Abstract
CS (chondroitin sulfate) is a glycosaminoglycan species that is widely distributed in the extracellular matrix. To understand the physiological roles of enzymes involved in CS synthesis, we produced CSGalNAcT1 (CS N-acetylgalactosaminyltransferase 1)-null mice. CS production was reduced by approximately half in CSGalNAcT1-null mice, and the amount of short-chain CS was also reduced. Moreover, the cartilage of the null mice was significantly smaller than that of wild-type mice. Additionally, type-II collagen fibres in developing cartilage were abnormally aggregated and disarranged in the homozygous mutant mice. These results suggest that CSGalNAcT1 is required for normal CS production in developing cartilage.
Keywords: cartilage, chondroitin sulfate, collagen fibre, N-acetylgalactosaminyltransferase (GalNAcT), gene knockout, glycosaminoglycan
Abbreviations: 2-AB, 2-aminobenzamide; C4st-1, chondrotin 4-sulfotransferase-1; ChPF, chondroitin polymerization factor; ChSy, chondroitin synthase; CS, chondroitin sulfate; CSGalNAcT, chondroitin sulfate N-acetylgalactosaminyltransferase; CSPG, chondroitin sulfate proteoglycan; E, embryonic day; ES, embryonic stem; Fam20b, family member 20B; G3pdh, glyceraldehyde-3-phosphate dehydrogenase; GAG, glycosaminoglycan; GlcUA, glucuronic acid; HRP, horseradish peroxidase; PCNA, proliferating cell nuclear antigen; PG, proteoglycan; RT, reverse transcription; TEM, transmission electron microscope
INTRODUCTION
CS (chondroitin sulfate) is a type of GAG (glycosaminoglycan), which are present in the extracellular matrices and on the surface of many cell types [1]. Similar to other GAGs, CS is attached to a serine residue of the core protein to form the CSPG [CS PG (proteoglycan)] [1]. After the formation of a common linkage region (GlcUAβ1-3Galβ1-3Galβ1-4Xylβ1-O-Ser) between sugar chains and the core protein, repeating disaccharide units of GalNAc (N-acetylgalactosamine) and GlcUA (glucuronic acid) residues with interspersed sulfate residues are synthesized [1]. Thus biosynthesis of a CS-specific polysaccharide chain is initiated by the transfer of a GalNAc residue to the tetrasaccharide linker, followed by the addition of GlcUA and GalNAc residues in reiterative chain elongation steps.
There are six glycosyltransferases known to be involved in CS synthesis [2–10]. Among them, CSGalNAcT1 (CS N-acetylgalactosaminyltransferase-1) and CSGalNAcT2 each have one glycosyltransferase domain, exhibit GalNAc transfer activity in both the initiation and elongation processes, and are thought to be responsible for the addition of the first GalNAc residue to the tetrasaccharide chain [4–7]. Although each of the six enzymes has been characterized biochemically in in vitro studies, the mechanism of in vivo CS biosynthesis, including roles for each glycosyltransferase, is poorly understood. Since cartilage contains the largest amount of CS of all body tissues, chondrocytes efficiently synthesize CS chains [11,12]. CS in cartilage is selectively linked to aggrecan, which can possess more than 100 CS chains; these CSPGs subsequently form multimolecular aggregates through interaction with hyaluronate and linker proteins [13]. CSGalNAcT1 is highly expressed in the developing cartilage, and this enzyme is thought to play a crucial role in CS biosynthesis on the basis of a study using cell lines [14]. Moreover, CSGalNAcT1 is thought to have important roles in chondrogenesis at early developmental stages [14].
To investigate the physiological role of CSGalNAcT1 in CS biosynthesis, we produced CSGalNAcT1-null mice and analysed the morphology and biochemistry of developing cartilage in these mutants. The mice were viable and fertile; however, the amount of CS was reduced by half relative to the amount in wild-type controls, and the null mice showed an abnormal profile of GAG chains in the biochemical analysis of CS. The thickness of the epiphyseal cartilage layer was decreased by 25% at E (embryonic day) 18.5. Moreover, the hindlimb and the body were also shorter. Taken together, our data suggest that CSGalNAcT1 plays a role in CS synthesis in the cartilage during embryonic development.
EXPERIMENTAL
Materials
Proteus vulgaris chondroitinase ABC (EC 4.2.2.4) was purchased from Seikagaku. The Superdex™ 200 10/300 GL column was obtained from Amersham Pharmacia Biotech.
Generation of CSGalNAcT1-null mice
All animal experiments were carried out in accordance with the guidelines laid down by the animal welfare committees of Niigata University. The CSGalNAcT1-null mice were produced by homologous recombination using a new ES (embryonic stem) cell line, RENKA, which we developed from the C57BL/6N strain [15].
The mouse CSGalNAcT1 gene (chondroitin 4-sulfotransferase-1) was identified by homology with the human CSGalNAcT1 gene (GenBank® accesion number NM_172753) [encoding 530 amino acids; 89% identity and 92% similarity to human EC 2.4.1.174 (NM_001130518)]. A 1.8-kb DNA fragment, which carried the 34-bp loxP sequence and Pgk-1 promoter-driven neomycin phosphotransferase gene (neo) flanked by two Flp recognition target (frt) sites [16], was inserted into a site 372 bp upstream of exon 7. The 34-bp loxP sequence was inserted into a site 249 bp downstream of exon 6. The targeting vector, ptv CSGALNACT1-flox, contained exon 7 of the CSGalNAcT1 gene flanked by loxP sequences, genomic sequences from 3.4 kb upstream and 7.1 kb downstream of exon 6, and a 4.3 kb pMC1DTpA vector [17].
ES cells were cultured on mitomycin C-treated neomycin-resistant fibroblasts in DMEM (Dulbecco's modified Eagle's medium; high glucose; Invitrogen) supplemented with 17.7% ES-cell-qualified fetal bovine serum (Invitrogen), 88.4 μM non-essential amino acids (Invitrogen), 884 μM sodium pyruvate (Sigma), 88.4 μM 2-mercaptoethanol (Sigma) and 884 units/ml of murine leukaemia inhibitory factor (ESGRO; Chemicon International). Linearized targeting vector was electroporated into RENKA cells, and G-418 (175 μg/ml)-resistant clones were picked. Recombinant clones were identified by Southern blot hybridization analysis. Recombinant ES cells were injected into eight-cell-stage embryos of the CD-1 mouse strain. The embryos were cultured to blastocysts and transferred to the uterus of a pseudopregnant CD-1 mouse. Resulting chimaeric mice were mated to C57BL/6N mice, and heterozygous offspring [CSGalNAcT1+/floxneo] were mated to telencephalin-cre mice [18,19]. The resulting heterozygous (CSGalNAcT1+/−; Cre+/−) mice were mated with C57BL/6N mice and the mice without the Cre gene were selected. Homozygous mutant mice and control mice were obtained by crossing heterozygous pairs.
Genotypes of the CSGalNAcT1+/floxneo mice and CSGalNAcT1+/− mice were identified by Southern blot hybridization analysis. Elimination of the Cre gene was confirmed by PCR using 5′-GCCTGCATTACCGGTCGATGCAACG-3′ (CreP1) and 5′-GCCCGGACCGACGATGAAGCATGTT-3′ (CreP2) [20] with internal control primers 5′-CCAGCTCCAGGGATCTAACA-3′ and 5′-ATTAAGGGCCAGCTCATTCC-3′ (glutamate receptor GluN2A subunit). Routine genotyping of CSGalNAcT1+/− animals was conducted by PCR using the primers 5′-CCAGCTCCAGGGATCTAACA-3′ and 5′-TGGTTTCCTCTAGCCATTGC-3′.
Derivatization of CS from articular cartilage using a fluorophore, 2-AB (2-aminobenzamide)
Articular cartilage CSPG from E18.5 embryos was extracted with 4 M guanidinium chloride and 0.05 M Tris/HCl, pH 8.0, containing proteinase inhibitors as described [21]. The extract was centrifuged at 15000 g for 10 min to remove insoluble material. The protein concentration of each sample was determined using a BCA (bicinchoninic acid) protein assay kit (Thermo Fisher Scientific) according to the manufacturer's instructions. The CSPG fractions were precipitated with 70% ethanol containing 5% sodium acetate. The partially purified CSPG fraction was digested with chondroitinase ABC, and the digests were then derivatized with 2-AB and analysed by HPLC as previously described [22].
Gel-filtration chromatography of CS
To determine the chain length of CS, the purified CSPG fraction was subjected to reductive β-elimination using NaBH4/NaOH, and then analysed by gel-filtration chromatography on a column (10 × 300 mm) of Superdex 200 eluted with 0.2 M ammonium bicarbonate at a flow rate of 0.4 ml/min. Fractions were collected at 3 min intervals, lyophilized and digested with chondroitinase ABC. The digests were derivatized with 2-AB, and then analysed by HPLC on an amine-bound PA-03 column [22]. The amounts of the 2-AB derivatives of unsaturated disaccharides were calculated based on fluorescence intensity.
Quantitative real-time RT (reverse transcription)–PCR
Total RNA was extracted from articular cartilage using RNeasy Lipid Tissue Mini kit (Qiagen) according to the manufacturer's instructions. The cDNA was synthesized from ~1 μg of total RNA using Moloney-murine-leukaemia virus reverse transcriptase (Promega) and an oligo(dT)20-M4 adaptor primer (Takara). The primer sequences used were as follows: CSGalNAcT1, forward primer 5′-CCAATTTCAGAAACTTCACCTTCAT-3′ and reverse primer 5′-TGTTCAGCCTACAAGTGTTGAG-3′; CSGalNAcT2, forward primer 5′-TTAATATCATTGTGCCACTTGCG-3′ and reverse primer 5′-TAGAATAGACTTGACTTTAGATAGTCCTT-3′; C4st-1 (chondroitin 4-sulfotransferase-1), forward primer 5′-ACCTCGTGGGCAAGTATGAG-3′ and reverse primer 5′-TCTGGAAGAACTCCGTGGTC-3′; Fam20B (family member 20B) [23], forward primer 5′-TTGTCTTTAAGCCTAAGCGGT-3′ and reverse primer 5′-GGCTTAACTTCTGTCCGCA-3′; and G3pdh (glyceraldehyde-3-phosphate dehydrogenase), forward primer 5′-CATCTGAGGGCCCACTG-3′ and reverse primer 5′-GAGGCCATGTAGGCCATGA-3′. Quantitative real-time RT–PCR was performed using a FastStart DNA Master plus SYBR Green I (Roche Diagnostics) in a LightCycler ST300 (Roche Diagnostics). The expression levels of CSGalNAcT1, CSGalNAcT2, C4st-1 and Fab20B mRNA were normalized to that of the G3pdh transcript.
Western blot analysis with anti-CSGalNAcT1 antibody
The anti-CSGalNAcT1 antibody was generated against recombinant GST (glutathione transferase)–CSGalNAcT1 (amino acids 261–534). E18.5 cartilage and brain extracts (0.1 mg of total protein) were subjected to SDS/PAGE (10% gels), followed by immunoblot analysis.
Body length and weight
Measurements were performed on adults from the dorsal tip of the nose to the dorsal base of the tail while mice were under anaesthesia. The body weight and body length measurements were based on data from seven male offspring from seven unique litters. Statistical analysis was performed using a Student's t test and the data are represented as the means±S.E.M. *P<0.05.
Skeletal analysis
Embryos were eviscerated under anaesthetic (isoflurane or pentobarbital) and fixed in 100% ethanol for 4 days. Toluidine Blue staining was performed in a solution of 80% ethanol, 20% acetic acid and 0.015% Toluidine Blue for 4 days at 37 °C. Specimens were rinsed and soaked in 100% ethanol for 3 days. Alizarin Red staining was then performed in a solution of 0.002% Alizarin Red and 1% KOH for 12 h at room temperature (25 °C). After rinsing with water, specimens were kept in 1% KOH solution until the skeletons become clearly visible. For storage, specimens were transferred sequentially into 50%, 80%, and finally 100% glycerol. Tibial length was determined by measuring the distance between the proximal and distal articular surfaces with a ruler under light microscopy.
Thickness of epiphyseal cartilage
To compare the thickness of epiphyseal cartilage of proximal femurs, formalin-fixed and paraffin-embedded bones of the limbs from E18.5 and at 4 weeks of postnatal development were used. The tissue material was sliced into serial sections and stained with haematoxylin/eosin. An SZX16 microscope (Olympus) with a digital camera Axiocam HRc (Zeiss) was used to collect image data from the serial sections, and the thickness of the epiphyseal cartilage was calculated from the length of the longitudinal centre axis by Imaris (Zeiss) and ImageJ (National Institutes of Health). A double-blind histological comparison between the null and the wild-type mice was performed. A Mann–Whitney U test was used to analyse the data.
Tissue preparation for mouse specimens
Mice were anaesthesitized with diethyl ether and pentobarbital (Nembutal; Dinabot) and then perfused with 4% paraformaldehyde solution (pH 7.4) under anaethestia tibiae of E18.5 CSGalNAcT1−/− fetuses were extracted, decalcified, and embedded in paraffin as previously described [24]. Immunostained sections of the mice were observed under a Nikon Eclipse E800 microscope, and light microscopic images were acquired with a digital camera (Nikon DXM1200C). For TEM (transmission electron microscope) observations, fixed specimens were decalcified, post-fixed with osmium tetroxide, dehydrated, and embedded in epoxy resin (Epon 812) as previously described [10]. Ultra-thin sections were obtained with an ultra microtome (Sorvall MT-5000) and stained with an aqueous solution of 1% tannic acid, 4% uranyl acetate and 2% lead citrate. These specimens were observed using a TEM (Hitachi H-7100) at 80 kV.
Immunostaining for type-II collagen and PCNA (proliferating cell nuclear antigen)
Dewaxed paraffin sections were pre-treated for endogenous peroxidase inhibition with 0.3% H2O2 in PBS for 20 min and treated as described previously with a 0.5% hyaluronidase solution (type I-S, from bovine testis; Sigma) for 10 min [24]. They were subjected to blocking for non-specific binding with BSA/PBS (1% BSA in PBS) for 30 min at room temperature. Sections were then incubated with rabbit anti-type-II or type-X collagen (1:100; LSL) overnight at 4 °C followed by incubation with HRP (horseradish peroxidase)-conjugated anti-(rabbit Igs) (Chemicon International) overnight at 4 °C. Other sections were incubated with mouse monoclonal antibody against PCNA (Oncogene), followed by incubation with HRP-conjugated anti-(mouse IgG) (Chemicon). Visualization of the immune complex was performed with diaminobenzidine tetrahydrochloride. All sections were faintly counterstained with Methyl Green.
RESULTS
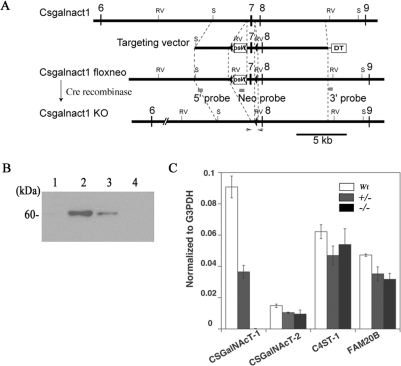
To investigate the role of CS synthesis in skeletal development, we inactivated the CSGalNAcT1 gene by targeted gene replacement in ES cells and subsequent gene knockout in transgenic mice. The DXD motif is present in most GalNAc/Gal transferases and is essential to the in vitro activity of these enzymes as the binding site for divalent metal cations such as Mn2+ [25–27]; therefore we focused on this motif in our gene targeting and gene knockout strategy. Since mouse CSGalNAcT1 has a DXD motif that is encoded in exon 7, we designed the targeting vector with exon 7 located between two loxP sites. Specifically, the neomycin-resistance cassette (neo-cassette) was inserted adjacent to exon 7 (Figure 1A). After the Cre recombinase-dependent excision of the exonic sequences flanked by loxP sites, a frame shift occurs that should prevent the production of normal CSGalNAcT1 enzyme (Figure 1A).
Figure 1. CSGalNAcT1-null mice did not express the CSGalNAcT1 enzyme.
(A) Construct of the targeting vector for producing the CSGalNAcT1-null mice. The numbers represent the exon number (exon 1 is defined as the first exon of this gene where the transcription starts). RV, EcoRV site; S, ScaI site. (B) Immunoblot analysis using an anti-CSGalNAcT1 antibody to probe head homogenate from wild-type mice (lane 2), heterozygous (CSGalNAcT1+/−) mice (lane 3) and null mice (CSGalNAcT1−/−) at E18.5. Lane 1 shows the negative control (wild-type homogenate after absorption by the recombinant CSGalNAcT1 protein). Note that no reactivity was evident in the homogenate from null mice (lane 4). (C) RT–PCR analysis of genes encoding several enzymes involved in CS synthesis. The expression level is shown as a ratio of each gene to G3pdh for normalization. Fam20B is an enzyme that phosphorylates xylose [23]. Note that no other specific enzymes showed elevated expression to compensate for the loss of CSGalNAcT1 protein.
The vector was introduced into the C57BL/6 ES cells, and chimaeric mice containing clonal recombinant ES cells were crossed with C57BL/6 mice. The complete null mice were obtained by mating the floxed mouse with a telencephalin-Cre mouse that expressed the Cre-recombinase in all cell types of early stage embryos (Figure 1A) [19].
The CSGalNAcT1 protein and CSGalNAcT1 mRNA was completely absent from CSGalNAcT1-null mice based on immunoblotting (Figure 1B) and RT–PCR (Figure 1C) analyses respectively. The CSGalNAcT1-null mice were viable and fertile. The protein and mRNA levels in heterozygous mice (CSGalNAcT1+/−) were decreased to 38% and 40% respectively of those in the wild-type controls (Figures 1B and 1C). Other enzymes related to CS synthesis, including CSGalNAcT2, a paralogue of CSGalNAcT1, were not up-regulated in the CSGalNAcT1-null mice (Figure 1C), indicating that no compensatory elevation in expression of related enzymes occurred.
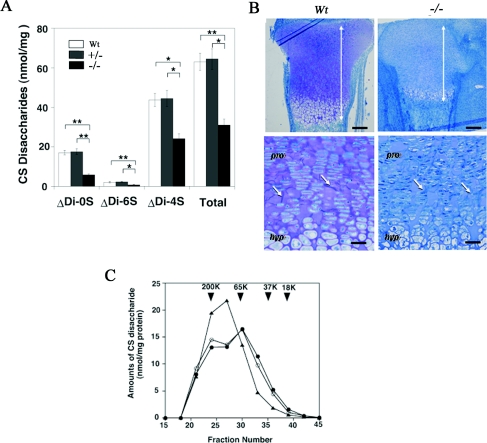
The null mice were viable and fertile; however, we found a large number of abnormalities in the cartilages of these mice. CS production in the cartilage of heterozygous mice (64.4±5.3 nmol/mg of protein) was not reduced compared with that of wild-type mice (63.0±4.4 nmol/mg of protein); however, total CS content in CSGalNAcT1-null mice was less than half (31.0±3.0 nmol/mg of protein; n=6 of each; Figure 2A) of that in the wild-type controls. Normally, CS appears metachromatically violet in Toluidine Blue-stained samples. On the basis of Toluidine Blue staining, the cartilage of wild-type controls was enriched with CS, and the metachromasia indicative of CS was not observed in the CSGalNAcT1-null mice (Figure 2B). Consistent with observation of aggregated collagen fibres under electron microscopic observation, there were many speculate fibres in the CSGalNAcT1-null mice (see arrows in Figure 1B; also see below and Figure 5). CS in the null mice was compositionally similar to that in the control mice except for a reduction in the proportion of the non-sulfated disaccharide (Figure 2A).
Figure 2. CS production in cartilage is reduced in CSGalNAcT1-null mice.
(A) The total amount and disaccharide analysis of the cartilage in wild-type (Wt), heterozygous (+/−; CSGalNAcT1+/−) and null (−/−; CSGalNAcT1−/−) mice. Student's t test was performed to compare the disaccharide amount derived from CS in the null mice with that in the wild-type or the heterozygous mice. Results are shown as mean±S.E.M. *P<0.05; **P<0.01 (n=6). Δdi-0S, ΔHexAα1-3GalNAc; Δdi-4S, ΔHexAα1-3GalNAc(4S); Δdi-6S, HexAα1-3GalNAc(6S). (B) Toluidine Blue staining of the epiphyseal cartilage from E18.5 wild-type (Wt; left) and CSGalNAcT1-null (−/−) fetuses. Metachromasia of Toluidine Blue (purple colour) can be seen in the wild-type cartilage, whereas no metachromasia is discernible in the null mice cartilage. Note the reduced size of the epiphyseal cartilage in the null mice, compared with their wild-type counterpart (bidirectional arrows, upper panels). The extracellular spaces in the cartilage of null mice have many spicules (arrows, lower panels), whereas the intercellular regions from wild-type mice do not. pro, proliferative zone; hyp, hypertrophic zone. Scale bars: in upper panels, 200 μm; in lower panels, 50 μm. (C) Gel-filtration analysis of the length of CS sugar chains in the E18.5 cartilage in wild-type (●), heterozygous (CSGalNAcT1+/−; ○) and null (CSGalNAcT1−/−; ▲) mice. There are no significant differences in the total amount of CS loaded on to the gels among groups. Note that the second peak between fraction numbers 30 and 35 is present both in wild-type and heterozygous mice, but not in CSGalNAcT1-null mice, indicating that the size of the GAG chains of CS changed in CSGalNAcT1-null mice. Arrowheads indicate the size of the molecular-mass-marker standards [mean molecular masses (K, kDa): 200, 65.5, 37.5 and 18.1 respectively; all from Sigma]. The calibration of the Superdex 200 column was performed using a series of size-defined commercial dextran polysaccharides. The results shown represent one of three series of independent experiments, where the three series of experiments gave essentially identical results.
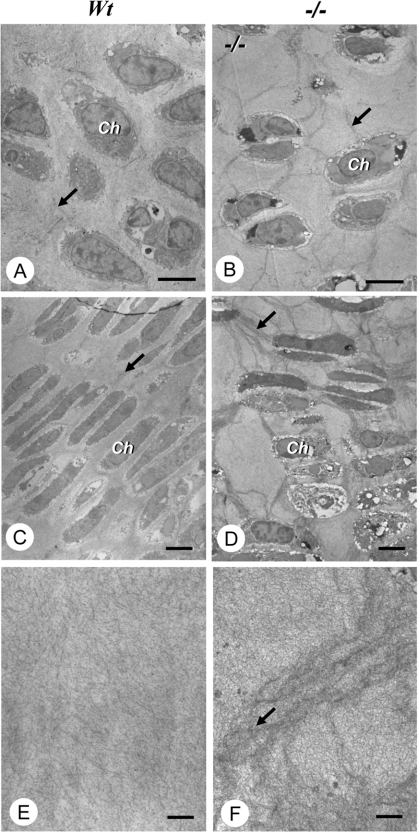
Figure 5. Abnormal ultrastructure of the type-II collagen is frequently observed in cartilage and chondrocytes of CSGalNAcT1-null mice.
(A, C and E) Wild-type (Wt) mice; (B, D and F) CSGalNAcT1-null (−/−) mice; at E18.5. Micrographs (A) and (B) were obtained from the resting zone, and (C) and (D) from the proliferative zones. Note that many electron-dense extracellular fibrils (arrows) are seen connecting chondrocytes in the resting (B) and proliferative (D) zones of the epiphysis from null mice, whereas only a few such fibrils are observed in both zones (A; resting zone, and C; proliferative zone) of wild-type mice. At a higher magnification, the wild-type cartilage matrix had collagen fibrils that spread radially (E), whereas cartilage of the null mice had a fine meshwork of twisted cartilaginous fibrils (F). Ch, chondrocytes. Scale bars: (A)–(D), 10 μm; (E)–(F), 1 μm.
Using gel filtration, we determined the size of the CS chains (Figure 2C). Unexpectedly, the CS chains from the cartilage in CSGalNAcT1-null mice were not the same in size as those from cartilage of wild-type or heterozygous mice. Specifically, CS chains of different lengths in the developing cartilage were identified as individual peaks in samples from wild-type mice (Figure 2C, closed circle). The longest CS chains were preserved in the null mice, but the amount of shorter chains was significantly reduced relative to the amount in wild-type animals (Figure 2C).
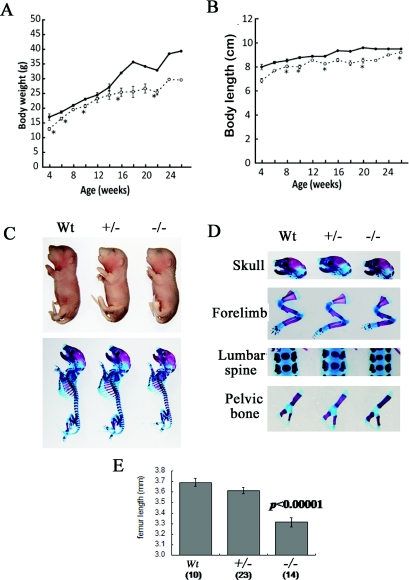
The CSGalNAcT1-null mice had a slightly but significantly shorter mean body length and smaller mean body weight than the wild-type controls (Figures 3A–3C); these differences were most likely due to the shorter limbs and axial skeleton of the null mice (Figure 3D). In contrast, the skulls of the mutant mice were similar in circumference and body weight to those of the heterozygous and wild-type mice (Figure 3D). The null mice had femurs that were 10% shorter than those of wild-type and heterozygous mice on E18.5 (Figure 3E).
Figure 3. CSGalNAcT1-null mice have reduced skeletal growth.
(A and B) The body weight (A) and the body length (B) in the wild-type (●) and CSGalNAcT1-null mice (○) during postnatal development. At 4 weeks after birth, the body mass and the body length of the null mice were slightly reduced compared with those of the wild-type.*P<0.05 (Student's t test). (C) The fetal body (top panel) and skeleton (bottom panel) of wild-type (Wt), heterozygous (+/−) and null (−/−) mice. (D) Various bone segments of the fetal mice. Note that only forelimb bones of the null mice are shorter than those of wild-type mice. (E) Measurements of femur length. The femurs at E18.5 of the null mice are significantly shorter than those of wild-type and heterozygous mice (Student's t test; P<0.00001). The number of the femurs measured for each genotype is shown in parentheses. The results are shown as means±S.E.M.
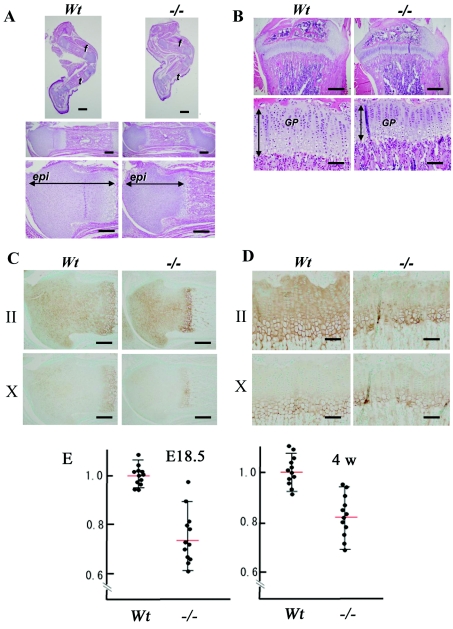
The epiphyseal cartilage of the femur in the CSGalNAcT1-null mice was apparently thinner than that of wild-type mice at E18.5 (Figure 4A). Type-II collagen staining also clearly showed thinning of the cartilage in the null mice (Figure 4C). At 4 weeks of postnatal development, the growth plate in null mice was still smaller than that in the wild-type animals (Figures 3B and 3D). In the null mice, the area of type-X collagen immunostaining, a marker of the hypertrophic zone in cartilage, was slightly smaller than that of wild-type animals at E18.5 and after 4 weeks of postnatal development (Figures 4C and 4D). The cartilage thickness at E18.5 was 25% narrower in CSGalNAcT1-null mice than in wild-type animals (Figure 3E). After 4 weeks of postnatal development, the cartilage of the null mice was still 15% thinner than that of the wild-type controls (Figure 4E). Staining for the cell proliferation marker PCNA was similar in chondrocytes of wild-type and null mice (results not shown), indicating that cell proliferation is not impaired in CSGalNAcT1-null mice and is not the cause of the cartilage abnormalities in the mutant animals (Figures 4C and 4D).
Figure 4. The thickness of the growth plate is reduced in CSGalNAcT1-null mice.
(A) Histological views of the epiphyses of E18.5 wild-type (Wt) and CSGalNAcT1-null (−/−) mice. Upper panels: hind limbs (f, femur; t, tibia), middle panels: the femurs of these littermates; lower panels: higher magnification views of the middle panels focusing on the epiphyseal cartilage. Consistent with the decreased size of femurs and tibiae of the null mice, the femoral epiphyseal cartilage (epi) was reduced in size in null mice compared with that of their wild-type counterparts. Scale bar: upper panels, 1 mm; middle panels, 500 μm; lower panels, 300 μm. (B) Histological findings on the growth plates of 4-week-old wild-type (Wt) and null (−/−) mice. Upper and lower panels show lower and higher magnification respectively. The longitudinal length of the growth plates (GP) was shortened in the null mice (bidirectional arrows, lower panels). Scale bars: upper panels, 800 μm; lower panels, 200 μm. (C and D) Immunodetection of type-II (II; upper panels) and type-X (X; lower panels) collagen in the epiphyses of the wild-type (Wt) and null (−/−) E18.5 fetuses (C) and 4-week-old mice (D). In (C), the type-II collagen-positive area (brown) was seen throughout the epiphyses of both Wt and null mice, despite the finding that cartilage area of the null mice was reduced (upper panels). Even in (D), the reduced size of the type-II collagen reactive-growth plate in the null mice was observed. Judging from the hypertrophic zone marker type-X collagen immunostaining, cartilage from E18.5 (C) and four-week-old (D) animals had a reduced hypertrophic zone. Scale bars: (C), 300 μm; (D), 100 μm. (E) Quantitative measurement of the epiphyseal cartilage in area. Both E18.5 and 4-week-old (4w) femurs were collected. (Mann–Whitney U test; P<0.05). The results are shown as means±S.E.M.
The extracellular matrix of cartilage is composed of two major structural components: collagen fibres and PG aggregates [12]. We examined the ultrastructure and arrangement of the collagen fibres in CSGalNAcT1-null mice using electron microscopy. Type-II collagen fibres were abnormally aggregated in the proliferative and resting layers of the cartilage (Figures 5A–5D). The fibres were thickened and convoluted in the cartilage substance (Figure 5F). The aggregated collagen fibres appeared to be associated with the chondrocytes of the null mice (Figure 5D), and this arrangement was not observed in wild-type tissue (Figures 5A, 5C and 5E). These results indicate that CSGalNAcT1 is important in establishing and/or maintaining normal cartilage ultrastructure including the arrangement of collagen fibres and the CSPG matrix.
DISCUSSION
In the present study, we demonstrated that CSGalNAcT1 is one of the key enzymes for CS biosynthesis in skeletal and cartilage development in vivo by generating and analysing CSGalNAcT1-null mice. The specific substrates of CS-synthesizing glycosyltransferases, including CSGalNAcT1, are firmly established based on in vitro studies [9,10,14]; however, no previous report definitively addresses the in vivo physiological function of any enzyme involved in CS synthesis. In the present study, we make two observations in CSGalNAcT1-null mice: (i) abnormalities in CS biochemistry; and (ii) defects in cartilage development, suggesting that this enzyme is essential for normal cartilage development and normal CS synthesis. During the early stages of embryonic cartilage development, this enzyme was required for at least 50% of the total CS synthesis (Figure 4A), indicating that other enzymes [5,7] cannot fully compensate for the absence of this enzyme (Figure 2B). CSGalNAcT1 is highly expressed in developing cartilage [14]; thus CSGalNAcT1 does have a non-redundant role in CS synthesis. The remaining 50% of CS synthesis in cartilage is probably dependent upon the activities of ChSy-1 (chondroitin synthase 1), ChSy-3 and ChPF (chondroitin polymerization factor) because they are expressed at high levels in this tissue, and any combinations of two of these enzymes can catalyse chondroitin polymerization in in vitro experiments [3,9]. CSGalNAcT2 is much less abundant than CSGalNAcT1, ChSy-1, ChSy-3 or ChPF in cartilage; thus we believe that ChSy-1, ChSy-3 and ChPF contributed more significantly to CS synthesis than CSGalNAcT2 did in the CSGalNAcT1-null mice [14]; however, it is possible that CSGalNAcT2 was more active than ChSy-1, ChSy-3 or ChPF in CS chain elongation rather than initiation of CS synthesis in cartilage of CSGalNAcT1-null mice.
These results also indicate that CSGalNAcT1 may be required for the production of specific short-chain CS species (Figures 2C and 2D). This is probably because CS GAG chains are synthesized differently depending upon the linkage position on the core protein, aggrecan. Other previous in vitro studies have demonstrated that CSGalNAcT1 and other CS-synthesizing enzymes have complex specificities, and these enzymes are thought to recognize the complicated three-dimensional structure of the sugar–core protein linkage to produce CS GAG chains in vivo [5,9]. However, it is also possible that the number of CS chains is determined by the level of initiating CSGalNAcT enzymes in the wild-type mice, but that chain length may be limited, to some extent, by availability of sugar precursors and/or elongating enzyme, leading to some different-sized chains in CSGalNAcT1-null mice. Further studies using these mice are needed to clarify the exact in vivo mechanisms that results in the absence of shorter CS chains in CSGalNAcT1-null mice.
The exact reason for the delay in cartilage development in the CSGalNAcT1-null mice is unknown [28]; however, it has been suggested that Indian Hedgehog and FGF (fibroblast growth factor), key signal molecules operating in early growth plate development, interact with CS [29]. Thus it is likely that the reduction of CS could affect normal chondrogenesis. In addition, type II collagen fibres in the cartilage of CSGalNAcT1-null mice also showed abnormal aggregation, probably due to a reduction in CS (Figure 5), indicating that this enzyme also affects the ultrastructure of cartilage during development. Since the network of type-II collagen fibres outlining the frame of the cartilage is filled with the CSPG gel, it is likely that electrostatic repulsion by negative charges derived from the large amount of CS in the wild-type animals prevents the collagen fibres from aggregating abnormally.
Many hereditary syndromes and idiopathic short stature patients are characterized by abnormal cartilage development, including those with PG defects [30]. Some of the hereditary syndromes and cases of idiopathic short stature might involve defects in CS synthesis and, more specifically, genetic errors in human CSGalNAcT1. We hope that our present study and the novel genetic model, the CSGalNAcT1-null mice, will contribute to the clinical investigations of these diseases.
AUTHOR CONTRIBUTION
Yumi Watanabe, Susumu Higa Onaga, Michiko Sato, Mika Tsujita, Manabu Abe, Rie Natsume, Ayumi Hasegawa, Minesuke Yokoyama and Kenji Sakimura produced CSGalNAcT1-null mice (Figure 1A), and Kosei Takeuchi performed the experiments and analysed the results shown in Figures 1(B), 3(A), 3(B) and 4(E). Minqi Li and Norio Amizuka performed the experiments shown in Figures 2(B), 4(A)–4(D) and 5. Mika Saeki, Tomomi Izumikawa and Hiroshi Kitagawa undertook the experiments shown in Figures 1(C), 2(A) and 2(C). Tatsuya Furuichi and Shiro Ikegawa performed the experiments shown in Figures 3(C)–3(E). Kenji Sakimura, Norio Amizuka, Shiro Ikegawa, Hiroshi Kitagawa, and Michihiro Igarashi designed the experiments. Michihiro Igarashi conceived the ideas and wrote the paper.
ACKNOWLEDGEMENTS
We thank the members of the Department of Animal Resources (Brain Research Institute, Niigata University, Niigata, Japan) for efficient production of the targeted mice.
FUNDING
This work was supported in part by the MEXT (Ministry of Education, Culture, Sports, Science and Technology) of Japan (KAKENHI) [grant numbers 17023019, 22240040 (to M.I.), 20570187 (to K.T.), 21380025 (to H.K), 21390488 (to N.A.), 21249080 (to S.I.), 21300118 (to K.S.)], the Naito Foundation (to H.K.), Research and Development Program for New Bio-industry Initiatives (to K.T.), and Niigata University (to M.I., Y.W. and S.H.O.). The production of the CSGalNAcT1-null mice was supported by TOGONO gene targeting support.
References
- 1.Gandhi N. S., Mancera R. L. The structure of glycosaminoglycans and their interactions with proteins. Chem. Biol. Drug Des. 2008;72:455–492. doi: 10.1111/j.1747-0285.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 2.Kitagawa H., Uyama T., Sugahara K. Molecular cloning and expression of a human chondroitin synthase. J. Biol. Chem. 2001;276:38721–38726. doi: 10.1074/jbc.M106871200. [DOI] [PubMed] [Google Scholar]
- 3.Kitagawa H., Izumikawa T., Uyama T., Sugahara K. Molecular cloning of a chondroitin polymerizing factor that cooperates with chondroitin synthase for chondroitin polymerization. J. Biol. Chem. 2003;278:23666–23671. doi: 10.1074/jbc.M302493200. [DOI] [PubMed] [Google Scholar]
- 4.Uyama T., Kitagawa H., Tamura J., Sugahara K. Molecular cloning and expression of human chondroitin N-acetylgalactosaminyltransferase: the key enzyme for chain initiation and elongation of chondroitin/dermatan sulfate on the protein linkage region tetrasaccharide shared by heparin/heparan sulfate. J. Biol. Chem. 2002;277:8841–8846. doi: 10.1074/jbc.M111434200. [DOI] [PubMed] [Google Scholar]
- 5.Uyama T., Kitagawa H., Tanaka J., Tamura J., Ogawa T., Sugahara K. Molecular cloning and expression of a second chondroitin N-acetylgalactosaminyltransferase involved in the initiation and elongation of chondroitin/dermatan sulfate. J. Biol. Chem. 2003;278:3072–3078. doi: 10.1074/jbc.M209446200. [DOI] [PubMed] [Google Scholar]
- 6.Gotoh M., Yada T., Sato T., Akashima T., Iwasaki H., Mochizuki H., Inaba N., Togayachi A., Kudo T., Watanabe H., et al. Enzymatic synthesis of chondroitin with a novel chondroitin sulfate N-acetylgalactosaminyltransferase that transfers N-acetylgalactosamine to glucuronic acid in initiation and elongation of chondroitin sulfate synthesis. J. Biol. Chem. 2002;277:38179–38188. doi: 10.1074/jbc.M203619200. [DOI] [PubMed] [Google Scholar]
- 7.Sato T., Gotoh M., Kiyohara K., Akashima T., Iwasaki H., Kameyama A., Mochizuki H., Yada T., Inaba N., Togayachi A., et al. Molecular cloning and characterization of a novel human β1,4-N-acetylgalactosaminyltransferase, β4GalNAc-T3, responsible for the synthesis of N,N′-diacetyllactosediamine, GalNAcβ1-4GlcNAc. J. Biol. Chem. 2003;278:3063–3071. doi: 10.1074/jbc.M308857200. [DOI] [PubMed] [Google Scholar]
- 8.Izumikawa T., Uyama T., Okuura Y., Sugahara K., Kitagawa H. Involvement of chondroitin sulfate synthase-3 (chondroitin synthase-2) in chondroitin polymerization through its interaction with chondroitin synthase-1 or chondroitin-polymerizing factor. Biochem. J. 2007;403:545–552. doi: 10.1042/BJ20061876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izumikawa T., Koike T., Shiozawa S., Sugahara K., Tamaura J., Kitagawa H. Identification of chondroitin sulfate glucuronyltransferase as chondroitin synthase-3 involved in chondroitin polymerization, chondroitin polymerization is achieved by multiple enzyme complexes consisting of chondroitin synthase family members. J. Biol. Chem. 2008;283:11396–11406. doi: 10.1074/jbc.M707549200. [DOI] [PubMed] [Google Scholar]
- 10.Sugahara K., Mikami T., Uyama T., Mizuguchi S., Nomura K., Kitagawa H. Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr. Opin. Struct. Biol. 2003;13:612–620. doi: 10.1016/j.sbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Burdan F., Szumilo J., Korobowicz A., Farooquee R., Patel S., Patel A., Dave A., Szumiko M., Solecki M., Klepacz R., Dudka J. Morphology and physiology of the epiphyseal growth plate. Folia Histochem. Cytobiol. 2009;47:5–16. doi: 10.2478/v10042-009-0007-1. [DOI] [PubMed] [Google Scholar]
- 12.Quintana L. M. S., zur Nieden N. I., Semino C. E. Morphogenetic and regulatory mechanisms during developmental chondrogenesis, new paradigms for cartilage tissue engineering. Tissue Eng. B. 2009;15:29–41. doi: 10.1089/ten.teb.2008.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiani C., Chen L., Wu Y. J., Yee A. J., Yang B. B. Roles of aggrecan domains in biosynthesis, modification by glycosaminoglycans and product secretion. Cell Res. 2002;12:19–32. doi: 10.1042/0264-6021:3540199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakai K., Kimata K., Sato T., Gotoh M., Narimatsu H., Shinomiya K., Watanabe H. Chondroitin sulfate N-acetylgalactosaminyltransferase-1 plays a critical role in chondroitin sulfate synthesis in cartilage. J. Biol. Chem. 2007;282:4152–4161. doi: 10.1074/jbc.M606870200. [DOI] [PubMed] [Google Scholar]
- 15.Mishina M., Sakimura K. Conditional gene targeting on the pure C57BL/6 genetic background. Neurosci. Res. 2007;58:105–112. doi: 10.1016/j.neures.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Takeuchi T., Nomura T., Tsujita M., Suzuki M., Fuse T., Mori H., Mishina M. Flp recombinase transgenic mice of C57BL/6 strain for conditional gene targeting. Biochem. Biophys. Res. Commun. 2002;293:953–957. doi: 10.1016/S0006-291X(02)00321-2. [DOI] [PubMed] [Google Scholar]
- 17.Kitayama K., Abe M., Kakizaki T., Honma D., Natsume R., Fukaya M., Watanabe M., Miyazaki J., Mishina M., Sakimura K. Purkinje cell-specific and inducible gene recombination system generated from C57BL/6 mouse ES cells. Biochem. Biophys. Res. Commun. 2001;281:1134–1140. doi: 10.1006/bbrc.2001.4492. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura K., Manabe T., Watanabe M., Mamiya T., Ichikawa R., Kiyama Y., Sanbo M., Yagi T., Inoue Y., Nabeshima T., et al. Enhancement of hippocampal LTP, reference memory and sensorimotor gating in mutant mice lacking a telencephalon-specific cell adhesion molecule. Eur. J. Neurosci. 2001;13:179–189. doi: 10.1046/j.0953-816x.2000.01366.x. [DOI] [PubMed] [Google Scholar]
- 19.Fuse T., Kanai Y., Kanai-Azuma M., Suzuki M., Nakamura K., Mori H., Hayashi Y., Mishina M. Conditional activation of RhoA suppresses the epithelial to mesenchymal transition at the primitive streak during mouse gastrulation. Biochem. Biophys. Res. Commun. 2004;318:665–672. doi: 10.1016/j.bbrc.2004.04.076. [DOI] [PubMed] [Google Scholar]
- 20.Tsujita M., Mori H., Watanabe M., Suzuki M., Miyazaki J., Mishina M. Cerebellar granule cell-specific and inducible expression of Cre recombinase in the mouse. J. Neurosci. 1999;19:10318–10323. doi: 10.1523/JNEUROSCI.19-23-10318.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez E., Roland S. K., Plaas A., Roughley P. J. The glycosaminoglycan attachment regions of human aggrecan. J. Biol. Chem. 2006;281:18444–18450. doi: 10.1074/jbc.M512531200. [DOI] [PubMed] [Google Scholar]
- 22.Kinoshita A., Sugahara K. Microanalysis of glycosaminoglycan-derived oligosaccharides labeled with a fluorophore 2-aminobenzamide by high-performance liquid chromatography: application to disaccharide composition analysis and exosequencing of oligosaccharides. Anal. Biochem. 1999;269:367–378. doi: 10.1006/abio.1999.4027. [DOI] [PubMed] [Google Scholar]
- 23.Koike T., Izumikawa T., Tamura J., Kitagawa H. FAM20B is a kinase that phosphorylates xylose in the glycosaminoglycan-protein linkage region. Biochem. J. 2009;421:157–162. doi: 10.1042/BJ20090474. [DOI] [PubMed] [Google Scholar]
- 24.Amizuka N., Davidson D., Liu H., Valverde-Franco G., Chai S., Maeda T., Ozawa H., Hammond V., Ornitz D. M., Goltzman D., Henderson J. E. Signalling by fibroblast growth factor receptor 3 and parathyroid hormone-related peptide coordinate cartilage and bone development. Bone. 2004;34:13–25. doi: 10.1016/j.bone.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Breton C., Bettler E., Joziasse D. H., Geremia R. A., Imberty A. Sequence-function relationships of prokaryotic and eukaryotic galactosyltransferases. J. Biochem. 1998;123:1000–1009. doi: 10.1093/oxfordjournals.jbchem.a022035. [DOI] [PubMed] [Google Scholar]
- 26.Qasba P. K., Ramakrishnan B., Boeggeman E. Substrate-induced conformational changes in glycosyltransferases. Trends Biochem. Sci. 2005;30:53–62. doi: 10.1016/j.tibs.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Lairson L. L., Henrissat B., Davies G. J., Withers S. G. Glycosyltransferases: structures, functions, and mechanisms. Annu. Rev. Biochem. 2008;77:521–555. doi: 10.1146/annurev.biochem.76.061005.092322. [DOI] [PubMed] [Google Scholar]
- 28.Hiraoka S., Furuichi T., Nishimura G., Shibata S., Yanagishita M., Rimoin D. L., Superti-Furga A., Nikkels P. G., Ogawa M., Katsuyama K., et al. Nucleotide-sugar transporter SLC35D1 is critical to chondroitin sulfate synthesis in cartilage and skeletal development in mouse and human. Nat. Med. 2007;13:1363–1367. doi: 10.1038/nm1655. [DOI] [PubMed] [Google Scholar]
- 29.Cortes M., Baria A. T., Schwartz N. B. Sulfation of chondroitin sulfate proteoglycans is necessary for proper Indian hedgehog signaling in the developing growth plate. Development. 2009;136:1697–1706. doi: 10.1242/dev.030742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz N. B., Domowicz M. Chondrodysplasias due to proteoglycan defects. Glycobiology. 2002;12:57R–68R. doi: 10.1093/glycob/12.4.57r. [DOI] [PubMed] [Google Scholar]