Abstract
This paper is about the taxonomy and genomics of herpesviruses. Each theme is presented as a digest of current information flanked by commentaries on past activities and future directions.
The International Committee on Taxonomy of Viruses recently instituted a major update of herpesvirus classification. The former family Herpesviridae was elevated to a new order, the Herpesvirales, which now accommodates 3 families, 3 subfamilies, 17 genera and 90 species. Future developments will include revisiting the herpesvirus species definition and the criteria used for taxonomic assignment, particularly in regard to the possibilities of classifying the large number of herpesviruses detected only as DNA sequences by polymerase chain reaction.
Nucleotide sequence accessions in primary databases, such as GenBank, consist of the sequences plus annotations of the genetic features. The quality of these accessions is important because they provide a knowledge base that is used widely by the research community. However, updating the accessions to take account of improved knowledge is essentially reserved to the original depositors, and this activity is rarely undertaken. Thus, the primary databases are likely to become antiquated. In contrast, secondary databases are open to curation by experts other than the original depositors, thus increasing the likelihood that they will remain up to date. One of the most promising secondary databases is RefSeq, which aims to furnish the best available annotations for complete genome sequences. Progress in regard to improving the RefSeq herpesvirus accessions is discussed, and insights into particular aspects of herpesvirus genomics arising from this work are reported.
Keywords: Herpesvirus, Classification, Genomics, Herpes simplex virus, Human cytomegalovirus
1. Introduction
Systematics is usually taken to be synonymous with the classification of organisms, but for the purposes of this paper I have employed a broader definition that includes both taxonomy and the systematic aspects of genomics. I address aspects of how the current situation in each area has been reached and how it might develop in future, and provide detailed summaries of current information. I also discuss a selection of new findings that have emerged from genomic studies. Readers should note that herpesvirus names are referred to in this paper not in full but by the acronyms listed in Table 1.
Table 1.
Herpesvirus classification and genome sequences.
| Taxon namea | Common nameb | Acronymc | Strain named | GenBank accession | RefSeq accession | Genome size (kb)e | Reference |
|---|---|---|---|---|---|---|---|
| OrderHerpesvirales | |||||||
| FamilyHerpesviridae | |||||||
| SubfamilyAlphaherpesvirinae | |||||||
| GenusIltovirus | |||||||
| Gallid herpesvirus 1* | Infectious laryngotracheitis virus | GaHV1 | Composite of 6 strains | NC_006623 | 148,687 | Thureen and Keeler (2006) | |
| Psittacid herpesvirus 1 | Pacheco's disease virus | PsHV1 | 97-0001 | AY372243 | NC_005264 | 163,025 | Thureen and Keeler (2006) |
| GenusMardivirus | |||||||
| Columbid herpesvirus 1 | Pigeon herpesvirus | CoHV1 | KP21/23 | ||||
| Gallid herpesvirus 2* | Marek's disease virus type 1 | GaHV2 | Md5 | AF243438 | NC_002229 | 177,874 | Tulman et al. (2000) |
| GA | AF147806 | 174,077 | Lee et al. (2000) | ||||
| CU-2 | EU499381 | 176,922 | Spatz and Rue (2008) | ||||
| Md11 [BAC] | AY510475 | Niikura et al. (2006) | |||||
| CVI988 [BAC] | DQ530348 | Spatz et al. (2007a) | |||||
| RB-1B [BAC] | EF523390 | Spatz et al. (2007b) | |||||
| 584A [BAC] | EU627065 | Spatz et al. (2008) | |||||
| Gallid herpesvirus 3 | Marek's disease virus type 2 | GaHV3 | HPRS24 | AB049735 | NC_002577 | 164,270 | Izumiya et al. (2001) |
| Meleagrid herpesvirus 1 | Turkey herpesvirus | MeHV1 | FC126 (Burmester) | AF291866 | NC_002641 | 159,160 | Afonso et al. (2001) |
| FC126 | AF282130 | 161,484f | Kingham et al. (2001) | ||||
| GenusSimplexvirus | |||||||
| Ateline herpesvirus 1 | Spider monkey herpesvirus | AtHV1 | |||||
| Bovine herpesvirus 2 | Bovine mammillitis virus | BoHV2 | |||||
| Cercopithecine herpesvirus 2 | Simian agent 8 | CeHV2 | B264 | AY714813 | NC_006560 | 150,715 | Tyler et al. (2005) |
| Human herpesvirus 1* | Herpes simplex virus type 1 | HHV1 | 17 | X14112 | NC_001806 | 152,261 | McGeoch et al. (1988) |
| 17 [BAC] | FJ593289 | Cunningham and Davison (unpublished) | |||||
| Human herpesvirus 2 | Herpes simplex virus type 2 | HHV2 | HG52 | Z86099 | NC_001798 | 154,746 | Dolan et al. (1998) |
| Leporid herpesvirus 4 | Leporid herpesvirus 4 | LeHV4 | |||||
| Macacine herpesvirus 1 | B virus | McHV1 | E2490 | AF533768 | NC_004812 | 156,789 | Perelygina et al. (2003) |
| Macropodid herpesvirus 1 | Parma wallaby herpesvirus | MaHV1 | |||||
| Macropodid herpesvirus 2 | Dorcopsis wallaby herpesvirus | MaHV2 | |||||
| Papiine herpesvirus 2 | Herpesvirus papio 2 | PaHV2 | X313 | DQ149153 | NC_007653 | 156,487 | Tyler and Severini (2006) |
| Saimiriine herpesvirus 1 | Marmoset herpesvirus | SaHV1 | |||||
| GenusVaricellovirus | |||||||
| Bovine herpesvirus 1 | Infectious bovine rhinotracheitis virus | BoHV1 | Composite of 5 strains | AJ004801 | NC_001847 | 135,301 | Schwyzer and Ackermann (1996) |
| Bovine herpesvirus 5 | Bovine encephalitis herpesvirus | BoHV5 | SSV507/99 | AY261359 | NC_005261 | 137,821 | Delhon et al. (2003) |
| Bubaline herpesvirus 1 | Water buffalo herpesvirus | BuHV1 | |||||
| Canid herpesvirus 1 | Canine herpesvirus | CaHV1 | |||||
| Caprine herpesvirus 1 | Goat herpesvirus | CpHV1 | |||||
| Cercopithecine herpesvirus 9 | Simian varicella virus | CeHV9 | Delta | AF275348 | NC_002686 | 124,784 | Gray et al. (2001) |
| Cervid herpesvirus 1 | Red deer herpesvirus | CvHV1 | |||||
| Cervid herpesvirus 2 | Reindeer herpesvirus | CvHV2 | |||||
| Equid herpesvirus 1 | Equine abortion virus | EHV1 | Ab4 | AY665713 | NC_001491 | 150,224 | Telford et al. (1992) |
| V592 | AY464052 | 149,430 | Nugent et al. (2006) | ||||
| Equid herpesvirus 3 | Equine coital exanthema virus | EHV3 | |||||
| Equid herpesvirus 4 | Equine rhinopneumonitis virus | EHV4 | NS80567 | AF030027 | NC_001844 | 145,597 | Telford et al. (1998) |
| Equid herpesvirus 9 | Gazelle herpesvirus | EHV9 | P19 | AP010838 | NC_011644 | 148,371 | Fukushi et al. (unpublished) |
| Felid herpesvirus 1 | Feline herpesvirus 1 | FHV1 | C-27 [BAC] | FJ478159 | NC_013590 | Tai et al. (unpublished) | |
| Human herpesvirus 3* | Varicella-zoster virus | HHV3 | Dumas | X04370 | NC_001348 | 124,884 | Davison and Scott (1986) |
| Oka vaccine | AB097932 | 125,078 | Gomi et al. (2002) | ||||
| Oka parental | AB097933 | 125,125 | Gomi et al. (2002) | ||||
| MSP | AY548170 | 124,883 | Grose et al. (2004) | ||||
| BC | AY548171 | 125,459 | Grose et al. (2004) | ||||
| SD | DQ479953 | 125,087 | Peters et al. (2006) | ||||
| Kel | DQ479954 | 125,374 | Peters et al. (2006) | ||||
| 11 | DQ479955 | 125,370 | Peters et al. (2006) | ||||
| 22 | DQ479956 | 124,868 | Peters et al. (2006) | ||||
| 03-500 | DQ479957 | 125,239 | Peters et al. (2006) | ||||
| 36 | DQ479958 | 125,030 | Peters et al. (2006) | ||||
| 49 | DQ479959 | 125,041 | Peters et al. (2006) | ||||
| 8 | DQ479960 | 125,451 | Peters et al. (2006) | ||||
| 32 passage 5 | DQ479961 | 124,945 | Peters et al. (2006) | ||||
| 32 passage 22 | DQ479962 | 125,084 | Peters et al. (2006) | ||||
| 32 passage 72 | DQ479963 | 125,169 | Peters et al. (2006) | ||||
| DR | DQ452050 | 124,770 | Norberg et al. (2006) | ||||
| CA123 | DQ457052 | 124,771 | Norberg et al. (2006) | ||||
| Oka Varilrix | DQ008354 | 124,821 | Tillieux et al. (2008) | ||||
| Oka Varivax | DQ008355 | 124,815 | Tillieux et al. (2008) | ||||
| NH293 | DQ674250 | 124,811 | Loparev et al. (2009) | ||||
| SVETA | EU154348 | 124,813 | Loparev et al. (2009) | ||||
| HJ0 | AJ871403 | 124,928 | Fickenscher et al. (unpublished) | ||||
| Phocid herpesvirus 1 | Harbour seal herpesvirus | PhoHV1 | |||||
| Suid herpesvirus 1 | Pseudorabies virus | SuHV1 | Composite of 6 strains | BK001744 | NC_006151 | 143,461 | Klupp et al. (2004) |
| Unassigned species in the subfamily | |||||||
| Chelonid herpesvirus 5 | Chelonid fibropapilloma-associated herpesvirus | ChHV5 | |||||
| Chelonid herpesvirus 6 | Lung-eye-trachea disease-associated virus | ChHV6 | |||||
| SubfamilyBetaherpesvirinae | |||||||
| GenusCytomegalovirus | |||||||
| Cercopithecine herpesvirus 5 | Simian cytomegalovirus | CeHV5 | 2715 | FJ483968 | NC_012783 | 226,204 | Dolan et al. (unpublished) |
| Colburn | FJ483969 | 219,524 | Dolan et al. (unpublished) | ||||
| Human herpesvirus 5* | Human cytomegalovirus | HHV5 | Merlin | AY446894 | NC_006273 | 235,646 | Dolan et al. (2004) |
| AD169 varUK | BK000394 | 230,290 | Chee et al. (1990) | ||||
| AD169 varUC | FJ527563 | 231,781 | Bradley et al. (2009) | ||||
| Towne varATCC | FJ616285 | 235,147 | Bradley et al. (2009) | ||||
| 3157 | GQ221974 | 235,154 | Cunningham et al. (2010) | ||||
| 3301 | GQ466044 | 235,694 | Cunningham et al. (2010) | ||||
| HAN13 | GQ221973 | 236,219 | Cunningham et al. (2010) | ||||
| HAN20 | GQ396663 | 235,728 | Cunningham et al. (2010) | ||||
| HAN38 | GQ396662 | 236,112 | Cunningham et al. (2010) | ||||
| JP | GQ221975 | 236,375 | Cunningham et al. (2010) | ||||
| Towne varS [BAC] | AY315197 | Dunn et al. (2003) | |||||
| AD169 varATCC [BAC] | AC146999 | Murphy et al. (2003) | |||||
| FIX [BAC] | AC146907 | Murphy et al. (2003) | |||||
| PH [BAC] | AC146904 | Murphy et al. (2003) | |||||
| Towne varS [BAC] | AC146851 | Murphy et al. (2003) | |||||
| Toledo [BAC] | AC146905 | Murphy et al. (2003) | |||||
| TR [BAC] | AC146906 | Murphy et al. (2003) | |||||
| TB40/E [BAC] | EF999921 | Sinzger et al. (2008) | |||||
| Towne varL [BAC] | GQ121041 | Cui et al. (unpublished) | |||||
| Merlin [BAC] | GU179001 | Stanton et al. (unpublished) | |||||
| Macacine herpesvirus 3 | Rhesus cytomegalovirus | McHV3 | 68-1 | AY186194 | NC_006150 | 221,454 | Hansen et al. (2003) |
| 180.92 | DQ120516 | 215,678 | Rivailler et al. (2006) | ||||
| Panine herpesvirus 2 | Chimpanzee cytomegalovirus | PnHV2 | Heberling | AF480884 | NC_003521 | 241,087 | Davison et al. (2003) |
| Unassigned viruses in the genus | |||||||
| Aotine herpesvirus 1 | Herpesvirus aotus type 1 | AoHV1 | S 34E | FJ483970 | 219,474 | Dolan et al. (unpublished) | |
| Aotine herpesvirus 3g | Herpesvirus aotus type 3 | AoHV3 | |||||
| Saimiriine herpesvirus 3 | Squirrel monkey cytomegalovirus | SaHV3 | SqSHV | FJ483967 | [189,956] | Dolan et al. (unpublished) | |
| GenusMuromegalovirus | |||||||
| Murid herpesvirus 1* | Murine cytomegalovirus | MuHV1 | Smith | U68299 | NC_004065 | 230,278 | Rawlinson et al. (1996) |
| C4A | EU579861 | 230,111 | Smith et al. (2008) | ||||
| G4 | EU579859 | 230,227 | Smith et al. (2008) | ||||
| WP15B | EU579860 | 230,118 | Smith et al. (2008) | ||||
| K181 [BAC] | AM886412 | Smith et al. (2008) | |||||
| Murid herpesvirus 2 | Rat cytomegalovirus | MuHV2 | Maastricht | AF232689 | NC_002512 | 230,138 | Vink et al. (2000) |
| GenusRoseolovirus | |||||||
| Human herpesvirus 6 | Human herpesvirus 6 | HHV6 | U1102 | X83413 | NC_001664 | 159,322 | Gompels et al. (1995) |
| Z29 | AF157706 | NC_000898 | 162,114 | Dominguez et al. (1999) | |||
| HST | AB021506 | 161,573 | Isegawa et al. (1999) | ||||
| Human herpesvirus 7 | Human herpesvirus 7 | HHV7 | RK | AF037218 | NC_001716 | 153,080 | Megaw et al. (1998) |
| JI | U43400 | 144,861 | Nicholas (1996) | ||||
| GenusProbiscivirus | |||||||
| Elephantid herpesvirus 1 | Elephant endotheliotropic herpesvirus | ElHV1 | |||||
| Unassigned species in the subfamily | |||||||
| Caviid herpesvirus 2 | Guinea pig cytomegalovirus | CavHV2 | 21222 [BAC] | FJ355434 | NC_011587 | Schleiss et al. (2008) | |
| Suid herpesvirus 2 | Pig cytomegalovirus | SuHV2 | |||||
| Tupaiid herpesvirus 1 | Tupaiid herpesvirus | TuHV1 | 2 | AF281817 | NC_002794 | 195,859 | Bahr and Darai (2001) |
| SubfamilyGammaherpesvirinae | |||||||
| GenusLymphocryptovirus | |||||||
| Callitrichine herpesvirus 3 | Marmoset lymphocryptovirus | CalHV3 | CJ0149 | AF319782 | NC_004367 | 149,696 | Rivailler et al. (2002a) |
| Cercopithecine herpesvirus 14 | African green monkey EBV-like virus | CeHV14 | |||||
| Gorilline herpesvirus 1 | Gorilla herpesvirus | GoHV1 | |||||
| Human herpesvirus 4* | Epstein–Barr virus | HHV4 | B95-8/Raji | AJ507799 | NC_007605 | 171,823 | Baer et al. (1984) and de Jesus et al. (2003) |
| GD1 | AY961628 | 171,657 | Zeng et al. (2005) | ||||
| AG876 | DQ279927 | 172,764 | Dolan et al. (2006) | ||||
| Macacine herpesvirus 4 | Rhesus lymphocryptovirus | McHV4 | LCL8664 | AY037858 | NC_006146 | 171,096 | Rivailler et al. (2002b) |
| Panine herpesvirus 1 | Herpesvirus pan | PnHV1 | |||||
| Papiine herpesvirus 1 | Herpesvirus papio | PaHV1 | |||||
| Pongine herpesvirus 2 | Orangutan herpesvirus | PoHV2 | |||||
| GenusMacavirus | |||||||
| Alcelaphine herpesvirus 1* | Wildebeest-associated malignant catarrhal fever virus | AlHV1 | C500 | AF005370 | NC_002531 | 130,608 | Ensser et al. (1997) |
| AF005368 | 1113 | ||||||
| Alcelaphine herpesvirus 2 | Hartebeest malignant catarrhal fever virus | AlHV2 | |||||
| Bovine herpesvirus 6 | Bovine lymphotropic herpesvirus | BoHV6 | |||||
| Caprine herpesvirus 2 | Caprine herpesvirus 2 | CpHV2 | |||||
| Hippotragine herpesvirus 1 | Roan antelope herpesvirus | HiHV1 | |||||
| Ovine herpesvirus 2 | Sheep-associated malignant catarrhal fever virus | OvHV2 | BJ1035 | AY839756 | NC_007646 | 135,135 | Hart et al. (2007) |
| Composite of several strains | DQ198083 | [131,621] | Taus et al. (2007) | ||||
| Suid herpesvirus 3 | Porcine lymphotropic herpesvirus 1 | SuHV3 | |||||
| Suid herpesvirus 4 | Porcine lymphotropic herpesvirus 2 | SuHV4 | |||||
| Suid herpesvirus 5 | Porcine lymphotropic herpesvirus 3 | SuHV5 | |||||
| GenusPercavirus | |||||||
| Equid herpesvirus 2* | Equine herpesvirus 2 | EHV2 | 86/67 | U20824 | NC_001650 | 184,427 | Telford et al. (1995) |
| Equid herpesvirus 5 | Equine herpesvirus 5 | EHV5 | |||||
| Mustelid herpesvirus 1 | Badger herpesvirus | MusHV1 | |||||
| GenusRhadinovirus | |||||||
| Ateline herpesvirus 2 | Herpesvirus ateles strain 810 | AtHV2 | |||||
| Ateline herpesvirus 3 | Herpesvirus ateles | AtHV3 | 73 | AF083424 | NC_001987 | 108,409 | Albrecht (2000) |
| AF126541 | 1582 | ||||||
| Bovine herpesvirus 4 | Bovine herpesvirus 4 | BoHV4 | 66-p-347 | AF318573 | NC_002665 | 108,873 | Zimmermann et al. (2001) |
| AF092919 | 2267 | ||||||
| Human herpesvirus 8 | Human herpesvirus 8 | HHV8 | GK18 | AF148805 | NC_009333 | 137,969 | Rezaee et al. (2006) |
| BC-1 | U75698 | [137,508] | Russo et al. (1996) | ||||
| U75699 | 801 | ||||||
| Composite of 2 strains | U93872 | [133,661] | Neipel et al. (1997) | ||||
| 219 [BAC] | GQ994935 | Brulois et al. (unpublished) | |||||
| Macacine herpesvirus 5 | Rhesus rhadinovirus | McHV5 | 17577 | AF083501 | NC_003401 | 133,719 | Searles et al. (1999) |
| 26-95 | AF210726 | 130,733 | Alexander et al. (2000) | ||||
| AY528864 | 131,217 | Hansen et al. (unpublished) | |||||
| Murid herpesvirus 4 | Murine herpesvirus 68 | MuHV4 | g2.4 (WUMS) | U97553 | NC_001826 | 119,450 | Virgin et al. (1997) |
| g2.4 | AF105037 | 119,550 | Nash et al. (2001) | ||||
| Saimiriine herpesvirus 2* | Herpesvirus saimiri | SaHV2 | A11 | X64346 | NC_001350 | 112,930 | Albrecht et al. (1992) |
| K03361 | 1444 | ||||||
| C488 | AJ410493 | 113,027 | Ensser et al. (2003) | ||||
| AJ410494 | 1458 | ||||||
| Unassigned viruses in the genus | |||||||
| Leporid herpesvirus 1 | Cottontail rabbit herpesvirus | LeHV1 | |||||
| Leporid herpesvirus 2 | Herpesvirus cuniculi | LeHV2 | |||||
| Leporid herpesvirus 3 | Herpesvirus sylvilagus | LeHV3 | |||||
| Marmodid herpesvirus 1 | Woodchuck herpesvirus | MarHV1 | |||||
| Unassigned species in the subfamily | |||||||
| Equid herpesvirus 7 | Asinine herpesvirus 2 | EHV7 | |||||
| Phocid herpesvirus 2 | Phocid herpesvirus 2 | PhoHV2 | |||||
| Saguinine herpesvirus 1 | Herpesvirus saguinus | SgHV1 | |||||
| Unassigned species in the family | |||||||
| Iguanid herpesvirus 2 | Iguana herpesvirus | IgHV2 | |||||
| Unassigned viruses in the family | |||||||
| Acciptrid herpesvirus 1 | Bald eagle herpesvirus | AcHV1 | |||||
| Anatid herpesvirus 1 | Duck enteritis virus | AnHV1 | VAC | EU082088 | NC_013036 | 158,091 | Li et al. (2009) |
| Boid herpesvirus 1 | Boa herpesvirus | BoiHV1 | |||||
| Callitrichine herpesvirus 2 | Marmoset cytomegalovirus | CalHV2 | |||||
| Caviid herpesvirus 1 | Guinea pig herpesvirus | CavHV1 | |||||
| Caviid herpesvirus 3 | Guinea pig herpesvirus 3 | CavHV3 | |||||
| Cebine herpesvirus 1 | Capuchin herpesvirus AL-5 | CbHV1 | |||||
| Cebine herpesvirus 2 | Capuchin herpesvirus AP-18 | CbHV2 | |||||
| Cercopithecine herpesvirus 3h | SA6 | CeHV3 | |||||
| Cercopithecine herpesvirus 4 | SA15 | CeHV4 | |||||
| Chelonid herpesvirus 1 | Grey patch disease-associated virus | ChHV1 | |||||
| Chelonid herpesvirus 2 | Pacific pond turtle herpesvirus | ChHV2 | |||||
| Chelonid herpesvirus 3 | Painted turtle herpesvirus | ChHV3 | |||||
| Chelonid herpesvirus 4 | Argentine turtle herpesvirus | ChHV4 | |||||
| Ciconiid herpesvirus 1 | Black stork herpesvirus | CiHV1 | |||||
| Cricetid herpesvirus | Hamster herpesvirus | CrHV1 | |||||
| Elapid herpesvirus 1 | Indian cobra herpesvirus | EpHV1 | |||||
| Erinaceid herpesvirus 1 | European hedgehog herpesvirus | ErHV1 | |||||
| Falconid herpesvirus 1i | Falcon inclusion body disease virus | FaHV1 | |||||
| Gruid herpesvirus 1 | Crane herpesvirus | GrHV1 | |||||
| Iguanid herpesvirus 1 | Green iguana herpesvirus | IgHV1 | |||||
| Lacertid herpesvirus | Green lizard herpesvirus | LaHV1 | |||||
| Macacine herpesvirus 6 | Rhesus leukocyte-associated herpesvirus strain 1 | McHV6 | |||||
| Macacine herpesvirus 7 | Herpesvirus cyclopis | McHV7 | |||||
| Murid herpesvirus 3 | Mouse thymic herpesvirus | MuHV3 | |||||
| Murid herpesvirus 5 | Field mouse herpesvirus | MuHV5 | |||||
| Murid herpesvirus 6 | Sand rat nuclear inclusion agent | MuHV6 | |||||
| Murid herpesvirus 7 | Wood mouse herpesvirus | MuHV7 | WM8 | GQ169129 | 120108 | Hughes et al. (2010) | |
| Ovine herpesvirus 1 | Sheep pulmonary adenomatosis-associated herpesvirus | OvHV1 | |||||
| Perdicid herpesvirus 1 | Bobwhite quail herpesvirus | PdHV1 | |||||
| Phalacrocoracid herpesvirus 1 | Cormorant herpesvirus | PhHV1 | |||||
| Procyonid herpesvirus 1 | Kinkajou herpesvirus | PrHV1 | |||||
| Sciurid herpesvirus 1 | Ground squirrel cytomegalovirus | ScHV1 | |||||
| Sciurid herpesvirus 2 | Ground squirrel herpesvirus | ScHV2 | |||||
| Sphenicid herpesvirus 1 | Black footed penguin herpesvirus | SpHV1 | |||||
| Strigid herpesvirus 1i | Owl hepatosplenitis virus | StHV1 | |||||
| FamilyAlloherpesviridae | |||||||
| GenusBatrachovirus | |||||||
| Ranid herpesvirus 1* | Lucké tumor herpesvirus | RaHV1 | McKinnell | DQ665917 | NC_008211 | 220,859 | Davison et al. (2006) |
| Ranid herpesvirus 2 | Frog virus 4 | RaHV2 | Rafferty | DQ665652 | NC_008210 | 231,801 | Davison et al. (2006) |
| GenusCyprinivirus | |||||||
| Cyprinid herpesvirus 1 | Carp pox herpesvirus | CyHV1 | |||||
| Cyprinid herpesvirus 2 | Goldfish haematopoietic necrosis virus | CyHV2 | |||||
| Cyprinid herpesvirus 3* | Koi herpesvirus | CyHV3 | U | DQ657948 | NC_009127 | 295,146 | Aoki et al. (2007) |
| J | AP008984 | 295,052 | Aoki et al. (2007) | ||||
| I | DQ177346 | 295,138 | Aoki et al. (2007) | ||||
| GenusIctalurivirus | |||||||
| Ictalurid herpesvirus 1* | Channel catfish virus | IcHV1 | Auburn 1 | M75136 | NC_001493 | 134,226 | Davison (1992) |
| Ictalurid herpesvirus 2 | Black bullhead herpesvirus | IcHV2 | |||||
| Acipenserid herpesvirus 2 | White sturgeon herpesvirus 2 | AciHV2 | |||||
| GenusSalmonivirus | |||||||
| Salmonid herpesvirus 1* | Herpesvirus salmonis | SalHV1 | |||||
| Salmonid herpesvirus 2 | Oncorhynchus masou herpesvirus | SalHV2 | |||||
| Salmonid herpesvirus 3 | Epizootic epitheliotropic disease virus | SalHV3 | |||||
| Unassigned viruses in the family | |||||||
| Acipenserid herpesvirus 1 | White sturgeon herpesvirus 1 | AciHV1 | |||||
| Anguillid herpesvirus 1 | Eel herpesvirus | AngHV1 | 500138 | FJ940765 | NC_013668 | 248,531 | van Beurden et al. (2010) |
| Esocid herpesvirus 1 | Northern pike herpesvirus | EsHV1 | |||||
| Percid herpesvirus 1 | Walleye epidermal hyperplasia herpesvirus | PeHV1 | |||||
| Pleuronectid herpesvirus 1 | Turbot herpesvirus | PlHV1 | |||||
| FamilyMalacoherpesviridae | |||||||
| GenusOstreavirus | |||||||
| Ostreid herpesvirus 1* | Oyster herpesvirus | OsHV1 | AY509253 | NC_005881 | 207,439 | Davison et al. (2005b) | |
The type species in each genus is indicated by an asterisk. Formal taxonomic names are italicized. The names of tentative species and unassigned viruses have no taxonomic standing are not italicized.
Virus common names have no taxonomic standing. Some viruses have more than one common name. A single, English, common name is given for each virus.
Acronyms or abbreviations may apply to viruses, not species, and have no taxonomic standing. A hyphen may precede the number.
Where a sequence was determined from a bacterial artificial chromosome (BAC), this is indicated in square brackets.
The sizes of complete or almost complete virus genomes are shown; the latter in square brackets. The sizes of BAC-derived sequences are not shown, since they may contain all or part of the sequence of the BAC vector. Sizes in square brackets indicate sequences that are incomplete in extent or construction. For members of the subfamily Gammaherpesvirinae other than EHV2, actual genome sizes are larger than those listed (150–180 kbp), owing to the presence of variable copy numbers of the terminal repeat flanking the unique region. A single size value represents either the unique region only or the unique region flanked by partial terminal repeats. Two values represent the unique region and the terminal repeat.
The size is that of the genome sequence reconstructed from the GenBank accession.
This is probably a strain of AoHV1 (Ebeling et al., 1983).
This is a strain of CeHV5 (G. Hayward, personal communication).
This is a strain of CoHV1 (Gailbreath and Oaks, 2008).
2. Herpesvirus taxonomy
2.1. Introduction
Taxonomy – the systematic classification of living organisms – is an exercise in categorization that helps human beings cope with the world. Of the taxa into which organisms may be classified, only those of species, genus, subfamily, family and order are presently applicable to viruses. The grouping of viruses into these taxa proceeds on the basis of identifying shared properties, and has applications in understanding virus evolution, identifying the origins of emerging diseases, and developing rational treatment strategies.
The criteria used to classify viruses have necessarily depended on the knowledge and technology available at the time. In early days, aspects of biology such as host range and broad pathogenic and epidemiological features of the disease constituted the majority of information available. With the advent of electron microscopy and physicochemical methods, data on the morphological properties and constituents of virus particles became accessible, and these are still important today in assigning viruses to higher taxa. Thus, for example, all herpesviruses share a capsid structure that consists of a DNA core surrounded by an icosahedral (20-faceted) capsid consisting of 12 pentavalent and 150 hexavalent capsomeres. The capsid is embedded in a proteinaceous matrix called the tegument, which in turn is invested in a glycoprotein-containing lipid envelope. The advent of specific antibodies facilitated the study of antigenic relationships, which are generally detectable only between closely related viruses; in the case of herpesviruses, those in the same genus. The era of nucleic acid sequencing has led to the wide application of sequence-based phylogeny to classification. Indeed, the quantitative basis and broad utility of this approach have resulted in this becoming a key taxonomical discriminator in all parts of the tree of life, to the point where it dominates all other criteria.
2.2. Resources
Advances in virus taxonomy have been recorded in a series of eight reports published at intervals since 1971 by the International Committee on Taxonomy of Viruses (ICTV). The latest report in book form was published in 2005 (Fauquet et al., 2005). In deference to the electronic age, annual updates of the list of virus species were published in 2007, 2008 and 2009 at the ICTV website (http://www.ictvonline.org).
Virus classification is advanced through a voting procedure involving the members of the ICTV. The proposals on which voting takes place are prepared by the ICTV Executive Committee from submissions made by individuals in the virological community, in particular those associated with Study Groups devoted to particular virus groups (usually families). The development of taxonomy as summarized in the ICTV reports is regularly promoted, supplemented and discussed by expert publications from Study Groups, including that focused on the herpesviruses.
2.3. Past
In the first ICTV report (Wildy, 1971), the genus Herpesvirus was established, consisting of 23 viruses and 4 groups of viruses named according to the usages of the day (e.g. herpesvirus of saimiri). In the second ICTV report (Fenner, 1976), this genus was elevated to the family Herpetoviridae, which, presumably because of the misleading association of this name with reptiles and amphibians, was renamed Herpesviridae in the third ICTV report (Matthews, 1979). Also, a formal system for naming herpesviruses was founded (Roizman et al., 1973), implemented (Fenner, 1976; Matthews, 1979), elaborated (Roizman et al., 1981) and consolidated (Francki et al., 1991; Roizman et al., 1992).
This naming system specified that each herpesvirus should be named after the taxon (family or subfamily) to which its primary natural host belongs. The subfamily name was used for viruses from members of the family Bovidae or from primates (the virus name ending in –ine, e.g. bovine), and the host family name for other viruses (ending in –id, e.g. equid). Human herpesviruses were treated as an exception (i.e. human rather than hominid). Following the host-derived term, the word herpesvirus was added, succeeded by an arabic number, which bore no implied meaning about the taxonomic or biological properties of the virus. Thus, the formal name of pseudorabies virus (also known as Aujeszky's disease virus) was established as suid herpesvirus 1. Since herpesviruses had previously been named on an ad hoc basis, sometimes with the effect that a virus might have several names, the formal system promised a degree of clarity and simplicity to students and scientists in the research field. However, a number of practical disadvantages of the formal naming system emerged. Most importantly, many virus names (e.g. Epstein–Barr virus) were so widely accepted that they could not be dislodged (e.g. in this case by human herpesvirus 4). This led to the use of a dual nomenclature in the literature for some herpesviruses.
Nonetheless, classification of herpesviruses continued and expanded. At the time of the third ICTV report (Matthews, 1979), the family Herpesviridae was divided into 3 subfamilies (Alphaherpesvirinae, Betaherpesvirinae and Gammaherpesvirinae) and 5 unnamed genera, and 21 viruses were listed. A subsequent list compiled by the Study Group contained 89 viruses (Roizman et al., 1981).
At the time of the seventh report (Minson et al., 2000), the ICTV adopted the species concept, which recognizes that a virus and the species to which it belongs fall into different categories, the real and the conceptual (Van Regenmortel, 1990). This sea change put the activities of the ICTV on a more logical footing, and also limited its authority to determining formal taxonomical nomenclature and classification, and not virus names, abbreviations and vernacular usages of formal names. It also brought about a simplification by effectively removing any implied taxonomical standing for viruses denoted previously as tentative species or unassigned viruses. Moreover, it reduced tensions concerning the pervasive use of a dual system for herpesvirus names, with the effect that the ICTV approach has been adopted for some (e.g. equine abortion virus is now well known as equid herpesvirus 1) and the ad hoc name for others (e.g. Kaposi's sarcoma-associated herpesvirus, rather than human herpesvirus 8). The rules for virus taxonomical names are that they are written in italics with the first letter capitalized, and never abbreviated. Thus, for example, pseudorabies virus belongs to the species Suid herpesvirus 1, genus Varicellovirus, subfamily Alphaherpesvirinae, family Herpesviridae. If an abbreviation based on the formal name is used (in this case, SuHV1 or SuHV-1), it represents the virus and not the species.
As the classification developed, it became clear from genome studies that IcHV1 and OsHV1 are very distant relatives of each other and other herpesviruses. For this reason, a new order, Herpesvirales, was created (Davison et al., 2009). This accommodates three families, namely the revised family Herpesviridae, which contains mammal, bird and reptile viruses, the new family Alloherpesviridae, which incorporates bony fish and frog viruses, and the new family Malacoherpesviridae, which contains OsHV1. Also, species representing viruses of non-human primates were renamed after the host genus rather than the subfamily, in order to cope with their rising number.
2.4. Present
The most recent update (2009) of herpesvirus taxonomy largely concerned the introduction of additional taxa to the family Alloherpesviridae. The current, complete list of herpesvirus taxa is given in the first column of Table 1. This table also provides the common names and acronyms (abbreviations) of the viruses. The right-hand part of the table conveys genomic information, which is discussed below. The order Herpesvirales now consists of 3 families, 3 subfamilies, 17 genera and 90 species. A total of 48 tentative species and unassigned viruses are also listed in Table 1, but, as discussed above, these viruses are not part of formal taxonomy and are included out of convenience rather than necessity. Many are longstanding entities that may no longer exist in laboratories and for which sequence data are not (and never will be) available. Moreover, some are now known or suspected to be strains of other viruses (see the footnotes to Table 1).
2.5. Future
The ICTV Herpesvirales Study Group is continuing to progress the classification of herpesviruses as they are identified and characterized. It is also taking forward discussions that will shape herpesvirus taxonomy in future. These include revisiting the herpesvirus species definition and the criteria used for taxonomic assignment, particularly in regard to the possibilities of classifying the large number of herpesviruses detected only as DNA sequences by polymerase chain reaction (PCR).
The ICTV follows the principle that “a virus species is a polythetic class of viruses that constitutes a replicating lineage and occupies a particular ecological niche” (Van Regenmortel, 1992). The polythetic nature of virus species (that is, having some but not all properties in common) implies that a species cannot be delineated on the basis of a single property. In line with this principle, herpesviruses are classified as distinct species if “(a) their nucleotide sequences differ in a readily assayable and distinctive manner across the entire genome and (b) they occupy different ecological niches by virtue of their distinct epidemiology and pathogenesis or their distinct natural hosts” (Roizman et al., 1992; Minson et al., 2000; Davison et al., 2005a). However, the dominant criterion in virus classification, as in the classification of all organisms, is now sequence-based phylogeny. The impact of this development upon the continuing viability of herpesvirus classification under the polythetic rule will need careful consideration. Discussions are likely to be driven to some extent by the ongoing PCR-based discovery of large numbers of herpesviruses that potentially belong to new taxa (e.g. Ehlers et al., 2008). Finally, the nomenclature of herpesvirus species is inherently unstable, because of its dependence on host nomenclature and the motivation to rename species that become very numerous in certain host families. It will be a challenge to maintain a balance between utility and stability.
3. Herpesvirus genomics
3.1. Introduction
Genomics – the study of the structure, function and evolution of genomes – underlies most discoveries in modern biology. Since the inception of nucleic acid sequencing, and increasingly since, interpretation of the data has lagged behind its generation. Hence, understanding the meaning of genomic data is at a premium. Interpretation of sequence data is served greatly by computer analyses in areas such as comparative genomics, pattern-based bioinformatics and proteomics. However, it is not best advanced when treated as a robotic exercise. Genomics, even of herpesviruses, has an actively speculative edge where new discoveries are being made and old models are being refined.
3.2. Resources
The primary nucleotide sequence databases, of which perhaps the most prominent is GenBank at the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov), are a vital resource to experimenters. NCBI is also putting effort into the Reference Sequence Project (RefSeq; http://www.ncbi.nlm.nih.gov/RefSeq), which aims to provide a comprehensive, integrated, non-redundant, well annotated set of sequences for major research organisms, including viruses (http://www.ncbi.nlm.nih.gov/genomes/GenomesHome.cgi?taxid=10239) (Pruitt et al., 2003). The herpesvirus RefSeqs are under development (http://www.ncbi.nlm.nih.gov/genomes/GenomesGroup.cgi?taxid=548681).
3.3. Past
The utility of the primary databases is limited by three major factors: the accuracy of the sequence, the quality of the annotation (i.e. the description of features in the genome—genes, RNAs, coding regions and so forth), and the improvements made to both over time. However, for understandable reasons, updating an accession is effectively restricted to the original depositor. The tendency is for this to be done rarely, if at all, and this is causing the primary databases to become antiquated. Against this background, an enterprise in updating herpesvirus accessions in primary sequence databases seems forlorn. RefSeq provides a way to counter the disadvantages of the primary databases by enabling updating and curation by experts other than the original depositor.
3.4. Present
The right-hand part of Table 1 lists information on the 51 herpesvirus species or potential species from which at least one virus has been sequenced to date. These species are represented by 122 complete or almost complete genome sequences. Partial sequence information is available on nearly all the other herpesviruses ranked in formal taxa; indeed, this is now a requirement for classification. At an experimental level, many herpesviruses are manipulated in the form of bacterial artificial chromosomes (BACs), the sequences of several of which are available; for example, those representing GaHV2, HHV1 and HHV5. The latest sequencing techniques are starting to be applied to herpesviruses, including the Roche 454 instrument (e.g. GaHV2 and MuHV1) or Illumina Genome Analyzer (e.g. HHV1 and HHV5).
I have been active in updating the herpesvirus RefSeqs, and thus far have processed all members of the subfamily Alphaherpesvirinae, plus some other viruses outside this group (e.g. HHV5). In addition to identifying potential sequence errors and upgrading the standard of annotation, one of the main tasks has been to start applying a systematic nomenclature to genome features. Members of the best studied family (Herpesviridae) in the order Herpesvirales share 44 genes apparently inherited from a common ancestor (core genes; Table 2), and yet the names of these orthologous genes and their encoded proteins vary from virus to virus. This has led to confusion in the research field. My tack has been to retain original gene names so that workers can find their way round an accession, and to apply standard names to the encoded proteins. This is aimed at improving the utility of the database accessions; for example, database searches would then return the same name for orthologous proteins from different viruses. Table 2 shows the current scheme used to annotate the RefSeqs for members of the subfamily Alphaherpesvirinae. A more extensive form of this database is available from me in spreadsheet form. As well as providing additional information, this database permits the genes and their encoded proteins to be sorted according to their order along a particular genome.
Table 2.
Names and conservation of proteins in members of the subfamily Alphaherpesvirinae.
 |
 |
 |
aThe viruses are grouped taxonomically, as indicated by the vertical lines, though AnHV1 has not yet been classified. The presence ( ) or absence () of apparent gene orthologues is indicated for each genome, with the UL and US regions oriented to correspond to the conventional arrangement in the varicelloviruses. Gene names differ between viruses, and are available in the RefSeqs.
) or absence () of apparent gene orthologues is indicated for each genome, with the UL and US regions oriented to correspond to the conventional arrangement in the varicelloviruses. Gene names differ between viruses, and are available in the RefSeqs.
bThe names of proteins encoded by genes in the inverted repeats in certain viruses, and which are therefore listed twice, are italicized.
cCore genes, inherited from an ancestor of alpha-, beta- and gammaherpesviruses; alpha genes, inherited from an ancestor of alphaherpesviruses. This is a definition based on evolution – certain core or alpha genes may have been lost in some lineages.
dGenes that have paralogues in the same or other herpesviruses belong to gene families. The paralogues have presumably arisen by gene duplication.
3.5. Future
The long term aim of updating herpesvirus RefSeqs is to ensure that are correct (free from errors), complete (adequately annotated), clear (using standard nomenclature) and current (up to date). From experience, I foresee that fulfilling this aim to an acceptable standard will be demanding and that many considerations will have to be weighed. Most importantly, the list of standard protein names must be considered as needing development in future, since the process of establishing names is fraught with pitfalls that are not developed further here. Thus, as with the names of herpesvirus species in Table 1, the protein names in Table 2 are provisional. It is intended that they will improve and harmonize as knowledge increases and data are imported from the other herpesvirus subfamilies. The substantial efforts of Mocaski (2007) to apply a standard nomenclature to the proteins encoded by core genes also deserve recognition in this regard.
4. Interpretations
4.1. Introduction
The process of systematizing information as described above tends to yield new insights, particularly those arising from comparative genomics. This section highlights three examples that were unearthed while reannotating genomes of members of the subfamily Alphaherpesvirinae and members of the genus Cytomegalovirus in the subfamily Betaherpesvirinae. These examples illustrate the fact that new discoveries remain to be made even with well characterized herpesviruses.
4.2. UL56 gene family
Previously, I reported that orthologues of gene UL56 are not confined to members of the genus Simplexvirus in the subfamily Alphaherpesvirinae, where they were first identified, but are also found among members of the genera Varicellovirus and Iltovirus (Davison, 2007). Subsequent analysis (Fig. 1 and Table 2) indicated that orthologues are also present in members of the genus Mardivirus. These studies indicate that all members of the subfamily Alphaherpesvirinae except BoHV1 and BoHV5 encode a UL56 orthologue. The appropriate amendments were applied to the RefSeqs by the middle of 2007.
Fig. 1.
Amino acid sequence alignment of the C-terminal regions of membrane protein UL56 and membrane protein UL56A from members of the subfamily Alphaherpesvirinae. The sequences are aligned only in the 15 residues at the left, as relationships among the sequences elsewhere are overall not detectable. The locations of PPXY (and related LPPY) motifs are highlighted in light grey, and other conserved residues located in or between the motifs are underlined. Additional PPXY and PPXY-related motifs in the N-terminal regions (indicated by >) are noted in square brackets. Predicted transmembrane domains are highlighted in dark grey. That for GaHV3 is in lower case to indicate that a correction of a putative frameshift (located approximately) was invoked. The total number of residues in each protein is indicated on the right.
All the versions of the UL56 product (membrane protein UL56) have a C-terminal hydrophobic domain, and sequence similarity is limited to a central region consisting in most instances of two PPXY motifs (Fig. 1). Most of the proteins have additional PPXY elements elsewhere in their sequences. Moreover, two viruses (CeHV9 and PsHV1) appear to have additional genes related to UL56, whose products are termed membrane protein UL56A. The existence of these paralogues gives rise to the novel UL56 gene family. This systematic evaluation provides a framework within which to view the possible roles of the encoded proteins.
In cellular proteins, PPXY motifs interact with the WW domain, which is 35–40 residues in length and structured as a 3-stranded, antiparallel β-sheet with two ligand-binding grooves (Macias et al., 1996). The WW domain is invariably joined to one of a wide variety of other protein modules, and is thus implicated in assembly of multiprotein complexes (Ingham et al., 2005). These processes encompass transcription, RNA processing, protein trafficking, receptor signaling, control of the cytoskeleton and vacuolar protein sorting (Hettema et al., 2004). Vacuolar protein sorting is exploited for budding by some enveloped viruses, utilizing PPXY motifs in virus proteins (Wills et al., 1994; Martin-Serrano et al., 2005). These observations from cellular proteins are in general accord with what is known about membrane protein UL56.
In HHV1, UL56 is not required for growth of virus in cell culture. However, mutants, including one lacking the C-terminal hydrophobic domain, are compromised in pathogenicity, including neuroinvasiveness (Peles et al., 1990; Rösen-Wolff et al., 1991; Berkowitz et al., 1994; Kehm et al., 1996), although this appears to depend on the system used (Nash and Spivack, 1994). Moreover, mutants with lesions in UL56 and other genes have been examined for utility in oncolytic viral therapy (Takakuwa et al., 2003; Sugiura et al., 2004; Ushijima et al., 2007). The protein has been detected in virions (Kehm et al., 1994, 1998).
Additional information is available for HHV2, in which UL56 has been shown to encode a type 2 membrane protein that localizes to the Golgi apparatus and cytoplasmic vesicles, and is tail-anchored by the C-terminal hydrophobic domain so that the N terminus (containing the PPXY motifs) is located in the cytoplasm (Koshizuka et al., 2002). An association of the protein has been reported with a neuron-specific kinesin (KIF1A) involved in transport of synaptic vesicle precursors in the axon, leading to the suggestion that it may function in vesicular transport (Koshizuka et al., 2005). These features prompted a comparison (Koshizuka et al., 2005) with membrane protein US9, which is a tail-anchored, type 2 membrane protein involved in transport of virus proteins towards the axon terminus, probably in vesicles (Brideau et al., 1998; Lyman et al., 2007). An interaction detected between membrane protein UL56 and myristylated tegument protein (encoded by gene UL49A in HHV2; alternatively named UL49.5), and colocalization of the complex to the Golgi apparatus and aggresome-like structures, suggests that it may have a role in virus maturation and egress (Koshizuka et al., 2006). Membrane protein UL56 also interacts with the ubiquitin ligase Nedd4 via its PPXY motifs and promotes its ubiquitination (Ushijima et al., 2008).
Some functional investigations have been carried out on other orthologues. Deletion of the EHV1 or SuHV1 gene has no effect on growth in cell culture (Sun and Brown, 1994; Baumeister et al., 1995), whereas the VZV protein is required for efficient growth of virus in cell culture and in an animal model system (Zhang et al., 2007).
4.3. Gene US8A
HHV1 gene US8A (alternatively named US8.5) appeared late on the scene. It was overlooked in the genome sequence analyses (McGeoch et al., 1985, 1988), and was discovered later by Georgopoulou et al. (1993). In a comparative description of the HHV2 gene content, Dolan et al. (1998) evaluated US8A as probably specific to the genus Simplexvirus, and noted a positional counterpart in EHV1, a member of the genus Varicellovirus. Georgopoulou et al. (1993) characterized an internally tagged form of the HHV1 protein as locating to nucleoli. However, as registered by Dolan et al. (1998), the sequence used in these experiments was frameshifted, so the 16 residues at the C terminus of the protein would have been replaced. This raises a question as to whether the mutated protein might have localized inappropriately. A comparative approach (Fig. 2) indicates that the US8A protein has the sequence properties of a tail-anchored, type 2 membrane protein (N terminus in the cytoplasm). It is notable that the adjacent gene (US9) also encodes a tail-anchored, type 2 membrane protein, though it is unrelated in sequence to membrane protein US8A. A gene that is positionally equivalent to gene US8A is present in members of other genera in the subfamily Alphaherpesvirinae, and the same protein name (membrane protein US8A) is currently assigned (Table 2).
Fig. 2.
Amino acid sequence alignment of protein US8A from the genus Simplexvirus. Conserved residues are highlighted in grey, and predicted transmembrane domains are boxed.
4.4. UL30A gene family
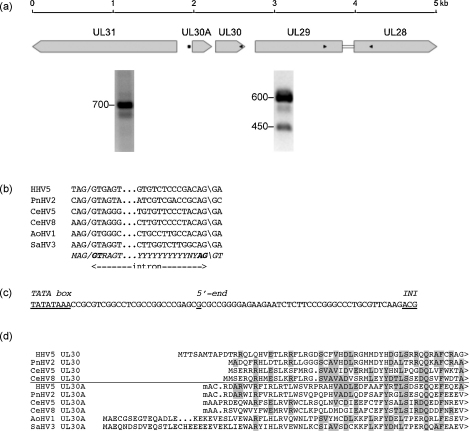
HHV5 and its closest relative (PnHV2) are members of the genus Cytomegalovirus in the subfamily Betaherpesvirinae. The UL28 coding region in these viruses was predicted several years ago to be spliced to an unidentified upstream exon (Davison et al., 2003). The layout of UL28 in relation to genes upstream is shown in Fig. 3a. A subsequent comparative analysis involving members or potential members of the genus Cytomegalovirus from Old and New World primates (HHV5, PnHV2, CeHV5 and CeHV8 for the former; AoHV1 and SaHV3 for the latter) strongly suggested that the upstream exon is UL29 (Fig. 3b). Reverse-transcription PCR (RT-PCR) confirmed the expression of the predicted spliced HHV5 RNA (as represented by the 450 bp RT-PCR product in Fig. 3a), as well as the unspliced RNA (as represented by the 600 bp product). The appropriate amendment to the HHV5 RefSeq was made early in 2009. The intron has also been detected independently (Mitchell et al., 2009). Thus, two previous coding regions (UL28 and UL29) present in primate members of the genus Cytomegalovirus have been conflated to a single gene (UL29).
Fig. 3.
Splicing of UL28 and UL29 and identification of the UL30 gene family in the genus Cytomegalovirus. (a) Layout of ORFs in the relevant region of the HHV5 genome (inverted from its standard depiction), with images of ethidium bromide-stained agarose gels showing RT-PCR and 5′-RACE products (sizes in bp) from total cell RNA harvested late during infection of human fibroblast cells by HHV5 strain Merlin. The RT-PCR products in the right panel relate to the region spanning UL28 and UL29 (primers 5-GTCGCCCAGCATGATGCCGTGCAG-3′ and 5′-CTTTCACCGCGTGCGGATTCTCTG-3′; black arrowheads). The 5′-RACE products in the left panel relate to the region upstream from UL30A (primer 5′-TCTACGGAGACCTGACAGCAGTTG-3′; black arrowhead). The mRNA 5′-end upstream from UL30 is marked with a black square (see panel (c) for details. (b) Nucleotide sequence alignment of predicted (confirmed in the case of HHV5) splice donor (/) and acceptor (\) sites flanking the intron linking UL28 and UL29. The italicized line shows the canonical sequences, with critical residues in bold type. (c) The proposed TATA box, mapped mRNA 5′-end and putative non-ATG (ACG) translational initiation codon (INI) for UL30A in HHV5 strain Merlin. The same 5′-end was mapped experimentally for HHV5 strain AD169. (d) Predicted amino acid sequence alignment of the N-terminal portion of the UL30A and UL30 proteins. The lower case residue at the start of the UL30A sequences indicates the proposed encoding of an initiating methionine residue by the non-ATG codon. Residues that are conserved respectively in at least two UL30A sequences and at least one UL30 sequence (or vice versa) are shaded. The C-termini of the proteins are indicated by >.
During transcript mapping experiments, we mapped the major 5′-end upstream from HHV5 UL29 to a nucleotide position approximately 800 bp upstream from the translational initiation codon, and 300 bp upstream from the UL30 translational initiation codon (as represented by the 700 bp 5′-RACE product in Fig. 3a). This transcriptional initiation site is located an appropriate distance downstream from a candidate TATA box (Fig. 3c). Comparative analysis then led to the discovery of a potential coding region (UL30A) located in the 300 bp region between the transcriptional initiation site and the UL30 translational initiation codon. This region potentially encodes a protein that is conserved in other Old World primate members of the genus Cytomegalovirus and is related to protein UL30 (Fig. 3d). However, none of the UL30A coding regions in these viruses possesses an appropriately positioned translational initiation codon (ATG). It is possible that translational initiation occurs from a non-ATG codon, in this case ACG, which has been shown to function as an initiation codon in eukaryotic systems (Anderson and Buzash-Pollert, 1985; Peabody, 1989) and viruses such as adeno-associated virus (Becerra et al., 1988) and Sendai virus (Curran and Kolakofsky, 1988). Indeed, a few respectable herpesvirus coding regions lack an ATG initiation codon and have been proposed to utilise a non-ATG codon; for example, that encoding HHV2 tegument protein UL16 (Dolan et al., 1998). The New World primate viruses that potentially belong to the genus Cytomegalovirus appear to have UL30A orthologues with ATG initiation codons, but lack UL30 orthologues (Fig. 3d). These findings indicate the presence of paralogues (UL30 and UL30A) that together constitute the novel UL30 gene family.
Conflict of interest
The author has no conflict of interest.
Acknowledgement
I am grateful to Charles Cunningham (MRC Virology Unit, Glasgow, UK) for providing RNA mapping data.
Footnotes
Systematicization proceeds best when interested people participate. I encourage those who wish to contribute to herpesvirus taxonomy to contact the chair of the Herpesvirales Study Group (currently Philip Pellett; ppellett@med.wayne.edu). Also, any with thoughts on annotating the herpesvirus RefSeqs should contact me.
References
- Afonso C.L., Tulman E.R., Lu Z., Zsak L., Rock D.L., Kutish G.F. The genome of turkey herpesvirus. J. Virol. 2001;75:971–978. doi: 10.1128/JVI.75.2.971-978.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht J.-C. Primary structure of the Herpesvirus ateles genome. J. Virol. 2000;74:1033–1037. doi: 10.1128/jvi.74.2.1033-1037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht J.-C., Nicholas J., Biller D., Cameron K.R., Biesinger B., Newman C., Wittmann S., Craxton M.A., Coleman H., Fleckenstein B., Honess R.W. Primary structure of the herpesvirus saimiri genome. J. Virol. 1992;66:5047–5058. doi: 10.1128/jvi.66.8.5047-5058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander L., Denekamp L., Knapp A., Auerbach M.R., Damania B., Desrosiers R.C. The primary sequence of rhesus monkey rhadinovirus isolate 26–95: sequence similarities to Kaposi's sarcoma-associated herpesvirus and rhesus monkey rhadinovirus isolate 17577. J. Virol. 2000;74:3388–3398. doi: 10.1128/jvi.74.7.3388-3398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C.W., Buzash-Pollert E. Can ACG serve as an initiation codon for protein synthesis in eucaryotic cells? Mol. Cell. Biol. 1985;5:3621–3624. doi: 10.1128/mcb.5.12.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T., Hirono I., Kurokawa K., Fukuda H., Nahary R., Eldar A., Davison A.J., Waltzek T.B., Bercovier H., Hedrick R.P. Genome sequences of three koi herpesvirus isolates representing the expanding distribution of an emerging disease threatening koi and common carp worldwide. J. Virol. 2007;81:5058–5065. doi: 10.1128/JVI.00146-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer R., Bankier A.T., Biggin M.D., Deininger P.L., Farrell P.J., Gibson T.J., Hatfull G., Hudson G.S., Satchwell S.C., Séguin C., Tuffnell P.S., Barrell B.G. DNA sequence and expression of the B95-8 Epstein–Barr virus genome. Nature. 1984;310:207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Bahr U., Darai G. Analysis and characterization of the complete genome of tupaia (tree shrew) herpesvirus. J. Virol. 2001;75:4854–4870. doi: 10.1128/JVI.75.10.4854-4870.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister J., Klupp B.G., Mettenleiter T.C. Pseudorabies virus and equine herpesvirus 1 share a nonessential gene which is absent in other herpesviruses and located adjacent to a highly conserved gene cluster. J. Virol. 1995;69:5560–5567. doi: 10.1128/jvi.69.9.5560-5567.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra S.P., Koczot F., Fabisch P., Rose J.A. Synthesis of adeno-associated virus structural proteins requires both alternative mRNA splicing and alternative initiations from a single transcript. J. Virol. 1988;62:2745–2754. doi: 10.1128/jvi.62.8.2745-2754.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz C., Moyal M., Rösen-Wolff A., Darai G., Becker Y. Herpes simplex virus type 1 (HSV-1) UL56 gene is involved in viral intraperitoneal pathogenicity to immunocompetent mice. Arch. Virol. 1994;134:73–83. doi: 10.1007/BF01379108. [DOI] [PubMed] [Google Scholar]
- Bradley A.J., Lurain N.S., Ghazal P., Trivedi U., Cunningham C., Baluchova K., Gatherer D., Wilkinson G.W.G., Dargan D.J., Davison A.J. High-throughput sequence analysis of variants of human cytomegalovirus strains Towne and AD169. J. Gen. Virol. 2009;90:2375–2380. doi: 10.1099/vir.0.013250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brideau A.D., Banfield B.W., Enquist L.W. The Us9 gene product of pseudorabies virus, an alphaherpesvirus, is a phosphorylated, tail-anchored type II membrane protein. J. Virol. 1998;72:4560–4570. doi: 10.1128/jvi.72.6.4560-4570.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M.S., Bankier A.T., Beck S., Bohni R., Brown C.M., Cerny R., Horsnell T., Hutchison C.A., III, Kouzarides T., Martignetti J.A., Preddie E., Satchwell S.C., Tomlinson P., Weston K.M., Barrell B.G. Analysis of the protein coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- Cunningham C., Gatherer D., Hilfrich B., Baluchova K., Dargan D.J., Thomson M., Griffiths P.D., Wilkinson G.W.G., Schulz T.F., Davison A.J. Sequences of complete human cytomegalovirus genomes from infected cell cultures and clinical specimens. J. Gen. Virol. 2010;91:605–615. doi: 10.1099/vir.0.015891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J., Kolakofsky D. Ribosomal initiation from an ACG codon in the Sendai virus P/C mRNA. EMBO J. 1988;7:245–251. doi: 10.1002/j.1460-2075.1988.tb02806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A.J. Channel catfish virus: a new type of herpesvirus. Virology. 1992;186:9–14. doi: 10.1016/0042-6822(92)90056-u. [DOI] [PubMed] [Google Scholar]
- Davison A.J. Introduction: definition and classification of the human herpesviruses, comparative analysis of the genomes. In: Arvin A., Campidelli-Fiume G., Mocarski E., Moore P.S., Roizman B., Whitley R., Yamanishi K., editors. Human Herpesviruses: Biology, Therapy and Immunoprophylaxis. Cambridge University Press; Cambridge: 2007. pp. 10–26. [PubMed] [Google Scholar]
- Davison A.J., Scott J.E. The complete DNA sequence of varicella-zoster virus. J. Gen. Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Davison A.J., Dolan A., Akter P., Addison C., Dargan D.J., Alcendor D.J., McGeoch D.J., Hayward G.S. The human cytomegalovirus genome revisited: comparison with the chimpanzee cytomegalovirus genome. J. Gen. Virol. 2003;84:17–28. doi: 10.1099/vir.0.18606-0. [DOI] [PubMed] [Google Scholar]
- Davison A., Eberle R., Hayward G.S., McGeoch D.J., Minson A.C., Pellett P.E., Roizman B., Studdert M.J., Thiry E. Herpesviridae. In: Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U., Ball L.A., editors. Virus Taxonomy: Classification and Nomenclature of Viruses, Eighth Report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press; London: 2005. pp. 193–212. [Google Scholar]
- Davison A.J., Trus B.L., Cheng N., Steven A.C., Watson M.S., Cunningham C., Le Deuff R.-M., Renault T. A novel class of herpesvirus with bivalve hosts. J. Gen. Virol. 2005;86:41–53. doi: 10.1099/vir.0.80382-0. [DOI] [PubMed] [Google Scholar]
- Davison A.J., Cunningham C., Sauerbier W., McKinnell R.G. Genome sequences of two frog herpesviruses. J. Gen. Virol. 2006;87:3509–3514. doi: 10.1099/vir.0.82291-0. [DOI] [PubMed] [Google Scholar]
- Davison A.J., Eberle R., Ehlers B., Hayward G.S., McGeoch D.J., Minson A.C., Pellett P.E., Roizman B., Studdert M.J., Thiry E. The order Herpesvirales. Arch. Virol. 2009;154:171–177. doi: 10.1007/s00705-008-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jesus O., Smith P.R., Spender L.C., Elgueta Karstegl C., Niller H.H., Huang D., Farrell P.J. Updated Epstein–Barr virus (EBV) DNA sequence and analysis of a promoter for the BART (CST, BARF0) RNAs of EBV. J. Gen. Virol. 2003;84:1443–1450. doi: 10.1099/vir.0.19054-0. [DOI] [PubMed] [Google Scholar]
- Delhon G., Moraes M.P., Lu Z., Afonso C.L., Flores E.F., Weiblen R., Kutish G.F., Rock D.L. Genome of bovine herpesvirus 5. J. Virol. 2003;77:10339–10347. doi: 10.1128/JVI.77.19.10339-10347.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan A., Jamieson F.E., Cunningham C., Barnett B.C., McGeoch D.J. The genome sequence of herpes simplex virus type 2. J. Virol. 1998;72:2010–2021. doi: 10.1128/jvi.72.3.2010-2021.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan A., Cunningham C., Hector R.D., Hassan-Walker A.F., Lee L., Addison C., Dargan D.J., McGeoch D.J., Gatherer D., Emery V.C., Griffiths P.D., Sinzger C., McSharry B.P., Wilkinson G.W.G., Davison A.J. Genetic content of wild type human cytomegalovirus. J. Gen. Virol. 2004;85:1301–1312. doi: 10.1099/vir.0.79888-0. [DOI] [PubMed] [Google Scholar]
- Dolan A., Addison C., Gatherer D., Davison A.J., McGeoch D.J. The genome of Epstein–Barr virus type 2 strain AG876. Virology. 2006;350:164–170. doi: 10.1016/j.virol.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Dominguez G., Dambaugh T.R., Stamey F.R., Dewhurst S., Inoue N., Pellett P.E. Human herpesvirus 6B genome sequence: coding content and comparison with human herpesvirus 6A. J. Virol. 1999;73:8040–8052. doi: 10.1128/jvi.73.10.8040-8052.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W., Chou C., Li H., Hai R., Patterson D., Stolc V., Zhu H., Liu F. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. U.S.A. 2003;100:14223–14228. doi: 10.1073/pnas.2334032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeling A., Keil G., Nowak B., Fleckenstein B., Berthelot N., Sheldrick P. Genome structure and virion polypeptides of the primate herpesviruses Herpesvirus aotus types 1 and 3: comparison with human cytomegalovirus. J. Virol. 1983;45:715–726. doi: 10.1128/jvi.45.2.715-726.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers B., Dural G., Yasmum N., Lembo T., de Thoisy B., Ryser-Degiorgis M.-P., Ulrich R.G., McGeoch D.J. Novel mammalian herpesviruses and lineages within the Gammaherpesvirinae: cospeciation and interspecies transfer. J. Virol. 2008;82:3509–3516. doi: 10.1128/JVI.02646-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensser A., Pflanz R., Fleckenstein B. Primary structure of the alcelaphine herpesvirus 1 genome. J. Virol. 1997;71:6517–6525. doi: 10.1128/jvi.71.9.6517-6525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensser A., Thurau M., Wittmann S., Fickenscher H. The genome of herpesvirus saimiri C488 which is capable of transforming human T cells. Virology. 2003;314:471–487. doi: 10.1016/s0042-6822(03)00449-5. [DOI] [PubMed] [Google Scholar]
- Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U., Ball L.A. Elsevier Academic Press; London: 2005. Virus Taxonomy: Classification and Nomenclature of Viruses, Eighth Report of the International Committee on Taxonony of Viruses. [Google Scholar]
- Fenner F. Classification and nomenclature of viruses. Second Report of the International Committee on Taxonomy of Viruses. Intervirology. 1976;7:1–115. doi: 10.1159/000149938. [DOI] [PubMed] [Google Scholar]
- Francki R.I.B., Fauquet C.M., Knudson D.L., Brown F. Classification and nomenclature of viruses, Fifth Report of the International Committee on Taxonomy of Viruses. Arch. Virol. 1991;Suppl. 2:103–110. [Google Scholar]
- Gailbreath K.L., Oaks J.L. Herpesviral inclusion body disease in owls and falcons is caused by the pigeon herpesvirus (columbid herpesvirus 1) J. Wildl. Dis. 2008;44:427–433. doi: 10.7589/0090-3558-44.2.427. [DOI] [PubMed] [Google Scholar]
- Georgopoulou U., Michaelidou B., Roizman B., Mavromara-Nazos P. Identification of a new transcriptional unit that yields a gene product within the unique sequences of the short component of the herpes simplex virus 1 genome. J. Virol. 1993;67:3961–3968. doi: 10.1128/jvi.67.7.3961-3968.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomi Y., Sunamachi H., Mori Y., Nagaike K., Takahashi M., Yamanishi K. Comparison of the complete DNA sequences of the Oka varicella vaccine and its parental virus. J. Virol. 2002;76:11447–11459. doi: 10.1128/JVI.76.22.11447-11459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompels U.A., Nicholas J., Lawrence G., Jones M., Thomson B.J., Martin M.E.D., Efstathiou S., Craxton M., Macaulay H.A. The DNA sequence of human herpesvirus-6: structure, coding content, and genome evolution. Virology. 1995;209:29–51. doi: 10.1006/viro.1995.1228. [DOI] [PubMed] [Google Scholar]
- Gray W.L., Starnes B., White M.W., Mahalingam R. The DNA sequence of the simian varicella virus genome. Virology. 2001;284:123–130. doi: 10.1006/viro.2001.0912. [DOI] [PubMed] [Google Scholar]
- Grose C., Tyler S., Peters G., Hiebert J., Stephens G.M., Ruyechan W.T., Jackson W., Storlie J., Tipples G.A. Complete DNA sequence analyses of the first two varicella–zoster virus glycoprotein E (D150N) mutant viruses found in North America: evolution of genotypes with an accelerated cell spread phenotype. J. Virol. 2004;78:6799–6807. doi: 10.1128/JVI.78.13.6799-6807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S.G., Strelow L.I., Franchi D.C., Anders D.G., Wong S.W. Complete sequence and genomic analysis of rhesus cytomegalovirus. J. Virol. 2003;77:6620–6636. doi: 10.1128/JVI.77.12.6620-6636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart J., Ackermann M., Jayawardane G., Russell G., Haig D.M., Reid H., Stewart J.P. Complete sequence and analysis of the ovine herpesvirus 2 genome. J. Gen. Virol. 2007;88:28–39. doi: 10.1099/vir.0.82284-0. [DOI] [PubMed] [Google Scholar]
- Hettema E.H., Valdez-Taubas J., Pelham H.R.B. Bsd2 binds the ubiquitin ligase Rsp5 and mediates the ubiquitination of transmembrane proteins. EMBO J. 2004;23:1279–1288. doi: 10.1038/sj.emboj.7600137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes D.J., Kipar A., Milligan S.G., Cunningham C., Sanders M., Quail M.A., Rajandream M.A., Efstathiou S., Bowden R.J., Chastel C., Bennett M., Sample J.T., Barrell B., Davison A.J., Stewart J.P. Characterization of a novel wood mouse virus related to murid herpesvirus 4. J. Gen. Virol. 2010;91:867–879. doi: 10.1099/vir.0.017327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham R.J., Colwill K., Howard C., Dettwiler S., Lim C.S.H., Yu J., Hersi K., Raaijmakers J., Gish G., Mbamalu G., Taylor L., Yeung B., Vassilovski G., Amin M., Chen F., Matskova L., Winberg G., Ernberg I., Linding R., O’Donnell P., Starostine A., Keller W., Metalnikov P., Stark C., Pawson T. WW domains provide a platform for the assembly of multiprotein networks. Mol. Cell. Biol. 2005;25:7092–7106. doi: 10.1128/MCB.25.16.7092-7106.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isegawa Y., Mukai T., Nakano K., Kagawa M., Chen J., Mori Y., Sunagawa T., Kawanishi K., Sashihara J., Hata A., Zou P., Kosuge H., Yamanishi K. Comparison of the complete DNA sequences of human herpesvirus 6 variants A and B. J. Virol. 1999;73:8053–8063. doi: 10.1128/jvi.73.10.8053-8063.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumiya Y., Jang H.K., Ono M., Mikami T. A complete genomic DNA sequence of Marek's disease virus type 2, strain HPRS24. Curr. Top. Microbiol. Immunol. 2001;255:191–221. doi: 10.1007/978-3-642-56863-3_8. [DOI] [PubMed] [Google Scholar]
- Kehm R., Lorentzen E., Rösen-Wolff A., Darai G. In vitro expression of UL56 gene of herpes simplex virus type 1; detection of UL56 gene product in infected cells and in virions. Virus Res. 1994;33:55–66. doi: 10.1016/0168-1702(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Kehm R., Rösen-Wolff A., Darai G. Restitution of the UL56 gene expression of HSV-1 HFEM led to restoration of virulent phenotype; deletion of the amino acids 217 to 234 of the UL56 protein abrogates the virulent phenotype. Virus Res. 1996;40:17–31. doi: 10.1016/0168-1702(96)80248-6. [DOI] [PubMed] [Google Scholar]
- Kehm R., Gelderblom H.R., Darai G. Identification of the UL56 protein of herpes simplex virus type 1 within the virion by immuno electron microscopy. Virus Genes. 1998;17:49–53. doi: 10.1023/a:1008053017716. [DOI] [PubMed] [Google Scholar]
- Kingham B.F., Zelník V., Kopáček J., Majerčiak V., Ney E., Schmidt C.J. The genome of herpesvirus of turkeys: comparative analysis with Marek's disease viruses. J. Gen. Virol. 2001;82:1123–1135. doi: 10.1099/0022-1317-82-5-1123. [DOI] [PubMed] [Google Scholar]
- Klupp B.G., Hengartner C.J., Mettenleiter T.C., Enquist L.W. Complete, annotated sequence of the pseudorabies virus genome. J. Virol. 2004;78:424–440. doi: 10.1128/JVI.78.1.424-440.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshizuka T., Goshima F., Takakuwa H., Nozawa N., Daikoku T., Koiwai O., Nishiyama Y. Identification and characterization of the UL56 gene product of herpes simplex virus type 2. J. Virol. 2002;76:6718–6728. doi: 10.1128/JVI.76.13.6718-6728.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshizuka T., Kawaguchi Y., Nishiyama Y. Herpes simplex virus type 2 membrane protein UL56 associates with the kinesin motor protein KIF1A. J. Gen. Virol. 2005;86:527–533. doi: 10.1099/vir.0.80633-0. [DOI] [PubMed] [Google Scholar]
- Koshizuka T., Kawaguchi Y., Goshima F., Mori I., Nishiyama Y. Association of two membrane proteins encoded by herpes simplex virus type 2, UL11 and UL56. Virus Genes. 2006;32:153–163. doi: 10.1007/s11262-005-6871-7. [DOI] [PubMed] [Google Scholar]
- Lee L.F., Wu P., Sui D., Ren D., Kamil J., Kung H.J., Witter R.L. The complete unique long sequence and the overall genomic organization of the GA strain of Marek's disease virus. Proc. Natl. Acad. Sci. U.S.A. 2000;97:6091–6096. doi: 10.1073/pnas.97.11.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Huang B., Ma X., Wu J., Li F., Ai W., Song M., Yang H. Molecular characterization of the genome of duck enteritis virus. Virology. 2009;391:151–161. doi: 10.1016/j.virol.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Loparev V.N., Rubtcova E.N., Bostik V., Tzaneva V., Sauerbrei A., Robo A., Sattler-Dornbacher E., Hanovcova I., Stepanova V., Splino M., Eremin V., Koskiniemi M., Vankova O.E., Schmid D.S. Distribution of varicella-zoster virus (VZV) wild-type genotypes in northern and southern Europe: evidence for high conservation of circulating genotypes. Virology. 2009;383:216–225. doi: 10.1016/j.virol.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Lyman M.G., Feierbach B., Curanovic D., Bisher M., Enquist L.W. Pseudorabies virus Us9 directs axonal sorting of viral capsids. J. Virol. 2007;81:11363–11371. doi: 10.1128/JVI.01281-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias M.J., Hyvonen M., Baraldi E., Schultz J., Sudol M., Saraste M., Oschkinat H. Structure of the WW domain of a kinase-associated protein complexed with a proline-rich peptide. Nature. 1996;382:646–649. doi: 10.1038/382646a0. [DOI] [PubMed] [Google Scholar]
- Martin-Serrano J., Eastman S.W., Chung W., Bieniasz P.D. HECT ubiquitin ligases link viral and cellular PPXY motifs to the vacuolar protein-sorting pathway. J. Cell Biol. 2005;168:89–101. doi: 10.1083/jcb.200408155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews R.E.F. Classification and nomenclature of viruses, Third Report of the International Committee on Taxonomy of Viruses. Intervirology. 1979;12:129–296. doi: 10.1159/000149081. [DOI] [PubMed] [Google Scholar]
- McGeoch D.J., Dolan A., Donald S., Rixon F.J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J. Mol. Biol. 1985;181:1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- McGeoch D.J., Dalrymple M.A., Davison A.J., Dolan A., Frame M.C., McNab D., Perry L.J., Scott J.E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- Megaw A.G., Rapaport D., Avidor B., Frenkel N., Davison A.J. The DNA sequence of the RK strain of human herpesvirus 7. Virology. 1998;244:119–132. doi: 10.1006/viro.1998.9105. [DOI] [PubMed] [Google Scholar]
- Minson A.C., Davison A., Eberle R., Desrosiers R.C., Fleckenstein B., McGeoch D.J., Pellett P.E., Roizman B., Studdert M.J. Family Herpesviridae. In: Van Regenmortel M.H.V., Fauquet C.M., Bishop D.H.L., Carstens E.B., Estes M.K., Lemon S.M., Maniloff J., Mayo M.A., McGeoch D.J., Pringle C.R., Wickner R.B., editors. Virus Taxonomy: Classification and Nomenclature of Viruses, Seventh Report of the International Committee on Taxonomy of Viruses. Academic Press; San Diego: 2000. pp. 203–225. [Google Scholar]
- Mitchell D.P., Savaryn J.P., Moorman N.J., Shenk T., Terhune S.S. Human cytomegalovirus UL28 and UL29 open reading frames encode a spliced mRNA and stimulate accumulation of immediate-early RNAs. J. Virol. 2009;83:10187–10197. doi: 10.1128/JVI.00396-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocaski E.S. Introduction: definition and classification of the human herpesviruses, comparative analysis of herpesvirus-common proteins. In: Arvin A., Campidelli-Fiume G., Mocarski E., Moore P.S., Roizman B., Whitley R., Yamanishi K., editors. Human Herpesviruses: Biology, Therapy and Immunoprophylaxis. Cambridge University Press; Cambridge: 2007. pp. 44–58. [Google Scholar]
- Murphy E., Yu D., Grimwood J., Schmutz J., Dickson M., Jarvis M.A., Hahn G., Nelson J.A., Myers R.M., Shenk T.E. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc. Natl. Acad. Sci. U.S.A. 2003;100:14976–14981. doi: 10.1073/pnas.2136652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash A.A., Dutia B.M., Stewart J.P., Davison A.J. Natural history of murine γ-herpesvirus infection. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2001;356:569–579. doi: 10.1098/rstb.2000.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash T.C., Spivack J.G. The UL55 and UL56 genes of herpes simplex virus type 1 are not required for viral replication, intraperitoneal virulence, or establishment of latency in mice. Virology. 1994;204:794–798. doi: 10.1006/viro.1994.1595. [DOI] [PubMed] [Google Scholar]
- Neipel F., Albrecht J.-C., Fleckenstein B. Cell-homologous genes in the Kaposi's sarcoma-associated rhadinovirus human herpesvirus 8: determinants of its pathogenicity? J. Virol. 1997;71:4187–4192. doi: 10.1128/jvi.71.6.4187-4192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas J. Determination and analysis of the complete nucleotide sequence of human herpesvirus 7. J. Virol. 1996;70:5975–5989. doi: 10.1128/jvi.70.9.5975-5989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niikura M., Dodgson J., Cheng H. Direct evidence of host genome acquisition by the alphaherpesvirus Marek's disease virus. Arch. Virol. 2006;151:537–549. doi: 10.1007/s00705-005-0633-7. [DOI] [PubMed] [Google Scholar]
- Norberg P., Liljeqvist J.-A., Bergström T., Sammons S., Schmid D.S., Loparev V.N. Complete-genome phylogenetic approach to varicella–zoster virus evolution: genetic divergence and evidence for recombination. J. Virol. 2006;80:9569–9576. doi: 10.1128/JVI.00835-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent J., Birch-Machin I., Smith K.C., Mumford J.A., Swann Z., Newton J.R., Bowden R.J., Allen G.P., Davis-Poynter N. Analysis of equid herpesvirus 1 strain variation reveals a point mutation of the DNA polymerase strongly associated with neuropathogenic versus nonneuropathogenic disease outbreaks. J. Virol. 2006;80:4047–4060. doi: 10.1128/JVI.80.8.4047-4060.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody D.S. Translation initiation at non-AUG triplets in mammalian cells. J. Biol. Chem. 1989;264:5031–5035. [PubMed] [Google Scholar]
- Peles E., Rosen H., Darai G., Rösen-Wolff A., Becker Y. Importance of the HpaI-P sequence for herpes simplex virus-1 replication in the adrenal glands. Arch. Virol. 1990;113:151–163. doi: 10.1007/BF01316669. [DOI] [PubMed] [Google Scholar]
- Perelygina L., Zhu L., Zurkuhlen H., Mills R., Borodovsky M., Hilliard J.K. Complete sequence and comparative analysis of the genome of herpes B virus (Cercopithecine herpesvirus 1) from a rhesus monkey. J. Virol. 2003;77:6167–6177. doi: 10.1128/JVI.77.11.6167-6177.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G.A., Tyler S.D., Grose C., Severini A., Gray M.J., Upton C., Tipples G.A. A full-genome phylogenetic analysis of varicella–zoster virus reveals a novel origin of replication-based genotyping scheme and evidence of recombination between major circulating clades. J. Virol. 2006;80:9850–9860. doi: 10.1128/JVI.00715-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt K.D., Tatusova T., Maglott D.R. NCBI Reference Sequence Project: update and current status. Nucleic Acids Res. 2003;31:34–37. doi: 10.1093/nar/gkg111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlinson W.D., Farrell H.E., Barrell B.G. Analysis of the complete DNA sequence of murine cytomegalovirus. J. Virol. 1996;70:8833–8849. doi: 10.1128/jvi.70.12.8833-8849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaee S.A.R., Cunningham C., Davison A.J., Blackbourn D.J. Kaposi's sarcoma-associated herpesvirus immune modulation: an overview. J. Gen. Virol. 2006;87:1781–1804. doi: 10.1099/vir.0.81919-0. [DOI] [PubMed] [Google Scholar]
- Rivailler P., Cho Y.-G., Wang F. Complete genomic sequence of an Epstein–Barr virus-related herpesvirus naturally infecting a new world primate: a defining point in the evolution of oncogenic lymphocryptoviruses. J. Virol. 2002;76:12055–12068. doi: 10.1128/JVI.76.23.12055-12068.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivailler P., Jiang H., Cho Y.-G., Quink C., Wang F. Complete nucleotide sequence of the rhesus lymphocryptovirus: genetic validation for an Epstein–Barr virus animal model. J. Virol. 2002;76:421–426. doi: 10.1128/JVI.76.1.421-426.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivailler P., Kaur A., Johnson R.P., Wang F. Genomic sequence of rhesus cytomegalovirus 180.92: insights into the coding potential of rhesus cytomegalovirus. J. Virol. 2006;80:4179–4182. doi: 10.1128/JVI.80.8.4179-4182.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B., Bartha A., Biggs P.M., Carmichael L.E., Granoff A., Hampar B., Kaplan A.S., Melendez L.V., Munk K., Nahmias A., Plummer G., Rajcani J., Rapp F., Terni M., de Thé G., Watson D.H., Wildy P. Provisional labels for herpesviruses. J. Gen. Virol. 1973;20:417–419. doi: 10.1099/0022-1317-20-3-417. [DOI] [PubMed] [Google Scholar]
- Roizman B., Carmichael L.E., Deinhardt F., de-Thé G., Nahmias A.J., Plowright W., Rapp F., Sheldrick P., Takahashi M., Wolf K. Herpesviridae: definition, provisional nomenclature, and taxonomy. Intervirology. 1981;16:201–217. doi: 10.1159/000149269. [DOI] [PubMed] [Google Scholar]
- Roizman B., Desrosiers R.C., Fleckenstein B., Lopez C., Minson A.C., Studdert M.J. The family Herpesviridae: an update. Arch. Virol. 1992;123:425–449. doi: 10.1007/BF01317276. [DOI] [PubMed] [Google Scholar]
- Rösen-Wolff A., Lamadé W., Berkowitz C., Becker Y., Darai G. Elimination of UL56 gene by insertion of LacZ cassette between nucleotide position 116030 to 121753 of the herpes simplex virus type 1 genome abrogates intraperitoneal pathogenicity in tree shrews and mice. Virus Res. 1991;20:205–221. doi: 10.1016/0168-1702(91)90076-8. [DOI] [PubMed] [Google Scholar]
- Russo J.J., Bohenzky R.A., Chien M.-C., Chen J., Yan M., Maddalena D., Parry J.P., Peruzzi D., Edelman I.S., Chang Y., Moore P.S. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc. Natl. Acad. Sci. U.S.A. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleiss M.R., McGregor A., Choi K.Y., Date S.V., Cui X., McVoy M.A. Analysis of the nucleotide sequence of the guinea pig cytomegalovirus (GPCMV) genome. Virol. J. 2008;5:139. doi: 10.1186/1743-422X-5-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyzer M., Ackermann M. Molecular virology of ruminant herpesviruses. Vet. Microbiol. 1996;53:17–29. doi: 10.1016/s0378-1135(96)01231-x. [DOI] [PubMed] [Google Scholar]
- Searles R.P., Bergquam E.P., Axthelm M.K., Wong S.W. Sequence and genomic analysis of a rhesus macaque rhadinovirus with similarity to Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8. J. Virol. 1999;73:3040–3053. doi: 10.1128/jvi.73.4.3040-3053.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinzger C., Hahn G., Digel M., Katona R., Sampaio K.L., Messerle M., Hengel H., Koszinowski U., Brune W., Adler B. Cloning and sequencing of a highly productive, endotheliotropic virus strain derived from human cytomegalovirus TB40/E. J. Gen. Virol. 2008;89:359–368. doi: 10.1099/vir.0.83286-0. [DOI] [PubMed] [Google Scholar]
- Smith L.M., McWhorter A.R., Masters L.L., Shellam G.R., Redwood A.J. Laboratory strains of murine cytomegalovirus are genetically similar to but phenotypically distinct from wild strains of virus. J. Virol. 2008;82:6689–6696. doi: 10.1128/JVI.00160-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatz S.J., Rue C.A. Sequence determination of a mildly virulent strain (CU-2) of Gallid herpesvirus type 2 using 454 pyrosequencing. Virus Genes. 2008;36:479–489. doi: 10.1007/s11262-008-0213-5. [DOI] [PubMed] [Google Scholar]
- Spatz S.J., Petherbridge L., Zhao Y., Nair V. Comparative full-length sequence analysis of oncogenic and vaccine (Rispens) strains of Marek's disease virus. J. Gen. Virol. 2007;88:1080–1096. doi: 10.1099/vir.0.82600-0. [DOI] [PubMed] [Google Scholar]
- Spatz S.J., Zhao Y., Petherbridge L., Smith L.P., Baigent S.J., Nair V. Comparative sequence analysis of a highly oncogenic but horizontal spread-defective clone of Marek's disease virus. Virus Genes. 2007;35:753–766. doi: 10.1007/s11262-007-0157-1. [DOI] [PubMed] [Google Scholar]
- Spatz S.J., Rue C., Schumacher D., Osterrieder N. Clustering of mutations within the inverted repeat regions of a serially passaged attenuated gallid herpesvirus type 2 strain. Virus Genes. 2008;37:69–80. doi: 10.1007/s11262-008-0242-0. [DOI] [PubMed] [Google Scholar]
- Sugiura S., Goshima F., Takakuwa H., Sata T., Nakashima T., Nishiyama Y. Treatment of solid sarcomas in immunocompetent mice with novel, oncolytic herpes simplex viruses. Otolaryngol. Head Neck Surg. 2004;130:470–478. doi: 10.1016/j.otohns.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Sun Y., Brown S.M. The open reading frames 1, 2, 71, and 75 are nonessential for the replication of equine herpesvirus type 1 in vitro. Virology. 1994;199:448–452. doi: 10.1006/viro.1994.1143. [DOI] [PubMed] [Google Scholar]
- Takakuwa H., Goshima F., Nozawa N., Yoshikawa T., Kimata H., Nakao A., Nawa A., Kurata T., Sata T., Nishiyama Y. Oncolytic viral therapy using a spontaneously generated herpes simplex virus type 1 variant for disseminated peritoneal tumor in immunocompetent mice. Arch. Virol. 2003;148:813–825. doi: 10.1007/s00705-002-0944-x. [DOI] [PubMed] [Google Scholar]
- Taus N.S., Herndon D.R., Traul D.L., Stewart J.P., Ackermann M., Li H., Knowles D.P., Lewis G.S., Brayton K.A. Comparison of ovine herpesvirus 2 genomes isolated from domestic sheep (Ovis aries) and a clinically affected cow (Bos bovis) J. Gen. Virol. 2007;88:40–45. doi: 10.1099/vir.0.82285-0. [DOI] [PubMed] [Google Scholar]
- Telford E.A.R., Watson M.S., McBride K., Davison A.J. The DNA sequence of equine herpesvirus-1. Virology. 1992;189:304–316. doi: 10.1016/0042-6822(92)90706-u. [DOI] [PubMed] [Google Scholar]
- Telford E.A.R., Watson M.S., Aird H.C., Perry J., Davison A.J. The DNA sequence of equine herpesvirus 2. J. Mol. Biol. 1995;249:520–528. doi: 10.1006/jmbi.1995.0314. [DOI] [PubMed] [Google Scholar]
- Telford E.A.R., Watson M.S., Perry J., Cullinane A.A., Davison A.J. The DNA sequence of equine herpesvirus-4. J. Gen. Virol. 1998;79:1197–1203. doi: 10.1099/0022-1317-79-5-1197. [DOI] [PubMed] [Google Scholar]
- Thureen D.R., Keeler C.L., Jr. Psittacid herpesvirus 1 and infectious laryngotracheitis virus: comparative genome sequence analysis of two avian alphaherpesviruses. J. Virol. 2006;80:7863–7872. doi: 10.1128/JVI.00134-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillieux S.L., Halsey W.S., Thomas E.S., Voycik J.J., Sathe G.M., Vassilev V. Complete DNA sequences of two Oka strain varicella-zoster virus genomes. J. Virol. 2008;82:11023–11044. doi: 10.1128/JVI.00777-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulman E.R., Afonso C.L., Lu Z., Zsak L., Rock D.L., Kutish G.F. The genome of a very virulent Marek's disease virus. J. Virol. 2000;74:7980–7988. doi: 10.1128/jvi.74.17.7980-7988.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler S.D., Peters G.A., Severini A. Complete genome sequence of cercopithecine herpesvirus 2 (SA8) and comparison with other simplexviruses. Virology. 2005;331:429–440. doi: 10.1016/j.virol.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Tyler S.D., Severini A. The complete genome sequence of herpesvirus papio 2 (Cercopithecine herpesvirus 16) shows evidence of recombination events among various progenitor herpesviruses. J. Virol. 2006;80:1214–1221. doi: 10.1128/JVI.80.3.1214-1221.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushijima Y., Luo C., Goshima F., Yamauchi Y., Kimura H., Nishiyama Y. Determination and analysis of the DNA sequence of highly attenuated herpes simplex virus type 1 mutant HF10, a potential oncolytic virus. Microbes Infect. 2007;9:142–149. doi: 10.1016/j.micinf.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Ushijima Y., Koshizuka T., Goshima F., Kimura H., Nishiyama Y. Herpes simplex virus type 2 UL56 interacts with the ubiquitin ligase Nedd4 and increases its ubiquitination. J. Virol. 2008;82:5220–5233. doi: 10.1128/JVI.02515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beurden S.J., Bossers A., Voorbergen-Laarman M.H., Haenen O.L., Peters S., Abma-Henkens M.H., Peeters B.P., Rottier P.J., Engelsma M.Y. Complete genome sequence and taxonomic position of anguillid herpesvirus 1. J. Gen. Virol. 2010;91:880–887. doi: 10.1099/vir.0.016261-0. [DOI] [PubMed] [Google Scholar]
- Van Regenmortel M.H. Virus species, a much overlooked but essential concept in virus classification. Intervirology. 1990;31:241–254. doi: 10.1159/000150159. [DOI] [PubMed] [Google Scholar]
- Van Regenmortel M.H. What is a virus? Arch. Virol. 1992;Suppl. 5:47–53. doi: 10.1007/978-3-7091-6920-9_5. [DOI] [PubMed] [Google Scholar]
- Vink C., Beuken E., Bruggeman C.A. Complete DNA sequence of the rat cytomegalovirus genome. J. Virol. 2000;74:7656–7665. doi: 10.1128/jvi.74.16.7656-7665.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin H.W., IV, Latreille P., Wamsley P., Hallsworth K., Weck K.E., Dal Canto A.J., Speck S.H. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildy, P., 1971. First Report of the International Committee on Nomenclature of Viruses. Monographs in Virology, vol. 5. Karger, Basel, pp. 33–34.
- Wills J.W., Cameron C.E., Wilson C.B., Xiang Y., Bennett R.P., Leis J. An assembly domain of the Rous sarcoma virus Gag protein required late in budding. J. Virol. 1994;68:6605–6618. doi: 10.1128/jvi.68.10.6605-6618.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng M.-S., Li D.-J., Liu Q.-L., Song L.-B., Li M.-Z., Zhang R.-H., Yu X.-J., Wang H.-M., Ernberg I., Zeng Y.-X. Genomic sequence analysis of Epstein–Barr virus strain GD1 from a nasopharyngeal carcinoma patient. J. Virol. 2005;79:15323–15330. doi: 10.1128/JVI.79.24.15323-15330.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Broll H., Ehlers B., Buhk H.-J., Rosenthal A., Goltz M. Genome sequence of bovine herpesvirus 4, a bovine Rhadinovirus, and identification of an origin of DNA replication. J. Virol. 2001;75:1186–1194. doi: 10.1128/JVI.75.3.1186-1194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Rowe J., Wang W., Sommer M., Arvin A., Moffat J., Zhu H. Genetic analysis of varicella-zoster virus ORF0 to ORF4 using a novel luciferase bacterial artificial chromosome system. J. Virol. 2007;81:9024–9033. doi: 10.1128/JVI.02666-06. [DOI] [PMC free article] [PubMed] [Google Scholar]