Abstract
Purpose
This study was conducted on the effects of vitrification cryotop method on gene expression of mature oocytes in Mus musculus.
Methods
Transcript analyses of three mouse genes, namely Mater, Hook1 and Sod1, were performed upon non-vitrified and vitrified oocytes with different concentrations of dimethyl sulfoxide (DMSO) and ethylene glycol (EG),15%: 7.5% DMSO + 7.5% EG, and 30%: 15% DMSO + 15% EG, using cryotop following normalization of transcripts with Hprt1 by nested quantitative PCR.
Results
Vitrification caused down-regulation of Mater and Hook1 and up-regulation of Sod1 when lower concentrations of cryoprotectants were used as opposed to the control group. The relative expression of Sod1 in vit2 (30% v/v) was significantly higher than vit1 (15% v/v). Quantitative transcript analysis of Mater and Hook1 for the vit2 condition failed to produce any data. Survival rates were the same for both vitrification treatments and significantly lower than control group.
Conclusions
Although vit1 treatment had lower survival rate compared to control group, it demonstrated better stability comparing to vit2 based on the transcript analysis.
Keyword: Cryotop, Gene expression, Nested quantitative PCR (nqPCR), Vitrified oocyte (MII)
Introduction
In the presence of cryoprotectants, oocytes can be cryopreserved through either controlled slow-freezing or fast-freezing techniques. Vitrification is a fast-freezing technique that preserves oocytes by preventing the formation of damaging ice crystals [1, 2]. Oocyte cryopreservation has become an important technique for females whose fertility may become compromised by medical treatment or the physiological effects of aging, or young women who wish to delay motherhood [3]. Despite the benefits that the cryopreservation seems to deliver, our knowledge towards the molecular responses of oocytes is somewhat obscure.
Comparative transcript analyses would be a major step towards understanding the systems biology in response to vitrification, possibly changing the way we treat oocytes during freeze-thaw procedure. Furthermore, we might be able to evaluate risks involved to the oocytes and to the future embryo. Additionally with thorough analyses of genetic responses, some insights might be gained concerning post-cryopreservation repair mechanisms [4].
The lack of enough molecular information on the effects of vitrification methods on mammalian oocytes encouraged us to pursue this avenue. This, in fact may unravel the genetic responses of cells to the cryopreservation process and consequently improve cryopreservation strategies for cells and tissues by applying better platforms. Therefore, we decided to evaluate for the first time the changes of gene expression post heating treatment of vitrified oocytes using cryotop.
For this purpose, three pivotal genes in oogenesis and embryo development, namely Mater, Sod1 and Hook1 were chosen for transcript analysis via nested quantitative PCR (nqPCR) and the data were normalized against Hprt1 [5, 6].
Mater (maternal effect gene) has a role in early embryo development and female fertility [7, 8]. Cu-Zn-superoxide dismutase (sod1) is an antioxidant enzyme that converts the superoxide oxygen anion (O−2) to H2O2, a less reactive oxygen species [9]. Sod1 is expressed at a relatively high level in human and mouse at GV (germinal vesicle) and MII stages (Metaphase II) of oocyte maturation. This enzyme probably plays a crucial role in protecting embryos against oxygen toxicity in vivo as well as in vitro [10].
Hook1 is related to ‘microtubule cytoskeleton’ and ‘chromosome segregation’ and is necessary for the correct positioning of microtubular structures within the haploid germ cell [11, 12]. Microarray transcript analysis of metaphase II oocytes in mouse demonstrated that the expression of Hook1 was decreased with maternal aging [12]. Since Hook1 is also associated with organizing chromosome segregation, and alteration of transcript levels may lead to chromosomal abnormalities [12].
Here, mouse oocytes (MII) were used for oocyte vitrification via cryotop. The effects of two different concentrations of cryoprotectants were evaluated on gene expression by nqPCR.
Sometimes the expression of some genes are low making quantification next to impossible. In such cases a prior PCR amplification is required to boost the template level for the following quantification via Real-Time PCR, a technique called “nested quantitative PCR”. It is noteworthy to mention that the use of PCR amplicons instead of cDNA for the absolute quantification is not as accurate. However in places where the relative quantification serves the purpose, nqPCR provides enough accuracy. Additionally, considering the number of cells or the quantity of RNA that is used for cDNA synthesis, the expression level can be calculated.
Materials and methods
All chemicals were purchased from Sigma Chemical (St Louis, MO, USA) unless it has been stated.
CD1 (ICR) female mice aged 8–10 weeks (Lisbon University, Portugal) were housed in polycarbonate cages (12 h light/dark; 22 ± 2°C), and were fed by standard food and fresh water.
Oocyte collection
Female mice were induced to superovulate by intraperitoneal injection of 10 IU pregnant mare serum gonadotropin (PMSG, G4877), and 48 h later, by 10 IU of human chorionic gonadotropin (hCG, CG5). Donor female mice were humanly killed by cervical dislocation at 14–16 h post hCG injection and cumulus oocyte complexes were collected from oviducts with subsequent removal of the cumulus cells using 0.1% bovine testicular hyaluronidase (H3506) in HTF medium for 30 s. Only those oocytes with normal morphology were selected for the subsequent steps. Oocytes were considered normal, if the zona pellucida and the plasma membrane were intact with an evenly distributed vitelline space [13, 14].
Study groups
The oocytes were vitrified in two different concentrations of cryoprotectants by cryotop and assessed the changes of Mater, Sod1 and Hook1 expression in vitrified and non-vitrified groups. The denuded oocytes were divided into two main groups, vitrified and control (non-vitrified) groups: The vitrified group was divided into two subgroups vit1 (15% v/v cryoprotectants) and vit2 (30% v/v cryoprotectants). Finally, all oocytes were washed three times in DNase and RNase-free water.
Each oocyte pool that contained ten cells was stored at −80°C in a minimum volume (2 μl) of RNase free water [5]. This procedure effectively inhibits RNase.
Vitrification & thawing solutions
As the base medium or washing solution (WS), modified Dulbecco’s phosphate-buffered saline solution or PBI containing 20% (v/v) fetal bovine serum (FBS, GIBCO) was used. The equilibration solution (ES) contained 7.5% (v/v) ethylene glycol (EG) and 7.5% (v/v) dimethyl sulphoxide (DMSO) in PBI with 20% FBS.
There were two vitrification solutions (VS) for two vitrified groups; VS1: 7.5% (v/v) EG, 7.5% (v/v) DMSO and 0.5 mol/l sucrose in PBI with 20% FBS and VS2: 15% (v/v) EG, 15% (v/v) DMSO and 0.5 mol/l sucrose in PBI with 20% FBS. Thawing solution (TS) contained 0.5M sucrose and diluents’ solutions (D1, D2, D3, D4, D5) contained 0.4, 0.3, 0.2, 0.1 and 0.05M sucrose in PBI, respectively.
Vitrification and thawing
Mouse oocytes were vitrified by two concentrations of vitrification solutions using cryotop. Oocytes of vit1 and vit2 groups were placed in three droplets of ES for 5 min to 7 min at 25°C. Subsequently, oocytes were transferred in vitrification solution, VS1 and VS2, respectively for less than 30 s. Oocytes were moved on a cryotop (<1 μl vitrification solution) and the cryotop was immediately submerged in filter-sterilized liquid nitrogen and incubated for at least 7 days. Samples were thawed by plunging the cryotop into 1 ml of TS at 37°C for 1 min. Rehydration and gradual removal of cryoprotectants were performed in D1, D2, D3, D4 and D5 for 3 min at every step. Thawed oocytes were washed three times in base medium (PBI) for 5 min at room temperature.
All survived oocytes (vitrified and non-vitrified) were stored as pools of ten oocytes at −80°C in a minimum volume of water (2 μl) until further use.
Definition of morphologically survived oocytes
Oocytes were defined “morphologically survived”, if the cells possessed an intact zona pellucida and plasma membrane and refractive cytoplasm. Following the thawing step, oocytes in 100 μl of sterilized HTF (EmbryoMax®, Human Tubal Fluid, Millipore, USA) and 5 mg/ml BSA were incubated under mineral oil with the availability of 5% (v/v) CO2, 5% (v/v) O2, and 90% (v/v) N2 for 1 h at 37°C. The morphologically survived oocytes [4, 13, 14] were counted under the microscope and recorded as survival rates.
The average percentages of cryosurvival rates in vitrified (vit1 and vit2) and control groups were compared with one way analysis of variance. Following analysis of variance, mean values were compared.
Gene expression
The relative quantification of gene transcripts was carried out by real-time PCR. Super Script™ III Platinum® Cells Direct Two-Step qRT-PCR Kit with SYBR® Green (Invitrogen) was used to provide cDNA synthesis and real time PCR.
Reverse transcription reaction (RT)
Oocytes were lysed in 1 μl lysis enhancer and 10 μl resuspension buffer for every PCR tube, which was incubated at 75°C for 10 min in Thermal Cycler (API 9700). To degrade any contaminating DNA, the cell lysates were treated with 5 μl DNase I and 1.6 μl DNase I buffer (10×) at 25°C for 5 min. They were added 4 μl of 25-mM EDTA and incubated at 70°C for 10 min.
For first-strand cDNA synthesis, 20 μl 2× RT Reaction Mix and 2 μl RT Enzyme Mix were added to each tube and was incubated at 25°C, 50°C and 85°C for 10 min, 20 min and 5 min, respectively.
Nested quantitative polymerase chain reaction (nqPCR)
In cases where cDNA copy number is very low, real-time PCR usually fails to produce any product. Generally, this can be resolved via performing a preliminary PCR to produce enough templates for the actual real-time PCR. The primer pairs could be the same or different in every step. This method is called nqPCR [15, 16].
The Primer pairs for each gene were designed, synthesized and validated by Molecular Diagnostic Center (MDC, Primer Design, and UK). The primer sequences, annealing temperatures and GenBank accession numbers are given in Table 1.
Table 1.
Primers and conditions used for quantification of gene expression by Real-Time PCR
| Gene symbol | GenBank accession | Sense primer (5′-3′) | Anti-Sense primer (5′-3′) | Tm (°C) | Amplicon size(bp) |
|---|---|---|---|---|---|
| Mater | NM_011860 | 5′AATACAAGTCTTCCATTCTCTGATAG3′ | 5′GCATCTCTTTCCCAGTTTCTTC3′ | 50 | 121 |
| Sod1 | NM_011434 | 5′TTGGGCAAAGGTGGAAATGAA3′ | 5′ACTCAGACCACACAGGGAAT 3′ | 48 | 109 |
| Hook1 | NM_030014 | 5′GGCAGATACACTAGCATTTGA3′ | 5′CTCCTCATTCGTCTCCTTCAG3′ | 48 | 116 |
| Hprt1 | NM_013556 | 5′TCCTCCTCAGACCGCTTTT3′ | 5′AGGTATACAAAACAAATCTAGGTCAT3′ | 48 | 118 |
Here, nqPCR was conducted in Real-Time Cycler (Applied Biosystems, 7500). To confirm the specificity and integrity of the PCR products, melt curve analyses were performed for all the real-time PCR reactions. Standard curves were generated using serial dilutions of cDNAs. The cDNA of every sample was used as template for the preliminary PCR by AmpliTaq Gold PCR Master Mix according to the manufacturer’s instruction. Reactions were performed in a final volume of 50 μl. The first-round PCR mix contained 2 μl specific primer mix (300 nM), 25 μl master mix, 5 μl cDNA and 18 μl sterile water.
The first-round PCR was performed in a thermal cycler (Applied Biosystem, 2720), by incubation at 95°C for 5 min, followed by 30 cycles of 95°C for 15 s, specific Tm for every gene for 15 s (Table 1), and 72°C for 60 s, and a final extension at 72°C for 7 min. The PCR products were separated on 3% agarose gel (Nusieve® GTC® Agarose, 50080).
Real time PCR was conducted on PCR products including, two no-template controls (NTC). Reactions (25 μl) were contained 12.5 μl Platinum® SYBR® green qPCR super mix-UDG, 0.5 μl Rox Reference dye, 0.5 μl primer mix (sense and antisense primers), 6.5 μl autoclaved distilled water, and 5 μl of the first-round PCR product.
Cycling parameters were 50°C for 2 min (UDG incubation), 95°C for 2 min, followed by 50 cycles of 95°C for 15 s, 60°C for 30 s. The melting curve was generated and analyzed at 95°C for 15 s, 50°C for 1 min, 95°C for 15 s and 60°C for 15 s. Every experiment repeated three times.
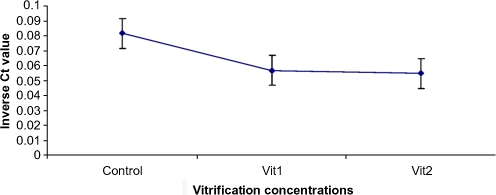
The data were analyzed with the integrated ABI 7500-V2.0.1 software and were normalized to Hprt1 within the log linear phase of the amplification curve using the comparative Ct (Fig. 1). The relative expression ratio (R) of target genes were measured based on a ΔCt formula [17–19] and normalized against Hprt1. PCR efficiencies [18, 19] of the genes ranged between 1.98 and 2.0. ΔCt was the difference between the Ct values of controls and samples.
Fig. 1.
Mean inverse Ct values of Hprt1 as the relevant abundance of transcript in three groups, Ct = threshold cycle, vit1 = vitrification with 7.5% DMSO and 7.5% EG, vit2 = vitrification with 15% DMSO and 15% EG
Result
The survival rates of vitrified and control groups were summarized in Table 2; with no difference between vitrified groups (p > 0.05) and significantly lower survival rates when compared with controls (p < 0.05).
Table 2.
The survival rates of oocytes in control and vitrified groups
| Groups | Concentration of cryoprotectans | No. of total oocytes | No. of survived oocytes | Mean of survival rate (%) | Standard deviation |
|---|---|---|---|---|---|
| cont | 0% | 70 | 70 | 100a | 3.69 |
| vit1 | 15% (7.5% EG + 7.5% DMSO) | 70 | 53 | 75.7b | 2.77 |
| vit2 | 30% (15% EG + 15% DMSO) | 70 | 60 | 85.7b | 1.31 |
DMSO dimethyl sulfoxide, EG ethylene glycol
a and b indicate significant difference between control with vitrified groups (P < 0.05)
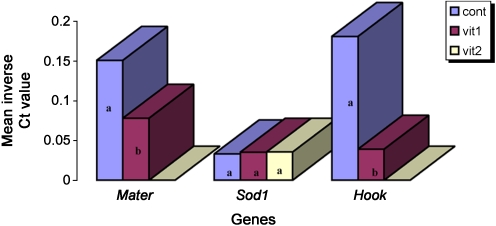
The relative abundance of Mater, Sod1, and Hook1 transcripts in vit1 and vit2 conditions were measured in relation to non-vitrified samples via nqPCR. Inverse Ct values of Mater and Hook1 in control group were significantly higher than vit1, while no significant difference was seen between control, vit1 and vit2 for Sod1 (Fig. 2). In comparison to control, significant down-regulation for Hook1 (5.54e-05 fold) and Mater (0.6 fold) was seen at vit1 condition (Table 3). No difference was detected between vit1 and vit2 when inverse Ct values for Sod1 were put under scrutiny (Fig. 2). However, normalized relative expression ratio of Sod1 demonstrated up-regulation in vit1 (143.7 fold) and it was even higher in vit2 (251.3 fold) (Table 3).
Fig. 2.
Mean inverse Ct values of Mater, Sod1 and Hook1 as the relevant abundance of transcripts in three groups, Ct = threshold cycle, cont = control group (non-vitrified), vit1 = vitrification with 7.5% DMSO and 7.5% EG, vit2 = vitrification with 15% DMSO and 15% EG. No detections were observed for Mater and Hook1 with vit2 treatment. Characters over the bars indicate the significant difference between inverse Ct value means. Bars with the same character are indicative of having no significant difference (P<0.05)
Table 3.
Relative quantification (fold change) in vit1 and vit2 compared to the control group after normalization with Hprt1
| Gene symbol | Groups | Relative quantification (fold change) |
|---|---|---|
| Mater | vit1 | 0.597* |
| vit2 | No detection | |
| Sod1 | vit1 | 143.686* |
| vit2 | 251.335* | |
| Hook1 | vit1 | 5.542E-05* |
| vit2 | No detection |
*P < 0.05
Although the nqPCR repeated thrice, transcript analysis of Mater and Hook1 for the vit2 condition failed to produce any data and nothing was detected in vit2 oocytes.
Discussion
Prior to the complete acceptance of oocyte cryopreservation and putting it into practice for humans, molecular studies are needed to uncover possible side effects. It is unfortunate that despite the significance of oocyte vitrification, the information regarding molecular events occurring subsequent to this process is limited [4, 20, 21]. Additionally, the common concentrations of cryoprotectants have been 30–40%, which has been demonstrated to have toxic effects on cellular process. In order to partly fill this gap, we decided to investigate the relative expression of three genes (Mater, Hook1 and Sod1) in two conditions; vitrified oocytes versus non-vitrified. Oocytes were vitrified with the common concentration (30%) and reduced to half (15%) concentration to evaluate whether the reduction of cryoprotectants has any role on diminishing the negative side effects.
Because of low cDNA copy number of target genes for oocyte, the amplification of cDNA unexpectedly failed for all genes and the use of more oocytes in cDNA synthesis was not helpful. As a result nqPCR was optimized and performed for quantification of all transcripts instead of regular real-time PCR.
Our results indicated that Mater and Hook1 were down-regulated in vit1 group. The role of Mater appears to be specific to very early development. In mice, Mater null mutants has no effects on oogenesis, folliculogenesis, oocyte maturation and ovulation, or fertilization [8]. Mater expression is detected in the cytoplasm of growing oocytes and remains present through the late blastocyst stage. It has been shown that Mater is essential for the pre-implantation development, because the development of Mater−/− embryos is arrested at two-cell stage [8, 12, 22]. It has been demonstrated that Mater is being down-regulated during aging of mouse oocyte [12].
By down-regulation of Mater, it can be expected a reduction in relevant protein synthesis leading to negative effects on pre-implantation development and thus after embryo arrest at two-cell stage. In this case, the rates of cleavage and blastocyst formation may decrease subsequent to vitrification. Accordingly, it has been noted a reduction in developmental rate of mouse vitrified oocytes (data not shown).
Hook1 is a structural gene that involves in the correct positioning of microtubular structures within the haploid germ cell and chromosome segregation [12]. Hook proteins have been shown to constitute a novel family of proteins that may link membrane compartments to microtubules. Furthermore, mutations in the Drosophila Hook showed that the deduced protein is required for formation or stabilization of mature multi-vesicular bodies [11].
Earlier studies suggest that high cooling rate and high cryoprotectant concentration alters the arrangement of chromosomes and microtubules of mouse oocytes [20, 23]. It seems that higher concentrations of cryoprotectants may have toxic effects on oocyte structure [2, 24, 25] and as a result our attempts to quantify Mater and Hook1 transcripts were not successful. Thus, despite the higher survival rate in higher concentrations of cryoprotectants (vit2: 86% vs. 75% for vit1) some genes where severely down-regulated or possibly vanished.
The expressions of Sod1 were up-regulated in vit1 and vit2, once compared with control. Moreover, the expression of sod1 was higher in vit2 than vit1.Sod1, among many others, is an oxidative stress/damage-related gene [12] and it may be expected an up-regulation upon imposing environmental stresses such as vitrification. Ho et al. (1998) suggested that both the Sod1 and Sod2 enzymes may be the primary targets of superoxide radicals generated during paraquat toxicity [26]. Accordingly, It was noticed that vitrification can induce oxidative stress on oocytes following warming step.
Conclusion
The results demonstrated that the cryotop vitrification causes down-regulation of selected genes such as Mater and Hook1 and up-regulation of Sod1. To understand the stability of these changes, more work has to be performed especially through analysis of the relevant proteins. It was also concluded that despite the lower survival rate of vit1 considering higher rates in the control group, the survived oocytes were more stable once compared to the vit2.
Acknowledgments
Special thanks to Raquel Fialho in Portugal for her advice in the Lab. This work was supported by CITA-A, University of the Azores, Angra do Hero´ısmo, Portugal and Cellular and Molecular Biology Researcher Center (CMBRC), Medical School of Shaheed Beheshti University of Medical Sciences and Health Services, Tehran, Iran.
Footnotes
Capsule
This research investigated the effects of vitrification cryotop method on gene expression (Mater, Sod1 and Hook1) in metaphase II mouse oocytes by nested quantitative PCR
Contributor Information
Afrooz Habibi, Email: Habibi_af@yahoo.com.
Ahmad Hosseini, Phone: +98-21-22439956, FAX: +98-21-22439956, Email: prof_hosseini@yahoo.com.
References
- 1.Fahy GM, MacFarlane DR, Angell DA, Meryman HT. Vitrification as an approach to cryopreservation. Cryobiology. 1984;21:407–426. doi: 10.1016/0011-2240(84)90079-8. [DOI] [PubMed] [Google Scholar]
- 2.Mukaida T, Wada S, Takahashi K, Pedro PB, An TZ, Kasai M. Vitrification of human embryos based on the assessment of suitable conditions for 8-cell mouse embryos. Hum Reprod. 1998;13(10):2874–2879. doi: 10.1093/humrep/13.10.2874. [DOI] [PubMed] [Google Scholar]
- 3.Cai XY, Chen GA, Lian Y, Zheng XY, Peng HM. Cryoloop vitrification of rabbit oocytes. Hum Reprod. 2005;20(7):1969–1974. doi: 10.1093/humrep/deh805. [DOI] [PubMed] [Google Scholar]
- 4.Mamo S, Bodo S, Kobolak J, Polgar Z, Tolgyesi G, Dinnyes A. Gene expression profiles of vitrified in vivo derived 8-cell stage mouse embryos detected by high density oligonucleotide microarrays. Mol Reprod Dev. 2006;73(11):1380–1392. doi: 10.1002/mrd.20588. [DOI] [PubMed] [Google Scholar]
- 5.Mamo S, Gal AB, Bodo S, Dinnyes A. Quantitative evaluation and selection of reference genes in mouse oocytes and embryos cultured in vivo and in vitro. BMC Dev Biol. 2007;7:14. doi: 10.1186/1471-213X-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mamo S, Gal AB, Polgar Z, Dinnyes A. Expression profiles of the pluripotency marker gene POU5F1 and validation of reference genes in rabbit oocytes and preimplantation stage embryos. BMC Mol Biol. 2008;9:67. doi: 10.1186/1471-2199-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong ZB, Gold L, Pfeifer KE, Dorward H, Lee E, Bondy CA, et al. Mater, a maternal effect gene required for early embryonic development in mice. Nat Genet. 2000;26(3):267–268. doi: 10.1038/81547. [DOI] [PubMed] [Google Scholar]
- 8.Tong ZB, Gold L, Pol A, Vanevski K, Dorward H, Sena P, et al. Developmental expression and subcellular localization of mouse MATER, an oocyte-specific protein essential for early development. Endocrinology. 2004;145(3):1427–1434. doi: 10.1210/en.2003-1160. [DOI] [PubMed] [Google Scholar]
- 9.Blomberg LA, Long EL, Sonstegard TS, VanTassell CP, Dobrinsky JR, Zuelke KA. Serial analysis of gene expression during elongation of the peri-implantation porcine trophectoderm (conceptus) Physiol Genomics. 2005;20:188–194. doi: 10.1152/physiolgenomics.00157.2004. [DOI] [PubMed] [Google Scholar]
- 10.Mouatassim S, Guérin P, Ménézo Y. Expression of genes encoding antioxidant enzymes in human and mouse oocytes during the final stages of maturation. Mol Hum Reprod. 1999;5(8):720–725. doi: 10.1093/molehr/5.8.720. [DOI] [PubMed] [Google Scholar]
- 11.Simpson F, Martin S, Evans TM, Kerr M, James DE, Parton RG, et al. A novel hook-related protein family and the characterization of hook-related protein 1. Traffic. 2005;6(6):442–458. doi: 10.1111/j.1600-0854.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 12.Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA, et al. Age-associated alteration of gene expression patterns in mouse oocytes. Hum Mol Genet. 2004;13(19):2263–2278. doi: 10.1093/hmg/ddh241. [DOI] [PubMed] [Google Scholar]
- 13.Chen SU, Lien YR, Cheng YY, Chen HF, Ho HN, Yang YS. Vitrification of mouse oocytes using closed pulled straws (CPS) achieves a high survival and preserves good patterns of meiotic spindles, compared with conventional straws, open pulled straws (OPS) and grids. Hum Reprod. 2001;16(11):2350–2356. doi: 10.1093/humrep/16.11.2350. [DOI] [PubMed] [Google Scholar]
- 14.Li XH, Chen SU, Zhang X, Tang M, Kui YR, Wu X, et al. Cryopreserved oocytes of infertile couples undergoing assisted reproductive technology could be an important source of oocyte donation: a clinical report of successful pregnancies. Hum Reprod. 2005;20(12):3390–3394. doi: 10.1093/humrep/dei262. [DOI] [PubMed] [Google Scholar]
- 15.Munoz-Fernandez MA, Gomez-Chacon GF, inventors; Genomadrid S.A. assignee. Method of in-vitro detection and quantification of HIV DNA by quantitative PCR. USA. 2008.
- 16.Forsman A, Uzameckis D, Ronnblom L, Baecklund E, Aleskog A, Bindra A, et al. Single-tube nested quantitative PCR: a rational and sensitive technique for detection of retroviral DNA. Application to RERV-H/HRV-5 and confirmation of its rabbit origin. J Virol Methods. 2003;111(1):1–11. doi: 10.1016/s0166-0934(03)00127-7. [DOI] [PubMed] [Google Scholar]
- 17.Avci ME, Konu O, Yagci T. Quantification of SLIT-ROBO transcripts in hepatocellular carcinoma reveals two groups of genes with coordinate expression. BMC Cancer. 2008;8:392–402. doi: 10.1186/1471-2407-8-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luciano P, Bertea CM, Temporale G, Maffei ME. DNA internal standard for the quantitative determination of hallucinogenic plants in plant mixtures. Genetics. 2007;1:262–266. doi: 10.1016/j.fsigen.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Pfaffl MW. A new mathematical model for relative quantification in real time RT-PCR. Nucleic Acids Res. 2001;29(9):2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boonkusol D, Gal AB, Bodo S, Gorhony B, Kitiyanant Y, Dinnyes A. Gene expression profiles and in vitro development following vitrification of pronuclear and 8-cell stage mouse embryos. Mol Reprod Dev. 2006;73(6):700–708. doi: 10.1002/mrd.20450. [DOI] [PubMed] [Google Scholar]
- 21.Mazoochi T, Salehnia M, Pourbeiranvand S, Forouzandeh M, Mowla SJ, Hajizadeh E. Analysis of apoptosis and expression of genes related to apoptosis in cultures of follicles derived from vitrified and non-vitrified ovaries. Mol Hum Reprod. 2009;15(3):155–164. doi: 10.1093/molehr/gap002. [DOI] [PubMed] [Google Scholar]
- 22.Tong ZB, Bondy CA, Zhou J, Nelson LM. A human homologue of mouse Mater, a maternal effect gene essential for early embryonic development. Hum Reprod. 2002;17(4):903–911. doi: 10.1093/humrep/17.4.903. [DOI] [PubMed] [Google Scholar]
- 23.Gomes CM, Silva CA, Acevedo N, Baracat E, Serafini P, Smith GD. Influence of vitrification on mouse metaphase II oocyte spindle dynamics and chromatin alignment. Fertil Steril. 2008;90(4):1396–1404. doi: 10.1016/j.fertnstert.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 24.Orief Y, Dafopoulos K, Schultze-Mosgau A, Al-Hasani S. Vitrification: will it replace the conventional gamete cryopreservation techniques? Middle East Fertil Soc J. 2005;10(3):171–184. [Google Scholar]
- 25.Picton HM, Gosden RG, Leibo SP. Cryopreservation of oocytes and ovarian tissue. Medical, Ethical and Social Aspects of Assisted Reproduction. Geneva: World Health Organization; 2001. pp. 142–151. [Google Scholar]
- 26.Ho YS, Gargano M, Cao J, Bronson RT, Heimler I, Hutz RJ. Reduced fertility in female mice lacking copper-zinc superoxide dismutase. J Biol Chem. 1998;273(13):7765–7769. doi: 10.1074/jbc.273.13.7765. [DOI] [PubMed] [Google Scholar]