteinase K and the RNA precipitated following phenol/chloroform extraction. Ligation of the 5′ terminus, reverse transcription and PCR amplification were as described previously [19]. PCR products were ligated into pCR4-TOPO and inserts sequenced from individual clones (ABI 3130XL).
In vivo mitochondrial protein synthesis
Analysis of mitochondrial protein synthesis in cultured cells was performed as described previously [20] after addition of emetine and was pulsed with [35S]methionine/cysteine for 30 min. Samples (50 μg) were separated by SDS/PAGE (15% gels) and gels were exposed to PhosphorImage cassettes and visualized using ImageQuant software (GE Healthcare).
Analysis of apoptosis
The proportion of apoptotic cells was analysed using the APO-DIRECT kit (BD Biosciences) following the manufacturer's protocol. Labelling reactions were performed on 0.5×106 cells and FACS analysis performed on a three laser beam (633 nm, 488 nm and 405 nm) FACSCanto II (Becton Dickinson). Terminal deoxynucleotidyl transferase, used to detect the DNA strand breaks, was conjugated to FITC and measured from the excitation of the 488 nm laser and collected using a 530/30 bandpass PMT (photomultiplier tube) filter; 5000–10000 cells were counted per analysis.
Statistical analysis
Where significance values are shown, P values were calculated using an unpaired Student's t test.
RESULTS
ERAL1 is a mitochondrial protein that associates with the small 28S subunit of the mitoribosome
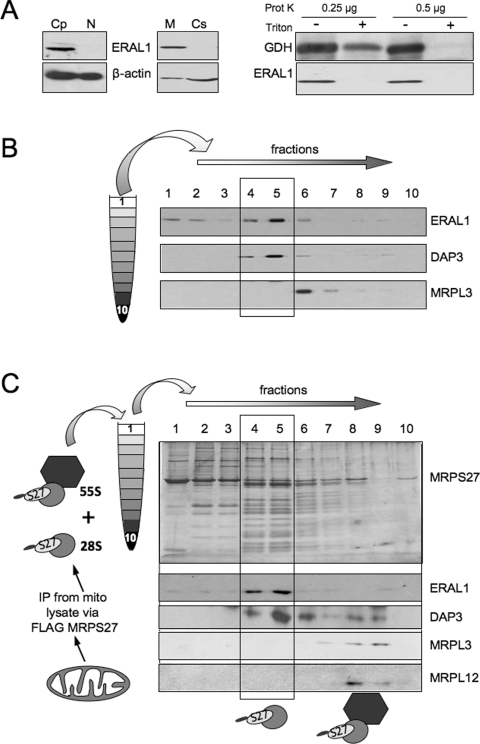
To assess the cellular distribution of ERAL1, human HEK-293T cells were separated into cytosolic and mitochondrial fractions. Western blots revealed a strong co-localization with the crude mitochondrial fraction with no signal in the cytosol or nucleus (Figure 1A, left-hand panels). As it is formally possible that ERAL1 localizes to the mitochondrial periphery and remains associated with the mitochondrial outer membrane on subfractionation, isolated intact mitochondria were subjected to increasing amounts of proteinase K to remove co-purifying contaminants. As shown in Figure 1(A) (right-hand panel), ERAL1 remains resistant to the protease, similar to the mitochondrial matrix marker, GDH. Both proteins were digested after detergent solubilization of the organelles. A similar pattern was observed with subfractionated HeLa cells (results not shown). These results are consistent with ERAL1 being a mitochondrial protein, potentially within the mitochondrial matrix.
Figure 1. ERAL1 associates with the 28S mt-SSU.
(A) Left-hand panel: Western blot analysis, with the indicated antibodies, of HEK-293T cells subfractionated into nuclear (N) or cytoplasmic (Cp) compartments, with the latter fraction further divided into mitochondrial (M) and cytosolic (Cs) fractions. Equivalent volumes of each fraction are loaded. Right-hand panel: mitochondria (30 μg) were pretreated with the indicated amounts of proteinase K and solubilized with Triton X-100 or subjected directly to Western blot analysis with the indicated antibodies. (B) HeLa cell lysate (0.7 mg) was separated by isokinetic gradient centrifugation as detailed in the Experimental section, prior to fractionation and Western blot analysis to indicate the position of mitoribosomal subunits (DAP3 for 28S mt-SSU; MRPL3 for 39S mt-LSU). The blot is representative of three independent repeats. (C) Eluted immunoprecipitate from mitochondria of cells expressing MRPS27–FLAG were separated by isokinetic gradient centrifugation and fractions were silver-stained (upper panel) or subjected to Western blotting with the indicated antibodies (lower panels). 28S mt-SSU, grey circle; 39S mt-LSU, black hexagon. Gels are representative of two independent repeats.
As Era, the bacterial orthologue of ERAL1, has been shown to play an important role in ribosome assembly, we next determined whether ERAL1 associates with mitoribosomal subunits. Following fractionation of HeLa cell lysates by sucrose gradients, a small population of ERAL1 was found in the lowest density fractions, whereas the majority appeared to co-localize with DAP3, a marker of the mt-SSU (Figure 1B). Separation of the 28S mt-SSU and 39S mt-LSU by isokinetic gradient centrifugation is robust, but the complete 55S monosome is often not detectable. However, by IP of the mitoribosome followed by similar gradient centrifugation, it has been recently reported that the monosome can be detected [20]. To assess whether ERAL1 binds only to the mt-SSU and not the intact monosome, a FLAG-tagged component of the 28S subunit, MRPS27 was inducibly expressed in human HEK-293T cells. Following IP, the bound complexes were eluted and separated through a similar 10–30% isokinetic gradient (Figure 1C). Under these conditions, ERAL1 was mainly visible in fractions 4 and 5, co-migrating with DAP3. As expected, no 39S subunit is precipitated alone, but DAP3, along with the 39S subunit markers MRPL3 and MRPL12, are visible in fractions 8 and 9, marking the 55S monosome (Figure 1C) with minimal ERAL1 associated.
Depletion causes a loss of de novo mt-SSU formation
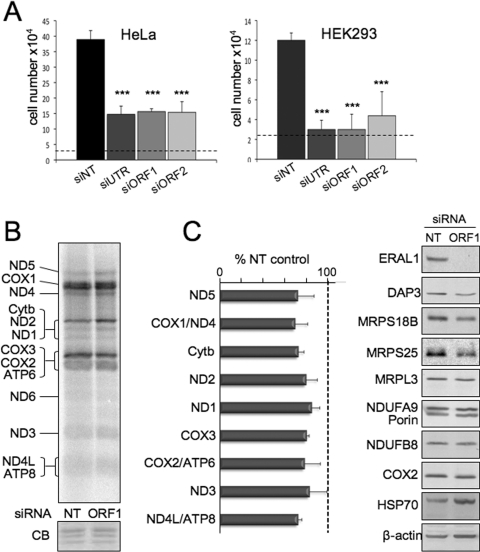
ERAL1 associates selectively with the mt-SSU, suggesting it may play a role in late 28S assembly. Depletion of ERAL1, would be predicted to result in a reduction of newly formed 28S, while having no effect on 39S mt-LSU formation. Therefore, to investigate the effect of ERAL1 loss on mitoribosome formation, siRNA was used to deplete the protein in cells prior to induction of either a FLAG-tagged component of the 28S (MRPS26) or 39S (ICT1) subunit. Three independent siRNA molecules were able to deplete ERAL1 (Figure 2A). Further experiments were performed with siORF1, directed at the open reading frame, after the likelihood of off-target effects was determined to be minimal (see below). Cells were treated with siORF1 for 24 h prior to induction of FLAG-tagged mitoribosomal protein for a further 2 days. Interestingly, similar signals were apparent in control and ERAL1-depleted samples for protein components of the 28S subunit following ICT1 IP. This suggests that the levels of complete mitoribosome in the control (NT) and ERAL1-depleted (ORF1) cells were similar (Figure 2C). In contrast, markers of both the small and large subunit in the MRPS26 IP were markedly decreased, consistent with a substantial reduction in 28S assembly in the absence of ERAL1 (Figure 2B). Taken together, these results infer that although little nascent 28S subunit is being made after loss of ERAL1, mitoribosome formation is still maintained during the depletion period, presumably due to re-use of 28S mt-SSU that had been assembled prior to ERAL1 depletion. How does ERAL depletion prevent 28S subunit assembly? In bacteria, Era may play a role in maturation of the 16S rRNA, through binding to the larger pre-16S species associated with the 30S ribosomal subunit and by promoting cleavage by various RNases [12]. Human mt-rRNAs are matured from a discrete polycistronic unit, although it is believed that this is mediated by folding of mt-tRNAs that flank both the 12S and 16S mt-rRNAs respectively [21]. To assess the quantity and quality of mitochondrial RNAs, Northern blots were performed after the siRNA treatment of cells (Figure 2D). Depletion of ERAL1 led to a minor increase in the steady-state levels of most mt-mRNAs analysed, with a substantial increase in MTND2 (mitochondrially encoded NADH dehydrogenase 2) and MTND3. Essentially no effect on stability could be measured (Supplementary Figure S1 at http://www.BiochemJ.org/bj/430/bj4300551add.htm). The most profound observation, however, was the selective loss of 12S mt-rRNA (Figure 2D, MT-RNR1), consistent with the reduction in 28S mitoribosomal subunit. As 16S mt-rRNA is not depleted (Figure 2D, MT-RNR2) and is produced from the same polycistronic unit, it can be concluded that loss of ERAL1 leads to a rapid and specific degradation of 12S mt-rRNA.
Figure 2. ERAL1 is required for assembly of the 28S small mitoribosomal subunit.
(A) Western blot of HEK-293T cell lysate (5, 10 or 15 μg) pretreated with NT or three independent ERAL1-targeted siRNAs for 3 days and probed with anti-ERAL1 or anti-β-actin antibodies. UTR, siRNA targeting the the 3′-UTR of Eral1; ORF1 and ORF2, siRNAs targeting regions of the open reading frame. In all further siRNA experiments, depletion of ERAL1 was confirmed by Western blotting. (B) Western blot of immunoprecipitate from cells expressing MRPS26–FLAG following 3 days of non-targeting (NT) or ERAL1-depletion (ORF1) siRNA. Position of mitoribosomal subunits are indicated with the markers DAP3, MRPS18B and MRPS25 for the28S mt-SSU, and MRPL3 and MRPL12 for the 39S mt-LSU. Quantification was performed on three independent experiments. On each occasion, signals were normalized to levels of MRPS26 in the immunoprecipitate. Results are means+S.D. (C) Western blot of immunoprecipitate from cells expressing ICT1–FLAG. Experimental details and data analysis as described for panel (A) (n=3). (D) Northern blot of 4 μg of RNA isolated from HEK-293T cells treated with si-NT or following ERAL1 depletion (si-ORF1) for 4 days. RNA from four independent experiments is shown. Probes highlight transcripts encoding components of complex I (MTND2), complex III (MTCYB; mitochondrially encoded cytochrome b) and complex IV (MTCO3, COX3) as well as both 16S (MT-RNR2) and 12S (MT-RNR1) mt-rRNAs. A probe to human 18S rRNA is shown as a loading and quality control (18S). The quantification is shown for 14 independent transcripts from four repeats. Results are means+S.D. ***P<0.001, **P<0.01, *P<0.05.
ERAL1 is an RNA chaperone for the 12S mt-rRNA
The degradation of 12S mt-rRNA may be mediated by the lack of mitoribosome assembly; conversely it may the loss of RNA stability that leads to a mitoribosomal assembly defect. The bacterial Era has been shown to bind 16S rRNA and the human orthologue ERAL1 contains an RNA-binding KH domain. Previously, this KH domain had been shown to be capable of binding RNA, although the target species was unknown [13]. In contrast, the RNA sequence to which the bacterial protein binds has been clearly identified. It is an unstructured nonanucleotide finishing only two nucleotides from the 16S 3′ terminus and contains the CCUCC anti-SD sequence that is not present in mammalian 12S mt-rRNA. To identify an RNA-binding footprint on mt-rRNA in vivo, we used the CLIP assay that has been established previously for use with cytosolic RNA-binding proteins [19]. Induction of FLAG-tagged ERAL1 was inefficient, resulting in levels similar to endo-genous ERAL1 (results not shown). Following induction, cells were UV-irradiated and the bound RNA cleaved by RNase T1 before rescue and identification. As shown in Figure 3(A), 12S mt-rRNA was found in 63% of sequenced clones (31 out of 49), revealing a minimal footprint of 33 nt. This region is close to the 3′ terminus of 12S mt-rRNA and spans a stem–loop that has a structural orthologue in bacterial 16S rRNA, but is distinct to the bacterial Era-binding site (Figure 3B). No other mt-rRNA sequence was identified, although several mt-tRNAPro sequences were recovered (Supplementary Table S1 at http://www.BiochemJ.org/bj/430/bj4300551add.htm). The significance of this is unclear; however, other mitochondrial RNA-binding proteins subjected to CLIP in our laboratory have also shown a similar association with the 3′ terminus of mt-tRNAPro (Mateusz Wydro, unpublished work). The remaining clones contained sequences from E. coli 23S rRNA (two clones), human transcripts (three clones) or could not be identified (six clones). We conclude that ERAL1 functions to protect the 12S rRNA by binding at its 3′ terminus, but that the position and sequence to which it binds differs from the bacterial protein.
Figure 3. ERAL1 binds in vivo to the predicted 3′ terminal stem–loop of 12S mt-rRNA.
Short RNA species bound by ERAL1 in vivo were identified following the CLIP assay as described in the Experimental section. (A) The sequence of the 50 nt 3′ terminal residues of 12S mt-rDNA, equivalent to nt 1551–1601 of the mitochondrial genome [1] is listed, along with the first four 5′ residues of mt-tRNAVal, immediately downstream. The entire insert of each clone is shown as a filled line spanning the corresponding part of the reference sequence, with the number of clones with an identical sequence indicated. The minimal ERAL1-binding site is boxed. The bold AA highlights the dimethylated adenine residues at nt 1582–1583. The sequences of all 31 clones are given in Supplementary Table S1 (at http://www.BiochemJ.org/bj/430/bj4300551add.htm). *, two clones were deleted for two A residues of the A triplet at nt 1582–1584. (B) The highly conserved 3′ terminal stem–loop structure in the mitochondrial 12S (left-hand side) or bacterial 16S (helix 45, right-hand side) [22] are shown, along with the terminal unstructured nucleotides. The minimal 33 nt binding site for ERAL1, along with the delineated nonamer for Aquifex aeolicius Era [12] is highlighted as a solid line. The highly conserved AUCA is shown in bold; the anti-SD region, CCUCC, is italicized. The two dimethylated adenines are shown in outline [23,24].
ERAL1 depletion induces cell death prior to mitochondrial translation or respiratory defects
ERAL1 plays an important role in the assembly of the 28S mt-SSU by acting as an RNA chaperone for the 12S mt-rRNA. Depletion with siORF1 resulted in a marked effect on normal (rho+) cell growth (Figure 4A), but had no effect on cells that did not require mtDNA expression for growth (rho0; Supplementary Figure S2 at http://www.BiochemJ.org/bj/430/bj4300551add.htm). A previous report used an inducible knockout technique in a chicken cell line to ablate the EraL1 gene [14]. This showed that loss of ERAL1 led to cell-cycle arrest and a substantial increase in the proportion of apoptotic cells in the population. We repeated this analysis on HEK-293T cells after 3 days of siRNA treatment and found a similar apoptotic response (with siORF1 35.9±9.5% were apoptotic compared with 0.3±0.4% with NT siRNA, n=4; Supplementary Figure S3 at http://www.BiochemJ.org/bj/430/bj4300551add.htm). Intriguingly, however, as shown in Figure 2, although ERAL1 depletion prevented the formation of nascent 28S, it did not reduce the amount of complete mitoribosome during the depletion time course. Consistent with this observation, metabolic labelling experiments showed that mitochondrial protein synthesis in HeLa cells was unaffected even after 4 days of depletion (Figure 4B) and was only partially affected in HEK-293T cells after 3 days of depletion (Figure 4C, left-hand panel). Furthermore, steady-state levels of proteins either directly encoded by mtDNA (COX2) or as markers of stable complexes that incorporate mtDNA-encoded polypeptides (NDUFB8) (Figure 4C, right-hand panel) were unaffected, although a consistent increase in HSP70 was found. These results infer that ERAL1 depletion does not induce apoptosis merely through the loss of mitochondrial translation products, but may involve a discrete signalling mechanism.
Figure 4. Depletion of ERAL1 leads to apoptosis prior to appreciable loss of mitochondrial protein synthesis.
(A) Counts of HEK-293T or HeLa cells were taken after siRNA treatment (3 or 4 days respectively) with an NT control or each of the three independent siRNAs targeted either to the EraL1 open reading frame (ORF1; ORF2) or to the corresponding 3′-UTR. Counts were performed on three (HeLa) or six (HEK-293T) independent repeats. ***P<0.001. (B) Metabolic radiolabelling of mitochondrial gene products was performed for 30 min in HeLa cells treated for three days with siRNA (NT or against ERAL1). Aliquots (50 μg) were separated by SDS/PAGE and visualized with a PhosphorImage as described in the Experimental section. Mitochondrial gene products are assigned from [20]. A small section of the gel is shown following exposure, rehydration and Coomassie Blue (CB)-staining to confirm equal loading. ATP8, mitochondrially encoded ATP synthase 8; Cytb, cytochrome b; ND, NADH dehydrogenase. (C) De novo synthesis (left-hand panel) and steady-state levels (right-hand panel) of mt-proteins in HEK-293T cell lysates following 3 days of siRNA treatment (siORF1 or NT control). Quantification of metabolic labelling is presented as a percentage of NT control for each translation product and is from three independent experiments. COX1/ND4, COX2/ATP6 and ND4L/ATP8 were quantified together as they could not confidently be quantified independently. The Western blot analysis visualized mitoribosomal proteins with antibodies as described in the text; markers used are: for complex I, NDUFB8 and NDUFA9; for complex IV, COX2; for the mitochondrial matrix, chaperone HSP70, and for the mitochondrial outer membrane, porin. Results are means+S.D.
DISCUSSION
ERAL1 is involved in the assembly of the mt-SSU. To date, only one other non-mitoribosomal protein, TFB1M (transcription factor B1, mitochondrial), has been shown to be important for 28S assembly, through its role in dimethylation of the 12S rRNA [25]. Completion of the assembly of the entire mitoribosome has been shown to require proteolytic maturation of MRPL32 by the mAAA protease, which promotes association of the mitoribosome with the inner mitochondrial membrane [26]. Our results show that ERAL1 is a mitochondrial protein whose function is to protect the 12S mt-rRNA on the 28S mitoribosomal subunit during SSU assembly. It therefore has some similar roles to its bacterial counterpart, where Era binding not only protects the 16S rRNA precursor, it also binds in a pocket that precludes the 50S subunit from associating with the 30S subunit. It also prevents mRNA forming the essential SD/anti-SD pairing necessary for protein synthesis initiation [11,12]. No SD motif exists in human mt-mRNA and so no such interaction occurs between the mt-rRNA and mRNA. The molecular mechanism by which the mitoribosome locates the mt-mRNA initiating codon is unclear [27]. Virtually all mt-mRNAs have three or fewer residues of 5′-UTR and for seven of the open reading frames the first ‘A’ of the initiating codon is the absolute 5′ terminal nucleotide [21,28]. Sequence analysis from our CLIP assays reflected physiological binding and demonstrated unequivocally that ERAL1 binds very close to the 3′ terminus of the 12S mt-rRNA, covering a sequence predicted to form a terminal stem–loop structure. This small 33 nt footprint includes two adjacent adenosine residues within the loop that are known to be dimethylated by the mitochondrial protein TFB1M in mammals [22,25]. In two of our clones, both of these nucleotides were missing, consistent with methylation of these residues potentially inducing deletions by the reverse transcriptase or indicating their covalent attachment to ERAL1 during UV-irradiation [19]. In cells from mice devoid of TFB1M, levels of the 12S mt-rRNA are dramatically reduced, whereas mt-mRNA levels remain largely unaffected or even increase; both of these results are similar to those noted in human cells depleted of ERAL1. It is tempting to suggest that ERAL1 binding may require adenine dimethylation in the loop. However, in both mice and humans, 12S adenine dimethylation is not absolute. Thus it is possible that ERAL1 has a greater affinity for the dimethylated stem–loop, but can still bind and protect unmethylated species albeit with a weaker affinity.
There is a marked variation between the RNA motif bound by Era in bacteria and ERAL1 in mitochondria. The 12S and bacterial 16S rRNA share sequence and structural similarity in the 3′ terminal stem–loop (helix 45 in bacteria), which is clearly identified as the 12S-binding site by our CLIP assays (Figure 3B). The 16S rRNA nucleotides that have been shown by X-ray crystallography to directly interact with the KH domain of Aquifex aeolicus Era are nts 1530–1534 (GAUCA) and the anti-SD sequence CCUCC immediately downstream at nts 1535–1539. The AUCA sequence is highly conserved across the domains of life, whereas the anti-SD is present but restricted to prokarya. Strikingly, however, this entire nonanucleotide primary sequence is absent from 12S mt-rRNA (Figure 3B). Therefore although ERAL1 is clearly a mitochondrial orthologue of Era, the RNA bound by the conserved KH domain is completely different. This may reflect the different functions that are required for these orthologues. Binding of Era promotes maturation of pre-16S rRNA by a currently unidentified RNase. Such processing is not required for the 12S equivalent, although binding is clearly needed to protect the 12S mt-rRNA from degradation. Furthermore, it is not clear from our results whether ERAL1 binding to 12S mt-rRNA on the mt-SSU completely precludes association with the mt-LSU as noted on Era binding to bacterial 30S. Clearly, the vast majority of ERAL1 is associated with isolated mt-SSU. However, LC-MS/MS data of complexes immunoprecipitated by ERAL1–FLAG does identify a small number of mt-LSU subunits and this is currently being explored (results not shown).
The protection of mt-mRNAs on ERAL1 depletion and in TFB1M-knockout mice that also prevents assembly of the 28S subunit, suggest that mt-mRNA is not initially associated with the mitoribosome or constituent subunits. Our preliminary results from RNA isolated from gradient fractions suggests that nascent mt-mRNA associates with a separate complex, which increases in mass proportionally with the size of the transcript and that the gradient profile for these mt-mRNAs remains unchanged in the ERAL1-depleted cells (S. Dennerlein, unpublished work). It is possible that initially after synthesis, mt-mRNA becomes associated with an RNA-binding complex and remains associated until the mt-mRNA is recruited to the mitoribosome for translation. The foundation for such a RNP complex has recently been reported [29]. This RNP contains two generic mitochondrial RNA-binding proteins, LRP130 (130 kDa leucine-rich protein) and SLIRP (stem–loop interacting RNA-binding protein). It is possible and even probable that this complex will associate with other mitochondrial RNA-binding proteins, such as those originally demonstrated in [30].
What is the cause of apoptosis in the ERAL1-depleted cell lines? Clearly, ERAL1 depletion leads to the loss of the newly assembled 28S subunit. However, the depleted lines appear to retain sufficient intact mitoribosomes to allow efficient mitochondrial protein synthesis up to the point when the cells die of apoptosis. This suggests that either the cells synthesize aberrant polypeptides as a function of having to recycle old 28S subunits, or there is an undefined retrograde signal from the mitochondrion to the nucleus that leads to the apoptosis. Polarographic analyses of ERAL1-depleted cells revealed no apparent difference in oxygen utilization or respiratory control ratios (results not shown). Furthermore, we were unable to measure any differences in ROS (reactive oxygen species) production or substantial changes in mitochondrial membrane potential. There have been numerous reports linking mitochondrial ribosomal subunits, such as DAP3, with apoptosis [31,32]. The lack of 28S assembly will result in more freely available polypeptides, but it would appear unlikely that such proteins could be exported from the matrix. Alternatively, perhaps it is possible that lack of SSU assembly leads to accumulation of nascent MRPs, such as DAP3 in the cytosol, leading to apoptosis. This intriguing area is currently being pursued.
Finally, we note that immediately prior to submission of this article, another manuscript was published on the function and localization of ERAL1 [33]. The authors also reported a 28S mitoribosomal association possibly mediated by 12S mt-rRNA-binding and a similar growth phenotype on depletion. However, their siRNA targeted a different sequence to the three siRNAs used in the present study, which we tested for both specific and off-target effects. Unlike the present study, their report indicated that depletion was shown to cause a lowered steady-state level of respiratory complexes, elevated ROS, lowered membrane potential and a substantial reduction in the level of most mtDNA-encoded transcripts. Their observation of this decrease in steady-state levels of mt-mRNA is surprising. It is in contrast with results with TFB1M-knockout mice that exhibit defective mt-SSU formation and in a patient where 12S mt-rRNA is lost as a consequence of a MRPS16 mutation [34], where in neither case is transcript loss noted. Irrespective, we are now able to report that the essential function of ERAL1 is as a 12S mt-rRNA chaperone and the identification of its binding site, in vivo.
Online data
AUTHOR CONTRIBUTION
Sven Dennerlein carried out the majority of the practical work with the exception that Agata Rozanska performed the CLIP assays and Mateusz Wydro generated the MRPS26 constructs and the HEK-293T cell line with inducible expression of MRPS26. Zofia Chrzanowska-Lightowlers and Robert Lightowlers provided the funding, intellectual direction and wrote the paper. All authors critically reviewed the paper.
ACKNOWLEDGEMENTS
We thank Joanna Rorbach, for helpful discussions and generating constructs, and Ian Dimmick and Rebecca Stewart (Newcastle University, Flow Cytometry Core Facility), for technical and data analysis advice.
FUNDING
This work was support by the The Wellcome Trust [grant number 074454/Z/04/Z], the Biotechnology and Biological Sciences Research Council [grant number BB/F011520/1] and the Medical Research Council [grant number G0700718].
References
- 1.Anderson S., Bankier A. T., Barrell B. G., De Bruijn M. H. L., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F., et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 2.Sharma M. R., Koc E. C., Datta P. P., Booth T. M., Spremulli L. L., Agrawal R. K. Structure of the mammalian mitochondrial ribosome reveals an expanded functional role for its component proteins. Cell. 2003;115:97–108. doi: 10.1016/s0092-8674(03)00762-1. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien T. W. Properties of human mitochondrial ribosomes. IUBMB Life. 2003;55:505–513. doi: 10.1080/15216540310001626610. [DOI] [PubMed] [Google Scholar]
- 4.Magalhaes P. J., Andreu A. L., Schon E. A. Evidence for the presence of 5S rRNA in mammalian mitochondria. Mol. Biol. Cell. 1998;9:2375–2382. doi: 10.1091/mbc.9.9.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smirnov A., Tarassov I., Mager-Heckel A. M., Letzelter M., Martin R. P., Krasheninnikov I. A., Entelis N. Two distinct structural elements of 5S rRNA are needed for its import into human mitochondria. RNA. 2008;14:749–759. doi: 10.1261/rna.952208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hage A. E., Tollervey D. A surfeit of factors: why is ribosome assembly so much more complicated in eukaryotes than bacteria? RNA Biol. 2004;1:10–15. [PubMed] [Google Scholar]
- 7.Britton R. A. Role of GTPases in bacterial ribosome assembly. Annu. Rev. Microbiol. 2009;63:155–176. doi: 10.1146/annurev.micro.091208.073225. [DOI] [PubMed] [Google Scholar]
- 8.Rorbach J., Richter R., Wessels H. J., Wydro M., Pekalski M., Farhoud M., Kuhl I., Gaisne M., Bonnefoy N., Smeitink J. A., et al. The human mitochondrial ribosome recycling factor is essential for cell viability. Nucleic Acids Res. 2008;36:5787–5799. doi: 10.1093/nar/gkn576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahnn J., March P. E., Takiff H. E., Inouye M. A GTP-binding protein of Escherichia coli has homology to yeast RAS proteins. Proc. Natl. Acad. Sci. U.S.A. 1986;83:8849–8853. doi: 10.1073/pnas.83.23.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Britton R. A., Powell B. S., Dasgupta S., Sun Q., Margolin W., Lupski J. R., Court D. L. Cell cycle arrest in Era GTPase mutants: a potential growth rate-regulated checkpoint in Escherichia coli. Mol. Microbiol. 1998;27:739–750. doi: 10.1046/j.1365-2958.1998.00719.x. [DOI] [PubMed] [Google Scholar]
- 11.Sharma M. R., Barat C., Wilson D. N., Booth T. M., Kawazoe M., Hori-Takemoto C., Shirouzu M., Yokoyama S., Fucini P., Agrawal R. K. Interaction of Era with the 30S ribosomal subunit implications for 30S subunit assembly. Mol. Cell. 2005;18:319–329. doi: 10.1016/j.molcel.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 12.Tu C., Zhou X., Tropea J. E., Austin B. P., Waugh D. S., Court D. L., Ji X. Structure of ERA in complex with the 3′ end of 16S rRNA: implications for ribosome biogenesis. Proc. Natl. Acad. Sci. U.S.A. 2009;106:14843–14848. doi: 10.1073/pnas.0904032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akiyama T., Gohda J., Shibata S., Nomura Y., Azuma S., Ohmori Y., Sugano S., Arai H., Yamamoto T., Inoue J. Mammalian homologue of Ecoli. Ras-like GTPase (ERA) is a possible apoptosis regulator with RNA binding activity. Genes Cells. 2001;6:987–1001. doi: 10.1046/j.1365-2443.2001.00480.x. [DOI] [PubMed] [Google Scholar]
- 14.Gohda J., Nomura Y., Suzuki H., Arai H., Akiyama T., Inoue J. Elimination of the vertebrate Escherichia coli Ras-like protein homologue leads to cell cycle arrest at G1 phase and apoptosis. Oncogene. 2003;22:1340–1348. doi: 10.1038/sj.onc.1206287. [DOI] [PubMed] [Google Scholar]
- 15.Richter R., Rorbach J., Pajak A., Smith P. M., Wessels H. J., Huynen M. A., Smeitink J. A., Lightowlers R. N., Chrzanowska-Lightowlers Z. M. A functional peptidyl-tRNA hydrolase, ICT1, has been recruited into the human mitochondrial ribosome. EMBO J. 2010;29:1116–1125. doi: 10.1038/emboj.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soleimanpour-Lichaei H. R., Kuhl I., Gaisne M., Passos J. F., Wydro M., Rorbach J., Temperley R., Bonnefoy N., Tate W., Lightowlers R., Chrzanowska-Lightowlers Z. mtRF1a is a human mitochondrial translation release factor decoding the major termination codons UAA and UAG. Mol. Cell. 2007;27:745–757. doi: 10.1016/j.molcel.2007.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ovcharenko D., Jarvis R., Hunicke-Smith S., Kelnar K., Brown D. Highthroughput RNAi screening in vitro: from cell lines to primary cells. RNA. 2005;11:985–993. doi: 10.1261/rna.7288405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chrzanowska-Lightowlers Z. M., Preiss T., Lightowlers R. N. Inhibition of mitochondrial protein synthesis promotes increased stability of nuclear-encoded respiratory gene transcripts. J. Biol. Chem. 1994;269:27322–27328. [PubMed] [Google Scholar]
- 19.Ule J., Jensen K., Mele A., Darnell R. B. CLIP: a method for identifying protein–RNA interaction sites in living cells. Methods. 2005;37:376–386. doi: 10.1016/j.ymeth.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Chomyn A. In vivo labeling and analysis of human mitochondrial translation products. Methods Enzymol. 1996;264:197–211. doi: 10.1016/s0076-6879(96)64020-8. [DOI] [PubMed] [Google Scholar]
- 21.Ojala D., Montoya J., Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981;290:470–474. doi: 10.1038/290470a0. [DOI] [PubMed] [Google Scholar]
- 22.Seidel-Rogol B. L., McCulloch V., Shadel G. S. Human mitochondrial transcription factor B1 methylates ribosomal RNA at a conserved stem-loop. Nat. Genet. 2003;33:23–24. doi: 10.1038/ng1064. [DOI] [PubMed] [Google Scholar]
- 23.Van Knippenberg P. H., Van Kimmenade J. M., Heus H. A. Phylogeny of the conserved 3′ terminal structure of the RNA of small ribosomal subunits. Nucleic Acids Res. 1984;12:2595–2604. doi: 10.1093/nar/12.6.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brimacombe R. The structure of ribosomal RNA: a three-dimensional jigsaw puzzle. Eur. J. Biochem. 1995;230:365–383. [PubMed] [Google Scholar]
- 25.Metodiev M. D., Lesko N., Park C. B., Camara Y., Shi Y., Wibom R., Hultenby K., Gustafsson C. M., Larsson N. G. Methylation of 12S rRNA is necessary for in vivo stability of the small subunit of the mammalian mitochondrial ribosome. Cell Metab. 2009;9:386–397. doi: 10.1016/j.cmet.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Nolden M., Ehses S., Koppen M., Bernacchia A., Rugarli E. I., Langer T. The m-AAA protease defective in hereditary spastic paraplegia controls ribosome assembly in mitochondria. Cell. 2005;123:277–289. doi: 10.1016/j.cell.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Jones C. N., Wilkinson K. A., Hung K. T., Weeks K. M., Spremulli L. L. Lack of secondary structure characterizes the 5′ ends of mammalian mitochondrial mRNAs. RNA. 2008;14:862–871. doi: 10.1261/rna.909208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Temperley R. J., Wydro M., Lightowlers R. N., Chrzanowska-Lightowlers Z. M. Human mitochondrial mRNAs-like members of all families, similar but different. Biochim. Biophys. Acta. 2010;1797:1081–1085. doi: 10.1016/j.bbabio.2010.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasarman F., Brunel-Guitton C., Antonicka H., Wai T., Shoubridge E. A. LRPPRC and SLIRP interact in a ribonucleoprotein complex that regulates posttranscriptional gene expression in mitochondria. Mol. Biol. Cell. 2010;21:1315–1323. doi: 10.1091/mbc.E10-01-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koc E. C., Spremulli L. L. RNA-binding proteins of mammalian mitochondria. Mitochondrion. 2003;2:277–291. doi: 10.1016/S1567-7249(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 31.Cavdar Koc E., Ranasinghe A., Burkhart W., Blackburn K., Koc H., Moseley A., Spremulli L. L. A new face on apoptosis: death-associated protein 3 and PDCD9 are mitochondrial ribosomal proteins. FEBS Lett. 2001;492:166–170. doi: 10.1016/s0014-5793(01)02250-5. [DOI] [PubMed] [Google Scholar]
- 32.Kissil J. L., Deiss L. P., Bayewitch M., Raveh T., Khaspekov G., Kimchi A. Isolation of DAP3, a novel mediator of interferon-gamma-induced cell death. J. Biol. Chem. 1995;270:27932–27936. doi: 10.1074/jbc.270.46.27932. [DOI] [PubMed] [Google Scholar]
- 33.Uchiumi T., Ohgaki K., Yagi M., Aoki Y., Sakai A., Matsumoto S., Kang D. ERAL1 is associated with mitochondrial ribosome and elimination of ERAL1 leads to mitochondrial dysfunction and growth retardation. Nucleic Acids Res. 2010 doi: 10.1093/nar/gkq305. doi:10.1093/nar/gkq305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller C., Saada A., Shaul N., Shabtai N., Ben-Shalom E., Shaag A., Hershkovitz E., Elpeleg O. Defective mitochondrial translation caused by a ribosomal protein (MRPS16) mutation. Ann. Neurol. 2004;56:734–738. doi: 10.1002/ana.20282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.