Abstract
Mammalian PKD (protein kinase D) isoforms have been implicated in the regulation of diverse biological processes in response to diacylglycerol and PKC (protein kinase C) signalling. To compare the functions of PKD1 and PKD2 in vivo, we generated mice deficient in either PKD1 or PKD2 enzymatic activity, via homozygous expression of PKD1S744A/S748A or PKD2S707A/S711A ‘knockin’ alleles. We also examined PKD2-deficient mice generated using ‘gene-trap’ technology. We demonstrate that, unlike PKD1, PKD2 catalytic activity is dispensable for normal embryogenesis. We also show that PKD2 is the major PKD isoform expressed in lymphoid tissues, but that PKD2 catalytic activity is not essential for the development of mature peripheral T- and B-lymphocytes. PKD2 catalytic activity is, however, required for efficient antigen receptor-induced cytokine production in T-lymphocytes and for optimal T-cell-dependent antibody responses in vivo. Our results reveal a key in vivo role for PKD2 in regulating the function of mature peripheral lymphocytes during adaptive immune responses. They also confirm the functional importance of PKC-mediated serine phosphorylation of the PKD catalytic domain for PKD activation and downstream signalling and reveal that different PKD family members have unique and non-redundant roles in vivo.
Keywords: cytokine, lymphocyte, protein kinase C (PKC), protein kinase D (PKD), T-cell antigen receptor (TCR)
Abbreviations: DAG, diacylglycerol; DN, double-negative; DP, double-positive; ERK1/2, extracellular-signal-regulated kinase 1/2; ES, embryonic stem; FBS, fetal bovine serum; IFNγ, interferon γ; IL-2, interleukin 2; NF-κB, nuclear factor κB; NP, 4-hydroxy-3-nitrophenyl acetyl; NP-40, Nonidet P40; PE, phycoerythrin; PKC, protein kinase C; PKD, protein kinase D; SP, single-positive; TCR, T-cell antigen receptor
INTRODUCTION
The mammalian serine/threonine PKD (protein kinase D) family comprises three different, but closely related, serine kinases, PKD1, PKD2 and PKD3, all of which have a highly conserved N-terminal regulatory domain containing two cysteine-rich DAG (diacylglycerol)-binding domains and an autoinhibitory PH (pleckstrin homology) domain. PKD family members are activated by binding DAG to their regulatory domain and by PKC (protein kinase C)-mediated phosphorylation of two conserved serine residues in their catalytic domain in response to phospholipase C and DAG signalling [1]. PKDs are highly conserved enzymes, with PKD1 and PKD2 being the most closely related of the three mammalian PKD isoforms, showing ~85% overall identity at the amino acid level. In particular, their catalytic domains and their regulatory DAG-binding domains, which are key for controlling the intracellular localization of these protein kinases, are highly homologous. One consequence of the high level of conservation of PKD1 and PKD2 is that it has been difficult to develop antibodies that can distinguish between these isoforms, although there are antibodies that selectively recognize PKD3. Accordingly, many studies of PKD function do not specify which PKD isoform is being studied. This is important as PKD1 and PKD2 share common regulatory mechanisms in many cell types. For example, ectopically expressed GFP (green fluorescent protein)-tagged PKD1 and endogenous PKD2 show identical mechanisms and kinetics of activation, inactivation and subcellular trafficking in response to antigen receptor triggering in the leukaemic Jurkat T-cell line and in A20 lymphoma B-cells [2,3].
Many adult tissues and cell lines show co-expression of the different PKD isoforms, and it is evident that there can be functional redundancy between these different PKD family members. For example, birds express two PKD isoforms that most closely resemble mammalian PKD1 and PKD3, and either of these two kinases can phosphorylate and regulate the nuclear export of the class II HDACs (histone deacetylases) HDAC5 and HDAC7 in avian B-cells [4]. Similarly, multiple PKD isoforms appear to redundantly regulate Golgi organization and protein transport [5–9], NF-κB (nuclear factor κB) activation and cell-survival responses [10–12], and chemokine release from Toll-like receptor-activated epithelial cells [13]. Functional redundancy between different PKD isoforms has also been observed for the phosphorylation of PKD substrates such as CERT (ceramide-transport protein) [14], PAR1b (partitioning defective 1) [15], sphingosine kinase 2 [16] and HSP27 (heat-shock protein 27) [17,18].
Functional redundancy between different PKD family members expressed in the same tissues/cells is not always the case, however. For example, PKD-mediated phosphorylation of phosphoinositide 4-kinase IIIβ is regulated by PKD1 and PKD2, but not by PKD3 [19]. In addition, there is differential timing of expression of PKD isoforms during early murine embryogenesis [20], and there are examples of cell types where expression of a single PKD isoform appears to dominate. Consequently, specific and non-redundant roles for PKD1 in regulating insulin secretion in pancreatic β-cells [21], TLR (Toll-like receptor)-dependent cytokine expression in macrophages [22], keratinocyte proliferation [23] and pathological cardiac remodelling in vivo [24] have been identified.
Our understanding of the functions of the different mammalian PKD isoforms is complicated further by the observation that distinct PKD protein pools have been observed at different intracellular sites, including the plasma membrane, cytoplasm, Golgi, mitochondria and nucleus [25]. In addition, different PKD isoforms can differentially localize within the same cell [26]. PKDs have also been shown to traffic between different cellular locations in response to specific stimuli [2,3,27,28], which has major consequences for the function of these enzymes. Thus Golgi-localized PKD regulates phosphoinositide 4-kinase IIIβ phosphorylation and vesicle trafficking [19], whereas mitochondrial PKD controls expression of SOD2 (superoxide dismutase 2) via activation of NF-κB [29]. Similarly, plasma-membrane-targeted and cytosolic PKD proteins regulate different aspects of T-cell differentiation [30].
Collectively, these data argue for unique functions for different PKD family members in different cellular contexts. An effective way to probe the physiological role of individual PKD isoforms is to look at the consequences of deleting the individual enzymes using gene-targeting strategies in mice. This approach has been used previously to probe the role of PKD1, revealing that homozygous germline deletion of PKD1 causes embryonic lethality [24]. Moreover, cardiomyocyte-specific deletion of PKD1 has also identified a unique and non-redundant role for PKD1 in pathological cardiac remodelling [24]. There has also been a mutant mouse strain described (129S5-Prkd3Gt(OST191038)Lex/Mmcd) that harbours a gene-trap deletion of PKD3. The mice are reportedly viable, and phenotypic analysis has revealed only a mild skeletal abnormality. To date, however, there have been no studies that have explored the role of PKD2 in vivo, although there are several in vitro studies using RNAi (RNA interference) techniques in cell lines that have proposed important non-redundant functions for PKD2. These include a key role for PKD2 in endothelial cells, to control cell proliferation and survival and to promote angiogenesis [31], and in monocytes, to control migratory responses [32]. Accordingly, the object of the present study was to produce mouse models that would allow an exploration of the role of PKD2 in physiological responses during embryogenesis and in adult mice.
The data show that mice deficient in PKD2 enzymatic activity were born at normal Mendelian frequency and were fertile and healthy. Strikingly, we observed that PKD2, but not PKD1, is selectively expressed in lymphoid cells in adult mice, but that the loss of PKD2 catalytic activity had no obvious effect on the development of mature T- and B-lymphocytes. PKD2 catalytic function was, however, important for effector cytokine production after TCR (T-cell antigen receptor) engagement and also for optimal induction of antibody responses to a model antigen. The data reveal that PKD1, but not PKD2, catalytic activity is essential for normal embryo development, and that PKD2, but not PKD1, has an important role in adult mice to control the function of lymphoid cells during adaptive immune responses.
MATERIALS AND METHODS
Generation of PKD2 and PKD1 transgenic mice
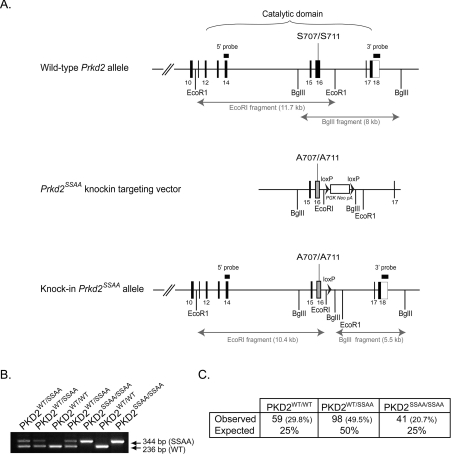
PKD2-knockin mice on a C57BL/6 background were generated by Ozgene. Briefly, genomic fragments containing exons 15–17 of the Prkd2 gene were amplified by PCR from C57BL/6 genomic DNA to generate 5′ and 3′ homology arms that were used to flank a loxP-PGK-NeoR-LoxP selection cassette. Mutations (underlined) within the 5′ homology arm that encoded alanine point mutations of Ser707 and Ser711 (5′-GAGAAGGCCTTCCGGCGCGCCGTG-3′) were introduced by PCR. Forward and reverse DNA sequencing and restriction enzyme digests confirmed the integrity of the final targeting construct. Following homologous recombination of the targeting vector in C57BL/6 ES (embryonic stem) cells, correctly targeted clones were identified by Southern blot analysis. The integrity of the Ser707 and Ser711 knockin point mutations in the targeted ES cells was confirmed by PCR screening and DNA sequencing. Targeted ES cell clones were microinjected into C57BL/6 blastocysts to generate chimaeric animals. After establishment of germline transmission, the PGK-NeoR cassette was deleted by breeding to a transgenic line containing Cre recombinase inserted into the Rosa26 locus (OZ Cre deleter mice). Selective breeding was used to eliminate the Cre gene and PKD2SSAA (PKD2S707A/S711A)-knockin mice were maintained on a pure C57BL/6 genetic background. Genotyping was carried out by PCR of genomic DNA using primers 671−5′armF (5′-AGTGGCACGTTCCCCTTCAATG-3′) and 671−3′armR (5′-CTTTGCCCAATCCCTTACAGCCT-3′), producing products of 236 bp [PKD2WT (wild-type PKD2)] and 344 bp (PKD2SSAA).
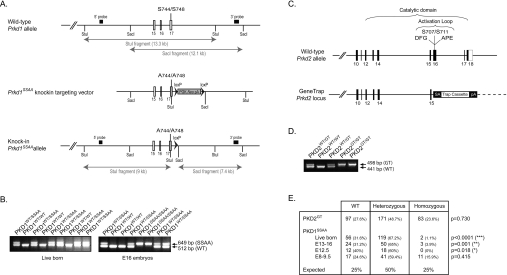
A similar methodology was followed to generate PKD1SSAA (PKD1S744A/S748A)-knockin mice, except that nucleotides encoding Ser744 and Ser748 were mutated to alanine (5′-GCCTTTAGGAGGGCC-3′). Genotyping was carried out by PCR of genomic DNA using primers 579−5′armF2 (5′ CAGCCTTCATGTATCCACCCAACC) and 579−3′armR (5′ TGAACAACAGCAGAGCCCTAACAG), producing products of 512 bp (PKD1WT) and 649 bp (PKD1SSAA).
Generation of PKD2 gene-trap mutant mice
PKD2-deficient mice were generated by a retrovirus-based gene-trap technique. An E14Tg2a.4 ES cell line (derived from the 129/OlaHsd mouse strain) containing a gene-trap cassette inserted into the Prkd2 locus (RRJ380, Bay Genomics) was injected into C57Bl/6J blastocysts, which were subsequently implanted into recipient female mice. The ES cells were subject to DNA sequence analysis before microinjection to confirm that the cell line contained only a single gene-trap cassette, located within the PKD2 locus. The resulting chimaeric mice were bred into a C57BL/6 background to obtain germline transmission. Genotyping was carried out by PCR of genomic DNA using primers PKD2-GTF6 (5′-GTTCCCCTTCCAGTCCATAATCCTCCT-3′), PKD2-GTR2 (5′-CGATGATGCGAGCGAAGCCAAA-3′) and B-geo5′R2 (5′-TGCCAGTTTGAGGGGACGACGACA-3′) in a single PCR, producing products of 498 bp [PKD2GT (PKD2 gene-trap)] and 441 bp (PKD2WT). All experiments on mice were conducted on a mixed 129-C57BL/6J background.
All mice were bred and maintained under specific pathogen-free conditions in the Wellcome Trust Biocentre at the University of Dundee in compliance with U.K. Home Office Animals (Scientific Procedures) Act 1986 guidelines.
Cell culture
Spleens, thymi and lymph nodes were removed from 2–4-month-old mice, mashed in cell strainers and disaggregated, and red blood cells were lysed as required before the lymphocytes were suspended in RPMI 1640 medium containing L-glutamine (Invitrogen) with 10% (v/v) heat-inactivated FBS (fetal bovine serum), 50 units/ml penicillin, 50 μg/ml streptomycin and 50 μM 2-mercaptoethanol. The cells were activated with 200 nM phorbol 12,13-dibutyrate, 2 μM OVA peptide (SIINFEKL) or the indicated concentrations of anti-CD3ϵ antibody (clone 2C11), as indicated, at a density of approx. (1–2) × 106 cells/ml.
Western blot analysis
Protein expression and phosphorylation was assessed using standard Western blotting protocols. Briefly, cell lysates (2 × 107 cells or 100 μg of tissue per ml of lysis buffer) were prepared on ice using NP-40 (Nonidet P40) lysis buffer (50 mM Hepes, pH 7.4, 75 mM NaCl, 1% NP-40, 10 mM NaF, 10 mM iodoacetimide, 1 mM EDTA, 40 mM 2-glycerophosphate, protease inhibitors and 1 mM PMSF) and then centrifuged at 16000 g for 10 min at 4 °C. Protein samples (0.25−0.5 million cell equivalents or 40 μg of tissue extract) were separated by SDS/PAGE (8% or 4–12% gels), transferred on to PVDF membranes and blocked with 5% (w/v) non-fat dried skimmed milk powder in PBS/Tween 20. Blots were probed with antibodies recognizing various phosphorylated and non-phosphorylated (total) proteins, as indicated.
Immunoprecipitation and in vitro kinase assays
Endogenous PKD isoforms were immunoprecipitated from cell lysates with either a PKD1/2-cross-reacting antibody [33] or with a PKD3-specific antibody [34] and recovered with Protein A−Sepharose beads. Exogenous substrate phosphorylation by immunoprecipitated PKD proteins was measured by the incorporation of [γ-32P]ATP into a synthetic PKD substrate peptide (KKLNRTLSVA), as described previously [34].
Flow cytometry
Unless otherwise stated, antibodies conjugated to FITC, PE (phycoerythrin), PE−Cy5.5 (indodicarbocyanine), PE−Cy7 (indotricarbocyanine) or APC (allophycocyanin) were from BD Pharmingen or eBioscience. Cells were stained for the cell-surface expression of the following antigens (clones are in parentheses): CD4 (RM4-5), CD8 (53-6.7), TCR-β (H57-597), B220 (RA3-6B2), IgD (11-26) and IgM (11/41). DN (double-negative) and DP (double-positive) thymocytes were defined by CD8 and CD4 staining. Mature SP (single-positive) thymocytes were defined as TCRβHi and positive for CD4 or CD8 expression. Where required, Fc receptors were blocked with mouse Fc block (CD16/CD32; FcγIII/II receptor; 2.4G2) (BD Pharmingen). Cells were stained with saturating concentrations of antibody in accordance with the manufacturer's instructions and were washed and resuspended in RPMI 1640 medium containing 1% FBS before acquisition. Live cells were gated according to their forward scatter and side scatter. Data were acquired on a FACSCalibur (Becton Dickinson) flow cytometer and analysed using FlowJo software (Treestar).
Cytokine ELISA
Lymph nodes were harvested and disaggregated. CD4+ and CD8+ T-cells were purified by negative selection using CD4+ T Cell Isolation Kit or CD8+ T Cell Isolation Kit (Miltenyi Biotec) by magnetic cell sorting (AutoMacs, Miltenyi Biotec). Cells were stimulated with different amounts of anti-CD3ϵ (clone 2C11). After 18 h, supernatants were collected and cytokine [IFNγ (interferon γ) and IL-2 (interleukin 2)] production was determined by ELISA using Mouse IFNγ ‘Femto-HS’-ELISA Ready-Set-Go and Mouse IL2-ELISA Ready-Set-Go kits (eBiosciences), according to the manufacturer's instructions.
Immunization and Ig ELISA
Sex- and age-matched mice at 8–12 weeks of age were immunized subcutaneously with 100 μg of NP (4-hydroxy-3-nitrophenyl acetyl)-OVA (Biosearch Technologies) pre-adsorbed to aluminium hydroxide (Pierce). Sera were collected after 0, 8, 14 and 29 days. Total and NP-specific Ig levels were determined by standard ELISA using plate-bound anti-mouse Ig (heavy and light chain) or NP−BSA33 and isotype-specific alkaline phosphatase-conjugated goat anti-mouse antibodies (SouthernBiotech). The reaction was revealed with pNPP (p-nitrophenyl phosphate) (Sigma), followed by absorbance reading at 405 nm. Purified Ig isotype standards were used to quantify specific Ig levels.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 5.00 for Macintosh (GraphPad Software). P<0.05 was considered to be statistically significant.
RESULTS
PKD1, but not PKD2, plays an important role in murine embryogenesis
PKD serine kinases are large proteins comprising multiple interaction domains, including both protein−protein- and protein−lipid-binding regions, and have the potential to function as scaffolds. To explore the physiological role of PKD2 in vivo, we therefore decided to produce mice where wild-type PKD2 alleles were replaced with mutant alleles encoding alanine substitutions for Ser707 and Ser711 in the PKD catalytic domain activation loop region. Phosphorylation of these two serine residues is critical for PKD2 catalytic activity [36], hence studies of PKD2SSAA mice allows an assessment of the importance of PKD2 catalytic activity in vivo while bypassing any impact of removing the scaffold function of PKD2. Accordingly, PKD2 S707A and S711A mutations were knocked into the wild-type Prkd2 locus in mouse ES cells by homologous recombination. The resulting ES cells were used to derive mice expressing PKD2SSAA under the control of its natural promoter (Figure 1). Previous studies have shown that homozygous deletion of PKD1 alleles causes embryonic lethality [24]. In striking contrast, homozygous PKD2SSAA-knockin mice were viable, fertile and were phenotypically indistinguishable from their wild-type littermates (Figure 1C and results not shown). This suggests that normal embryonic development is not perturbed by the loss of PKD2 catalytic function.
Figure 1. Generation of mice with a knockin PKD2 mutation.
(A) Depiction of the endogenous mouse Prkd2 allele containing exons 10–18, the knockin construct and the targeted allele with the neomycin cassette removed by Cre recombinase. The black/grey rectangles represent exons and the black arrowheads represent LoxP sites. Thick black lines indicate the positions of the probes used for Southern blot analysis. The knockin allele containing the Ser707/Ser711 mutation in exon 16 is illustrated as a grey rectangle. (B) Genotypes of PKD2SSAA mutant mice were determined by PCR amplification of genomic DNA. The wild-type allele generates a 236 bp product, whereas the knockin allele generates a 344 bp product. The larger knockin allele product is due to the presence of the 108 bp LoxP site and flanking region, which remains in an intronic region following Cre-mediated excision of the neomycin-selection cassette. (C) Heterozygous matings for PKD2SSAA mice were set up and the progeny were genotyped as described in the Materials and methods section. The number (and percentage) of each genotype observed followed by its expected Mendelian frequency. P=0.482 (χ2 test).
We considered the possibility that the small amount of residual catalytic activity still present in the PKD2SSAA mutant protein (see Figure 4A) might be sufficient to permit normal embryogenesis. Therefore, to validate our hypothesis, we generated two additional PKD mutant mouse models. The first had wild-type PKD1 alleles replaced by mutant PKD1 alleles lacking the critical PKC-dependent serine phosphorylation sites, Ser744 and Ser748 (Figure 2A). We also generated PKD2-deficient mice (PKD2GT mice) using an ES cell line containing a gene-trap cassette inserted into the Prkd2 locus (Figure 2C). Our DNA sequence analysis of this ES cell line confirmed that the cell line contained only a single gene-trap cassette, located within the PKD2 locus. We mapped the gene-trap insertion site to intron 15, which disrupts and eliminates the catalytic domain of PKD2 (Figure 2C). Importantly, PKD2GT/GT mice were born at the normal expected frequency (Figure 2E) and were viable, fertile and phenotypically indistinguishable from their wild-type littermates. In contrast, when heterozygous PKD1SSAA mice were inter-crossed, wild-type and heterozygous newborn mice, but not homozygous PKD1SSAA mice, were easily identified (Figure 2E). Of the 177 observed live births, two mice were homozygous for the PKD1SSAA allele (~1%). This indicates that homozygous expression of the PKD1SSAA allele causes embryonic lethality with incomplete penetrance. This is consistent with the report by Olson and colleagues that deletion of exons 12–14 of PKD1, which eliminates the PKD1 catalytic domain, also causes embryonic lethality with incomplete penetrance [24]. Analysis of the stage of embryogenesis that required PKD1 catalytic activity revealed that normal Mendelian frequencies of homozygous PKD1SSAA fetuses could be identified before day 9.5 of embryogenesis, but not later (Figure 2E). Hence the majority of embryos with homozygous substitutions for PKD1SSAA alleles perish early in development. Collectively, these results demonstrate that the catalytic activity of PKD1, but not PKD2, is essential for normal mouse embryogenesis.
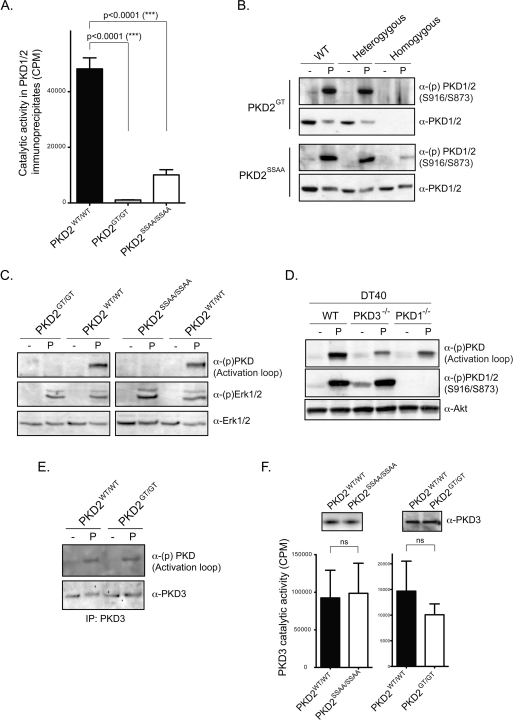
Figure 4. PKD2 is the dominant PKD isoform expressed in murine lymphoid tissue and cells.
(A) Global PKD1/2 catalytic activity in wild-type and PKD2 mutant thymocytes. PKD1/2 proteins were immunoprecipitated from phorbol-ester-activated thymocytes using pan-PKD1/2 antisera [33] before PKD catalytic activity was measured in in vitro peptide substrate kinases assays, as described in the Materials and methods section. Data are from eight wild-type, eight PKD2GT/GT and six PKD2SSAA/SSAA thymi, assayed in three separate experiments. Results are means + S.E.M.; statistical significance was calculated by a Student's t test. (B) PKD1/2 autophosphorylation on Ser916 and Ser873 respectively in whole-cell extracts prepared from control and phorbol-ester-activated wild-type, heterozygous and homozygous PKD2GT and PKD2SSAA thymocytes. Blots are representative of two independent experiments. (C) Analysis of PKD activation loop and ERK1/2 phosphorylation in whole-cell extracts prepared from wild-type, PKD2GT/GT and PKD2SSAA/SSAA splenocytes activated with phorbol ester for 15 min. Similar results were observed in phorbol-ester-activated thymocytes. (D) PKD activation loop and Ser916 phosphorylation in wild-type, PKD1−/− and PKD3−/− DT40 B-cells activated with phorbol ester for 15 min. (E) Analysis of PKD activation loop phosphorylation in PKD3 immunoprecipitates prepared from untreated and phorbol-ester-activated wild-type and PKD2GT/GT thymocytes. (F) PKD3 expression and catalytic activity in primary wild-type and homozygous PKD2GT and PKD2SSAA lymphoid cells. PKD3 proteins were immunoprecipitated from phorbol-ester-activated thymocytes using a specific anti-PKD3 antibody [34] before PKD3 catalytic activity was measured in in vitro peptide substrate kinases assays, as described in the Materials and methods section. Results are from four to six wild-type, PKD2GT/GT and PKD2SSAA/SSAA thymi, assayed in three separate experiments and are means+S.E.M. Student's t test was used to calculate statistical significance.
Figure 2. Generation of PKD2 gene-trap mutant mice and PKD1-knockin mutant mice.
(A) Depiction of the endogenous mouse Prkd1 allele containing exons 15–17, the knockin construct and the targeted allele with the neomycin cassette removed by Cre recombinase. The white/grey rectangles represent exons and the black arrowheads represent LoxP sites. Thick black lines indicate the positions of the probes used for Southern blot analysis. The knockin allele containing the Ser744/Ser748 mutation in exon 16 is illustrated as a grey rectangle. (B) PKD1SSAA-knockin mice were genotyped by PCR-amplification of genomic DNA over the LoxP insertion site as described in the Materials and methods section. (C) Depiction of the wild-type Prkd2 allele and the gene-trap-targeted Prkd2 allele present in the E14Tg2a.4 ES cell line. This insertion disrupts the catalytic domain structure and deletes several key motifs that are important for catalysis and substrate binding (the DFG and APE motifs) as well as the Ser707 and Ser711 phosphorylation sites that are important for PKD2 activation. (D) PKD2GT mice were genotyped by PCR amplification of genomic DNA as described in the Materials and methods section. (E) Heterozygous matings for PKD2GT mice and for PKD1SSAA mice were set up, and the live progeny and PKD1SSAA-knockin embryos were genotyped as described in the Materials and methods section. The number (and percentage) of each genotype observed is shown, followed by its expected Mendelian frequency. The data were analysed using a χ2 test to determine statistical significance.
PKD1 and PKD2 expression patterns in adult mice
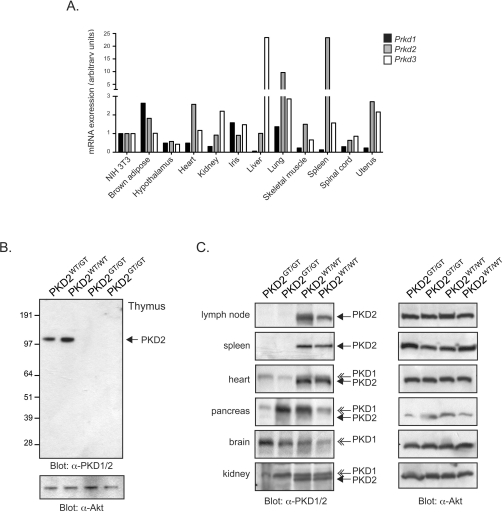
Analysis of EST (expressed sequence tag)/gene expression databases indicates that many adult tissues show co-expression of different PKD isoforms at the mRNA level (Figure 3A). However, the relative expression of specific PKD isoforms at the protein level in different tissues and cells is not well defined. In the context of PKD1 and PKD2, which are highly homologous, most antibodies generated against these kinases cannot discriminate between the two isoforms. For example, the most commonly used ‘pan’-PKD antisera used to detect PKD1 in variety of tissues was raised against the peptide sequence EEREMKALSERVSIL (corresponding to amino acids 904–918 in murine PKD1), but reacts equally well with PKD2. In SDS/PAGE analysis, it is theoretically possible to discriminate PKD1 and PKD2 on the basis of subtle differences in their electrophoretic mobility [10]. However, because the electrophoretic mobility of PKD isoforms can be altered by protein phosphorylation [37], it is not possible to use differential migration on SDS/PAGE gels as a reliable criterion to distinguish PKD1 from PKD2 expression.
Figure 3. Differential expression of PKD1 and PKD2 isoforms in adult tissues.
(A) Prkd1, Prkd2 and Prkd3 gene expression analysis in adult murine tissues. mRNA expression data in the GeneAtlas MOE430 (gcrma) data set was downloaded from the BioGPS gene portal hub (http://www.biogps.gnf.org) and expressed as the fold change in expression in the indicated tissues, compared with that expressed in NIH 3T3 cells. (B) Western blot analysis of PKD1, PKD2 and Akt expression in wild-type compared with PKD2GT/GT thymocytes. Blots are representative of three or more separate experiments. Molecular masses are indicated in kDa. (C) Western blot analysis of PKD1, PKD2 and Akt expression in wild-type compared with PKD2GT/GT adult tissues. Blots are from two separate PKD2SSAA/SSAA mice and their wild-type littermates. Closed arrows indicate PKD2; open arrows indicate PKD1.
In this respect, the exons encoding the region of the protein recognized by the pan-PKD antisera will not be expressed in PKD2GT/GT mice. Accordingly, Western blot analysis with the pan-PKD antisera of tissues from wild-type compared with PKD2GT/GT mice will allow an assessment of which adult tissues express PKD1 and PKD2. Western blot analysis of lymphoid tissue extracts from PKD2GT/GT mice, notably the thymus (Figure 3B), lymph nodes and spleen (Figure 3C), reveal that these tissues do not contain any protein reactive with the pan-PKD1/2 antisera, indicating that T- and B-lymphocytes, which comprise the majority of cells within these tissues, specifically express PKD2, but not PKD1. In contrast, deletion of PKD2 causes little or no loss of total PKD1/2 protein in the brain or the pancreas, but reduces overall PKD1/2 proteins levels in heart and kidney (Figure 3C). Hence PKD1 and PKD2 are co-expressed in many adult tissues, albeit at differing levels, but one exception is in lymphoid cells, which appear to only express PKD2.
PKD2 is the major PKD isoform expressed in murine lymphocytes
The data suggesting that PKD2, but not PKD1, is selectively expressed in lymphoid cells in adult mice were confirmed by in vitro kinase assays, comparing total PKD1/2 catalytic activity in wild-type and PKD2GT/GT thymocytes. Substantial peptide substrate phosphorylation was detected in PKD1/2 immunoprecipitates prepared from phorbol-ester-activated wild-type thymocytes, but not in those prepared from PKD2GT/GT thymocytes (Figure 4A). Another well-characterized marker of endogenous PKD1/PKD2 catalytic activity is the phosphorylation status of a conserved C-terminal autophosphorylation site (Ser916 in PKD1; Ser873 in PKD2). As shown in Figure 4(B), phorbol-ester-activated wild-type, but not PKD2GT/GT, thymocytes contain active autophosphorylated PKD1/2 proteins.
We also examined total PKD1/2 catalytic activity in the PKD2SSAA-knockin lymphocytes. There was no loss of PKD2 protein expression in homozygous PKD2SSAA lymphocytes, but total PKD1/2 catalytic activity was severely impaired, as assessed by in vitro kinase assays of PKD2 proteins immunoprecipitated from phorbol-ester-stimulated wild-type compared with PKD2SSAA/SSAA thymocytes (Figure 4A). Moreover, PKD2SSAA mutant proteins could not effectively autophosphorylate on Ser873 in response to phorbol ester stimulation (Figure 4B). We did, however, detect a small amount of residual PKD1/2 catalytic activity in activated PKD2SSAA/SSAA thymocytes compared with PKD2GT/GT thymocytes. As lymphoid cells do not express PKD1 (see above), low levels of PKD2 catalytic activity can apparently be induced in PKD2SSAA mutant thymocytes independently of PKC-mediated phosphorylation of the PKD2 catalytic domain.
Strikingly, we observed that, although phorbol ester treatment induced significant PKD activation loop phosphorylation in wild-type lymphocytes, this was undetectable in whole-cell extracts prepared from phorbol-ester-stimulated PKD2SSAA/SSAA or PKD2GT/GT lymphocytes, despite normal induction of ERK1/2 (extracellular-signal-regulated kinase 1/2) phosphorylation (Figure 4C). This was surprising as PKD3 is expressed in mammalian lymphocytes [34] (Figure 4E) and the PKD activation loop segment is fully conserved in all three mammalian PKD isoforms. Hence PKD ‘activation loop’ phospho-specific antibodies should cross-react equally with PKD1 (phosphorylated on Ser744/Ser748), PKD2 (phosphorylated on Ser707/Ser711) and PKD3 (phosphorylated on Ser730/Ser734). To clarify whether the PKD activation loop phospho-specific antibody could indeed detect active phosphorylated PKD3 proteins, we analysed avian DT40 B-lymphocytes, which are known to express PKD3 and PKD1 at approximately equimolar levels, but which do not express PKD2 [4]. The data in Figure 4(D) show that deletion of PKD1 in DT40 B-lymphocytes results in a complete loss of PKD1 Ser916 phosphorylation, but only a 2–3-fold reduction in total PKD activation loop phosphorylation levels, after phorbol ester stimulation. Similarly, in PKD3−/− DT40 cells, there was no change in PKD1 Ser916 phosphorylation and again only a 2–3-fold reduction in PKD activation loop phosphorylation levels after phorbol ester stimulation (Figure 4D). Thus PKD activation loop phospho-specific antibodies can very efficiently detect active phosphorylated PKD3 in cells that express high PKD3 protein levels. Indeed, when we immunoprecipitated PKD3 proteins from phorbol-ester-activated PKD2GT/GT thymocytes, we were now able to detect PKD3 activation loop phosphorylation (Figure 4E). Accordingly, the failure to detect any PKD activation loop phosphorylation in whole-cell extracts prepared from PKD2 mutant lymphocytes argues that PKD3 does not make a large quantitative contribution to the total PKD protein pool in murine lymphoid cells/tissues.
Although PKD3 protein expression is very low in murine lymphocytes, it was important to assess whether the loss of PKD2 catalytic activity had any impact on PKD3 expression or activity. We therefore immunoprecipitated PKD3 from control and phorbol-ester-stimulated wild-type and PKD2 mutant thymocytes. In these experiments, we found that PKD3 expression levels and catalytic activity were normal in wild-type, PKD2GT/GT and PKD2SSAA/SSAA thymocytes (Figure 4F). Thus the loss of PKD2 expression and or catalytic activity in lymphoid cells is not compensated for by increased expression/activity of other PKD isoforms. Nor is there any evidence that expression of a catalytically inactive PKD2 has any dominant-negative effect on PKD3 expression or activity.
PKD2 is dispensable for the development of mature T-lymphocytes
Since PKD2 is the major PKD isoform expressed in the thymus and in mature T-lymphocytes (results not shown), we asked whether PKD2 catalytic function is essential for normal T-lymphocyte development. Previous studies have shown that PKD can be activated by the pre-TCR in T-cell progenitors in the thymus and by the mature α/β TCR complex in peripheral T-cells [30,33]. To date, experiments exploring the consequence of PKD activity for TCR function have used transgenesis techniques to target an activated PKD mutant protein (comprising the catalytic domain of PKD1) to either to the membrane or cytosol of pre-T-cells. These experiments demonstrate that constitutively active PKD signalling can substitute for the pre-TCR to drive T-cell proliferation and differentiation in recombinase-gene-null mice [30]. However, experiments with such gain-of-function mutants can inform about the functional capacity of a protein kinase, but they do not assess whether the kinase is essential for a particular biological process.
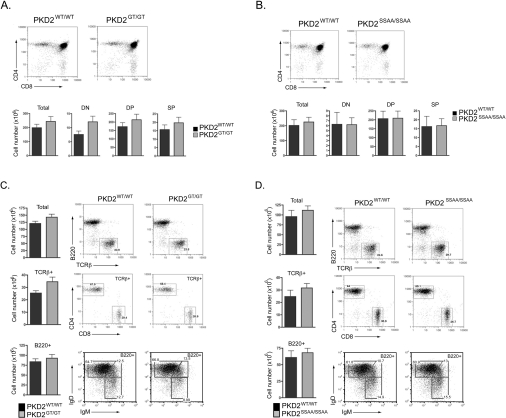
We therefore investigated T-cell development in PKD2GT/GT and PKD2SSAA/SSAA mice using flow cytometry to define the major thymic and peripheral T-cell subpopulations. Early T-lymphocyte progenitors in the thymus are DN for the MHC co-receptors CD4 and CD8. These DN progenitors undergo TCRβ locus rearrangements to produce a TCRβ polypeptide that permits cell-surface expression of the pre-TCR complex. The pre-TCR then supports the survival and rapid clonal expansion of DN progenitors along with their differentiation into CD4+CD8+ DP thymocytes. TCRα chain gene rearrangements then occur, and cells that express a functional, but non-self-reactive, α/β TCR complex differentiate into either CD4+ or CD8+ SP T-lymphocytes. Thymi from PKD2SSAA/SSAA and PKD2GT/GT mice did not display any obvious abnormalities and contained normal numbers of DN, DP and mature SP thymocytes that express high levels of the mature α/β TCR complex (Figures 5A and 5B). Moreover, these mature SP thymocytes were able to exit the thymus and populate peripheral tissues normally, since the frequencies and total numbers of peripheral CD4+ and CD8+ T-lymphocytes in the lymph nodes and spleen of wild-type, PKD2SSAA/SSAA and PKD2GT/GT mice were comparable (Figures 5C and 5D, and results not shown). Similarly, B-lymphocyte development within the bone marrow occurred normally in the absence of PKD2 catalytic activity (results not shown), resulting in normal total numbers of mature IgM/IgD-expressing B-lymphocytes in the spleens and lymph nodes of PKD2SSAA/SSAA and PKD2GT/GT mice (Figures 5C and 5D, and results not shown). Thus PKD2 kinase activity is dispensable for the normal development of mature, peripheral T- and B-lymphocytes.
Figure 5. PKD2 is dispensable for development of mature T- and B-lymphocytes.
Expression of CD4/CD8 DP and SP subsets in thymi from wild-type compared with PKD2GT/GT (A) or wild-type compared with PKD2SSAA/SSAA mice (B). Analysis of peripheral T-cell (TCRβ+CD4+ and TCRβ+CD8+) and B-cell (B220+IgMlo/+IgDlo/+) subsets in spleens from wild-type compared with PKD2GT/GT (C) or wild-type compared with PKD2SSAA/SSAA mice (D). Tissues from the PKD2 mutant mice and wild-type littermate controls were harvested at 8–12 weeks, stained with specific antibodies and analysed by flow cytometry as described in the Materials and methods section. Results are means + S.E.M. (n ≥ 7 mice).
PKD2 catalytic activity is important for mature peripheral T-lymphocytes
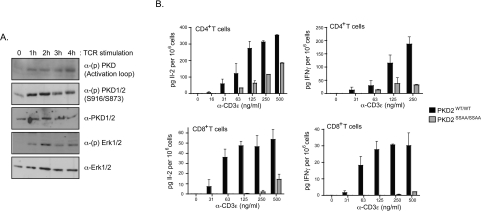
The next question addressed was whether or not PKD2 has any role in peripheral T-cells. As shown in Figure 6(A), TCR engagement induces robust and sustained phosphorylation of PKD2 on Ser707/Ser711, and correspondingly increases PKD2 catalytic activity, resulting in increased autophosphorylation on Ser873. It is well established that phosphorylation of Ser707/Ser711 within the PKD2 catalytic domain is mediated by PKCs [36]. In this context, PKC isoforms that have been implicated in the regulation of PKD activation loop phosphorylation and activation in response to antigen receptor ligation include PKCδ in B-lymphocytes [38] and a redundant role for PKCα and PKCθ in T-lymphocytes (G. Baier, personal communication). It is also known that PKC enzymes have a key role in T-lymphocytes to control expression of cytokine genes in TCR-triggered cells [39–41]. It has also been reported that PKD2 can activate reporter constructs for the cytokine IL-2 in the leukaemic Jurkat T-cell line [42]. We therefore asked whether PKD2 catalytic activity is required to support normal lymphocyte activation responses.
Figure 6. PKD2 catalytic activity is important for peripheral T-lymphocyte function.
(A) CD8+ naïve T-cells were purified from OTI-TCR transgenic lymph node cells and stimulated in vitro with specific OVA peptide. At different time points, cells were lysed and protein phosphorylation and expression was assessed by Western blotting. (B) CD4+ and CD8+ naïve T-cells were purified separately from wild-type, PKD2SSAA/SSAA and PKD2GT/GT lymph nodes and stimulated in vitro via the TCR for 18 h with increasing concentrations of an anti-CD3ϵ monoclonal antibody. IL-2 and IFNγ levels in the culture supernatant were determined by ELISA. Results are means + S.E.M. for triplicate samples and are representative of two separate experiments.
We initially compared the proliferative responses of wild-type and PKD2SSAA/SSAA lymphocytes. However, we observed no major differences in the proliferative capability of mutant PKD2 lymphocytes compared with wild-type controls after in vitro stimulation of the TCR (results not shown). However, it was striking that the ability of both CD4+ and CD8+ T-cells to produce the effector cytokines IL-2 and IFNγ was severely attenuated in PKD2SSAA/SSAA T-lymphocytes that had been stimulated in vitro via the TCR (Figure 6B). Thus PKD2 catalytic activity is required for efficient antigen receptor-induced cytokine production in mature peripheral T-lymphocytes.
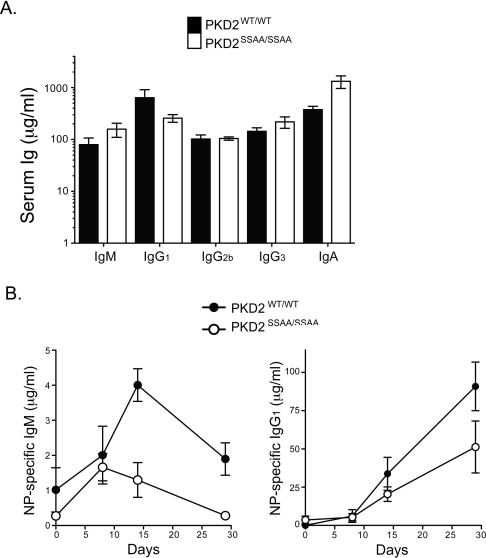
We explored further the in vivo function of PKD2 catalytic activity by looking at the ability of PKD2 mutant mice to mount an immune response to a model antigen. We immunized control and PKD2SSAA/SSAA mice with NP-OVA, an antigen that elicits antibody production in a T-cell-dependent fashion. At different time points after immunization, the serum levels of antigen-specific Igs were determined by ELISA. As shown in Figure 7(A), baseline serum Ig titres were normal in PKD2SSAA/SSAA mice. However, PKD2SSAA/SSAA mice showed reduced antigen-specific IgM and IgG1 responses compared with those of wild-type mice (Figure 7B). Thus PKD2 catalytic activity is important for optimal T-cell-dependent antibody responses in vivo.
Figure 7. PKD2 catalytic activity is important for efficient humoral immune responses against T-cell-dependent antigens.
(A) ELISA of basal serum Ig levels in control and PKD2SSAA-knockin mice. Results are means±S.E.M. (n=4 mice per genotype). (B) PKD2SSAA/SSAA and PKD2WT/WT mice were immunized with NP-OVA on day 0. Serum anti-NP IgM and IgG1 levels were determined on days 8, 14 and 29 after immunization by ELISA of serial dilutions of serum. Results are means±S.E.M. (n=4 mice per genotype). Statistical significance was calculated by two-way ANOVA. P=0.001 and P=0.081 for NP-specific IgM and NP-specific IgG1 levels respectively. Results are representative of two independent experiments.
DISCUSSION
To explore the role of PKD1 and PKD2 in vivo, we have generated mice in which wild-type PKD1 and PKD2 alleles were replaced with mutants with alanine substitutions for Ser744/Ser748 and Ser707/Ser711 respectively. These serine residues are located within the PKD catalytic domains, and in vitro studies have shown that they are phosphorylated by classical/novel PKC isoforms, thereby activating PKD catalytic activity [36,43]. The importance of these phosphorylation events for PKD regulation in vivo was not known. However, the inability of the PKD1SSAA/SSAA mutant to support embryo development now shows that phosphoryation of Ser744/Ser748 is essential for PKD1 function in vivo. The data also show that PKD1 and PKD2 isoforms have unique functions in vivo: loss of PKD1 expression [24] or catalytic function (the present study) results in early embryonic lethality. In contrast, the loss of PKD2 expression or catalytic function in vivo has no impact on normal embryonic development. Moreover, adult mice in which wild-type PKD2 alleles are deleted or replaced with catalytically inactive PKD2 mutants are viable and fertile.
A major aim of the present study was to explore whether there is any unique role for PKD2 in vivo. A striking finding in the present study is our discovery that PKD2 is the major PKD isoform expressed in murine T- and B-lymphocytes; PKD1 is not expressed in these cells, whereas PKD3 is apparently expressed only at very low levels in primary murine lymphoid cells. Moreover, PKD2 catalytic activity was important for effector cytokine production after TCR engagement. PKD2 was also important for optimal induction of antibody responses to a model antigen. PKD2 thus has a unique role in adult mice to control the function of lymphoid cells.
PKDs are highly conserved enzymes, but the evolution of multiple isoforms seems to be associated with evolution of vertebrates, hence amphibians, fish and birds express two PKD isoforms, whereas mammals express three PKD family members. The invertebrate PKD found in Drosophila is most closely homologous with the mammalian PKD3 isoform; the second PKD isoform found in fish and amphibians most closely resembles PKD1; in contrast, PKD2 appears to have evolved with mammals. The present data show that there is differential expression of PKD isoforms in different tissues, in particular the unique expression of PKD2 in primary and secondary lymphoid tissues. Exactly what cellular mechanisms control the differential expression levels of different PKD isoforms in different tissues and at different stages of development remains unclear. Why lymphocytes would express PKD2 rather than PKD1 when they have shared substrate specificities and are regulated by identical intracellular signalling mechanisms is also unclear. Nevertheless, the failure of T-cells to express PKD1 allows PKD2 to have a unique non-redundant role in T-cells to mediate TCR-induced production of the essential cytokines IL-2 and IFNγ.
The induction of IL-2 and IFNγ production in response to TCR engagement is mediated by DAG signalling pathways and PKCs, notably PKCα and PKCθ [39–41]. In vitro studies have also shown that several different PKC isoforms can phosphorylate Ser707 and Ser711 in PKD2, thereby activating the kinase [36], but the importance of this pathway for PKD regulation in vivo was not previously known. The failure of PKD2SSAA/SSAA T-lymphocytes to effectively produce IL-2 and IFNγ thus reveals that PKD functions downstream of PKCs to control cytokine production in T-cells. The cytokines IL-2 and IFNγ play a critical role in adaptive immune responses and the present study thus also provides evidence that PKD2 has a unique non-redundant role to control T-cell functions during adaptive immune responses. A role for PKD2 in mammalian adaptive immune responses is intriguing because DKF-2, a Caenorhabditis elegans PKD acts downstream of DAG and PKCs to regulate innate immunity in the nematode [44]. The participation of PKDs in the mechanisms that control immune responses is therefore a conserved one.
In summary, our in vivo data confirm the functional importance of the PKC-mediated serine phosphorylation sites located within the PKD catalytic domain for PKD1 and PKD2 activation and downstream signalling. They also provide further evidence that PKD isoforms may act as downstream effectors of DAG-regulated PKC enzymes in vivo.
AUTHOR CONTRIBUTION
Sharon Matthews, Maria Navarro, Linda Sinclair, Elizabeth Emslie and Carmen Feijoo-Carnero performed the experiments; Sharon Matthews, Maria Navarro and Doreen Cantrell designed the experiments and analysed the results; Sharon Matthews and Doreen Cantrell wrote the paper.
ACKNOWLEDGEMENTS
We thank members of the Biological Services Resource Unit for mouse care and members of the Cantrell laboratory for the critical reading of the paper.
FUNDING
This project was supported by a Wellcome Trust Principal Research Fellowship (to D.C.) and a Programme Grant [grant number 065975/Z/01/A].
References
- 1.Rozengurt E., Rey O., Waldron R. T. Protein kinase D signaling. J. Biol. Chem. 2005;280:13205–13208. doi: 10.1074/jbc.R500002200. [DOI] [PubMed] [Google Scholar]
- 2.Spitaler M., Emslie E., Wood C. D., Cantrell D. Diacylglycerol and protein kinase D localization during T lymphocyte activation. Immunity. 2006;24:535–546. doi: 10.1016/j.immuni.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Matthews S. A., Iglesias T., Rozengurt E., Cantrell D. Spatial and temporal regulation of protein kinase D (PKD) EMBO J. 2000;19:2935–2945. doi: 10.1093/emboj/19.12.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthews S. A., Liu P., Spitaler M., Olson E. N., McKinsey T. A., Cantrell D. A., Scharenberg A. M. Essential role for protein kinase D family kinases in the regulation of class II histone deacetylases in B lymphocytes. Mol. Cell. Biol. 2006;26:1569–1577. doi: 10.1128/MCB.26.4.1569-1577.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bossard C., Bresson D., Polishchuk R. S., Malhotra V. Dimeric PKD regulates membrane fission to form transport carriers at the TGN. J. Cell Biol. 2007;179:1123–1131. doi: 10.1083/jcb.200703166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J., O'Connor K. L., Hellmich M. R., Greeley G. H., Jr., Townsend C. M., Jr, Evers B. M. The role of protein kinase D in neurotensin secretion mediated by protein kinase C-α/-δ and Rho/Rho kinase. J. Biol. Chem. 2004;279:28466–28474. doi: 10.1074/jbc.M314307200. [DOI] [PubMed] [Google Scholar]
- 7.von Wichert G., Edenfeld T., von Blume J., Krisp H., Krndija D., Schmid H., Oswald F., Lother U., Walther P., Adler G., Seufferlein T. Protein kinase D2 regulates chromogranin A secretion in human BON neuroendocrine tumour cells. Cell. Signalling. 2008;20:925–934. doi: 10.1016/j.cellsig.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Yeaman C., Ayala M. I., Wright J. R., Bard F., Bossard C., Ang A., Maeda Y., Seufferlein T., Mellman I., Nelson W. J., Malhotra V. Protein kinase D regulates basolateral membrane protein exit from trans-Golgi network. Nat. Cell Biol. 2004;6:106–112. doi: 10.1038/ncb1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J., Chen L. A., Townsend C. M., Jr, Evers B. M. PKD1, PKD2, and their substrate Kidins220 regulate neurotensin secretion in the BON human endocrine cell line. J. Biol. Chem. 2008;283:2614–2621. doi: 10.1074/jbc.M707513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mihailovic T., Marx M., Auer A., Van Lint J., Schmid M., Weber C., Seufferlein T. Protein kinase D2 mediates activation of nuclear factor κB by Bcr-Abl in Bcr-Abl+ human myeloid leukemia cells. Cancer Res. 2004;64:8939–8944. doi: 10.1158/0008-5472.CAN-04-0981. [DOI] [PubMed] [Google Scholar]
- 11.Storz P., Doppler H., Toker A. Protein kinase Cδ selectively regulates protein kinase D-dependent activation of NF-κB in oxidative stress signaling. Mol. Cell. Biol. 2004;24:2614–2626. doi: 10.1128/MCB.24.7.2614-2626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Storz P., Toker A. Protein kinase D mediates a stress-induced NF-κB activation and survival pathway. EMBO J. 2003;22:109–120. doi: 10.1093/emboj/cdg009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steiner T. S., Ivison S. M., Yao Y., Kifayet A. Protein kinase D1 and D2 are involved in chemokine release induced by Toll-like receptors 2, 4, and 5. Cell. Immunol. 2010;264:135–142. doi: 10.1016/j.cellimm.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Fugmann T., Hausser A., Schoffler P., Schmid S., Pfizenmaier K., Olayioye M. A. Regulation of secretory transport by protein kinase D-mediated phosphorylation of the ceramide transfer protein. J. Cell Biol. 2007;178:15–22. doi: 10.1083/jcb.200612017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watkins J. L., Lewandowski K. T., Meek S. E., Storz P., Toker A., Piwnica-Worms H. Phosphorylation of the Par-1 polarity kinase by protein kinase D regulates 14-3-3 binding and membrane association. Proc. Natl. Acad. Sci. U.S.A. 2008;105:18378–18383. doi: 10.1073/pnas.0809661105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding G., Sonoda H., Yu H., Kajimoto T., Goparaju S. K., Jahangeer S., Okada T., Nakamura S. Protein kinase D-mediated phosphorylation and nuclear export of sphingosine kinase 2. J. Biol. Chem. 2007;282:27493–27502. doi: 10.1074/jbc.M701641200. [DOI] [PubMed] [Google Scholar]
- 17.Liu P., Scharenberg A. M., Cantrell D. A., Matthews S. A. Protein kinase D enzymes are dispensable for proliferation, survival and antigen receptor-regulated NFκB activity in vertebrate B-cells. FEBS Lett. 2007;581:1377–1382. doi: 10.1016/j.febslet.2007.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan J., Rozengurt E. PKD, PKD2, and p38 MAPK mediate Hsp27 serine-82 phosphorylation induced by neurotensin in pancreatic cancer PANC-1 cells. J. Cell. Biochem. 2008;103:648–662. doi: 10.1002/jcb.21439. [DOI] [PubMed] [Google Scholar]
- 19.Hausser A., Storz P., Martens S., Link G., Toker A., Pfizenmaier K. Protein kinase D regulates vesicular transport by phosphorylating and activating phosphatidylinositol-4 kinase IIIβ at the Golgi complex. Nat. Cell Biol. 2005;7:880–886. doi: 10.1038/ncb1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oster H., Abraham D., Leitges M. Expression of the protein kinase D (PKD) family during mouse embryogenesis. Gene Expression Patterns. 2006;6:400–408. doi: 10.1016/j.modgep.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Sumara G., Formentini I., Collins S., Sumara I., Windak R., Bodenmiller B., Ramracheya R., Caille D., Jiang H., Platt K. A., et al. Regulation of PKD by the MAPK p38δ in insulin secretion and glucose homeostasis. Cell. 2009;136:235–248. doi: 10.1016/j.cell.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park J. E., Kim Y. I., Yi A. K. Protein kinase D1 is essential for MyD88-dependent TLR signaling pathway. J. Immunol. 2009;182:6316–6327. doi: 10.4049/jimmunol.0804239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jadali A., Ghazizadeh S. Protein kinase D is implicated in the reversible commitment to differentiation in primary cultures of mouse keratinocytes. J. Biol. Chem. 2010;285:23387–23397. doi: 10.1074/jbc.M110.105619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fielitz J., Kim M. S., Shelton J. M., Qi X., Hill J. A., Richardson J. A., Bassel-Duby R., Olson E. N. Requirement of protein kinase D1 for pathological cardiac remodeling. Proc. Natl. Acad. Sci. U.S.A. 2008;105:3059–3063. doi: 10.1073/pnas.0712265105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Q. J. PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol. Sci. 2006;27:317–323. doi: 10.1016/j.tips.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Rey O., Yuan J., Young S. H., Rozengurt E. Protein kinase Cν/protein kinase D3 nuclear localization, catalytic activation, and intracellular redistribution in response to G protein-coupled receptor agonists. J. Biol. Chem. 2003;278:23773–23785. doi: 10.1074/jbc.M300226200. [DOI] [PubMed] [Google Scholar]
- 27.Oancea E., Meyer T. Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals. Cell. 1998;95:307–318. doi: 10.1016/s0092-8674(00)81763-8. [DOI] [PubMed] [Google Scholar]
- 28.Rey O., Sinnett-Smith J., Zhukova E., Rozengurt E. Regulated nucleocytoplasmic transport of protein kinase D in response to G protein-coupled receptor activation. J. Biol. Chem. 2001;276:49228–49235. doi: 10.1074/jbc.M109395200. [DOI] [PubMed] [Google Scholar]
- 29.Storz P., Doppler H., Toker A. Protein kinase D mediates mitochondrion-to-nucleus signaling and detoxification from mitochondrial reactive oxygen species. Mol. Cell. Biol. 2005;25:8520–8530. doi: 10.1128/MCB.25.19.8520-8530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marklund U., Lightfoot K., Cantrell D. Intracellular location and cell context-dependent function of protein kinase D. Immunity. 2003;19:491–501. doi: 10.1016/s1074-7613(03)00260-7. [DOI] [PubMed] [Google Scholar]
- 31.Hao Q., Wang L., Zhao Z. J., Tang H. Identification of protein kinase D2 as a pivotal regulator of endothelial cell proliferation, migration, and angiogenesis. J. Biol. Chem. 2009;284:799–806. doi: 10.1074/jbc.M807546200. [DOI] [PubMed] [Google Scholar]
- 32.Tan M., Hao F., Xu X., Chisolm G. M., Cui M. Z. Lysophosphatidylcholine activates a novel PKD2-mediated signaling pathway that controls monocyte migration. Arterioscler. Thromb. Vasc. Biol. 2009;29:1376–1382. doi: 10.1161/ATVBAHA.109.191585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Lint J. V., Sinnett-Smith J., Rozengurt E. Expression and characterization of PKD, a phorbol ester and diacylglycerol-stimulated serine protein kinase. J. Biol. Chem. 1995;270:1455–1461. doi: 10.1074/jbc.270.3.1455. [DOI] [PubMed] [Google Scholar]
- 34.Matthews S. A., Dayalu R., Thompson L. J., Scharenberg A. M. Regulation of protein kinase Cν by the B-cell antigen receptor. J. Biol. Chem. 2003;278:9086–9091. doi: 10.1074/jbc.M211295200. [DOI] [PubMed] [Google Scholar]
- 35.Matthews S. A., Rozengurt E., Cantrell D. Protein kinase D: a selective target for antigen receptors and a downstream target for protein kinase C in lymphocytes. J. Exp. Med. 2000;191:2075–2082. doi: 10.1084/jem.191.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sturany S., Van Lint J., Gilchrist A., Vandenheede J. R., Adler G., Seufferlein T. Mechanism of activation of protein kinase D2 (PKD2) by the CCKB/gastrin receptor. J. Biol. Chem. 2002;277:29431–29436. doi: 10.1074/jbc.M200934200. [DOI] [PubMed] [Google Scholar]
- 37.Matthews S. A., Rozengurt E., Cantrell D. Characterization of serine 916 as an in vivo autophosphorylation site for protein kinase D/protein kinase Cμ. J. Biol. Chem. 1999;274:26543–26549. doi: 10.1074/jbc.274.37.26543. [DOI] [PubMed] [Google Scholar]
- 38.Pracht C., Minguet S., Leitges M., Reth M., Huber M. Association of protein kinase C with the B cell antigen receptor complex. Cell. Signalling. 2006;19:715–722. doi: 10.1016/j.cellsig.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 39.Pfeifhofer C., Kofler K., Gruber T., Tabrizi N. G., Lutz C., Maly K., Leitges M., Baier G. Protein kinase Cθ affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells. J. Exp. Med. 2003;197:1525–1535. doi: 10.1084/jem.20020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfeifhofer C., Gruber T., Letschka T., Thuille N., Lutz-Nicoladoni C., Hermann-Kleiter N., Braun U., Leitges M., Baier G. Defective IgG2a/2b class switching in PKCα−/− mice. J. Immunol. 2006;176:6004–6011. doi: 10.4049/jimmunol.176.10.6004. [DOI] [PubMed] [Google Scholar]
- 41.Gruber T., Hermann-Kleiter N., Pfeifhofer-Obermair C., Lutz-Nicoladoni C., Thuille N., Letschka T., Barsig J., Baudler M., Li J., Metzler B., et al. PKCθ cooperates with PKCα in alloimmune responses of T cells in vivo. Mol. Immunol. 2009;46:2071–2079. doi: 10.1016/j.molimm.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 42.Irie A., Harada K., Tsukamoto H., Kim J. R., Araki N., Nishimura Y. Protein kinase D2 contributes to either IL-2 promoter regulation or induction of cell death upon TCR stimulation depending on its activity in Jurkat cells. Int. Immunol. 2006;18:1737–1747. doi: 10.1093/intimm/dxl108. [DOI] [PubMed] [Google Scholar]
- 43.Iglesias T., Waldron R. T., Rozengurt E. Identification of in vivo phosphorylation sites required for protein kinase D activation. J. Biol. Chem. 1998;273:27662–27667. doi: 10.1074/jbc.273.42.27662. [DOI] [PubMed] [Google Scholar]
- 44.Ren M., Feng H., Fu Y., Land M., Rubin C. S. Protein kinase D is an essential regulator of C. elegans innate immunity. Immunity. 2009;30:521–532. doi: 10.1016/j.immuni.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]