Abstract
Introduction
We serendipitously observed a protective effect of tamoxifen against depletion of ovarian follicles by 7,12-dimethylbenzanthracene (DMBA), a chemical carcinogen, during a cancer prevention study. Such ovarian protection is being sought as an alternative approach to fertility preservation in human cancer patients.
Methods
Rats received tamoxifen (0, 1 mg or 2.5 mg/kg/d) and DMBA (0, 1, 2 mg/kg/wk) or cyclophosphamide (0, 35, 50 mg/kg/wk). Ovarian follicles were quantified and effects on fertility and litter size were tested. Cultured oocytes were exposed to chemotherapy drug doxorubicin, with or without 4-hydroxytamoxifen (4HT).
Results
DMBA and cyclophosphamide decreased the number of primordial and total follicles, and this reduction was prevented by tamoxifen. Cyclophosphamide tended to reduce fertility and lessened neonatal survival. Tamoxifen reversed these defects. Doxorubicin caused oocyte fragmentation which was prevented by 4HT.
Conclusions
Tamoxifen decreases follicle loss and improves reproductive function following exposure to ovarian toxicants including chemotherapy drugs in the female rat.
Keywords: Cancer, Chemotherapy, Ovary, Rat, Toxicology
Introduction
Standard cytotoxic cancer therapy can destroy ovarian follicles and predisposes women to premature menopause and infertility [1, 2]. Previously this infertility and iatrogenic menopause were viewed by physicians and patients as an acceptable cost of curative chemotherapy and radiation regimens. However, with earlier diagnoses and higher survival rates, reproductive side effects from cancer therapy have become major survivorship issues. Although some patients are currently able to preserve their fertility during cancer treatment by embryonic cryopreservation [3–5], ovaries remain in situ and exposed to chemotherapy drugs in nearly all patients. GnRH agents have been tested as ovarian protectants with mixed results [6–10]. An alternative conservative strategy to protect the ovary from cytotoxic therapy is needed to maintain not only oocyte quality but also hormone production for the support of follicle growth and pregnancy.
In a preclinical ovarian cancer prevention study, our laboratory serendipitously observed a protective effect of tamoxifen against ovarian follicular loss, a side effect caused by the carcinogen 7,12-dimethylbenzanthracene (DMBA, [11]). Cancer chemotherapy drugs cyclophosphamide (Cy) and doxorubicin (DOX) share similar mechanisms of action to DMBA and are among the most studied ovarian toxicants. In the current study, we tested a novel use of tamoxifen to protect ovarian reserve and fertility from DMBA-, Cy- and DOX-induced damage in female Sprague Dawley rats.
Materials and methods
Animals and treatments
Four- to six-week old virgin female Sprague Dawley rats (Harlan Breeding Laboratories, Indianapolis, IN) were housed in a climate- and light- (12L:12D) controlled environment and fed food and water ad libitum. Following arrival rats acclimated for 1 week before beginning treatment. All experimental protocols are approved by the University of Kansas Medical Center Animal Care and Use Committee.
DMBA in vivo In order to expand our initial observation of expanded follicular reserves in a chronic cancer prevention study utilizing tamoxifen and the ovotoxic carcinogen DMBA, rats (n = 6 per treatment group, 4 weeks old) were anesthetized with ketamine hydrochloride and xylazine (80 and 8 mg/kg, respectively) and sustained release pellets containing TAM (1 mg/kg/d, refs. [11, 12]) or matching placebos (Hormone Pellet Press, Leawood, KS) were implanted subcutaneously between the scapulae on day 0. Rats received DMBA (0, 30 or 50 mg/kg in 0.5 ml corn oil, p.o., Sigma St. Louis, MO; refs. [13–16]) on days 2 and 9. One week following the final DMBA treatment, rats were sacrificed by decapitation and the left ovary was excised, weighed, fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) and embedded in paraffin.
Cy in vivo Rats (n = 6 per treatment group, 5 weeks old) were anesthetized and implanted with pellets containing TAM or matching placebos (1 mg/kg/d; Innovative Research of America, Sarasota, FL) on day 0 as described above. On day 2 and weekly thereafter, rats were treated with Cy (0, 35 or 50 mg/kg/wk in 0.9% NaCl, i.p., Sigma, St. Louis, MO [17, 18]) or vehicle injections for 4 weeks. Three days following the last Cy injection, rats were sacrificed by decapitation. The left ovary was collected, processed and embedded in paraffin.
Cy mating experiment An additional experiment was carried out to assess the effect of tamoxifen on reproductive function following Cy treatment. Rats (n = 6 per treatment group, 6 weeks old) received 1 or 2.5 mg/kg/d TAM or matching placebos on day 0 of week 1 as described above. Rats were treated with Cy (50 mg/kg) twice a week beginning on d7 and continuing for 2 weeks. During week 4, rats were hemiovariectomized to assess and further deplete ovarian follicular reserves, better modeling the perimenopausal status of most cancer patients [2]. Pellets were also removed at this time. Ovaries collected were fixed and embedded in paraffin. Rats were allowed to recover for 2 weeks (weeks 5 and 6) and vaginal cytologies were documented each day during week 7. Rats were mated on the day of estrus with fertile males starting in week 8. Mating was confirmed by sperm positive vaginal smears on the following morning. Rats with three failed attempts to become pregnant after mating with different males were considered infertile. Gestation (length) and offspring (number and sex ratio) records were documented. Treatment groups included: 1) controls, 2) 1 mg/kg/d or low-dose TAM (LDTAM), 3) 2.5 mg/kg/d or high-dose TAM (HDTAM), 4) 50 mg/kg Cy, 5) Cy+LDTAM, and 6) Cy+HDTAM (Table 1).
Table 1.
Fertility rates and average litter sizes
| Groups | N | # of fertile rats | Average litter size | |
|---|---|---|---|---|
| Day 0 | Day 10 | |||
| CONT | 6 | 6 (100%) | 8.3 ± 1.7 | 8.3 ± 1.7 |
| LDTAM | 6 | 6 (100%) | 11.0 ± 1.9 | 11.0 ± 1.9 |
| HDTAM | 6 | 6 (100%) | 9.7 ± 0.7 | 9.7 ± 0.7 |
| Cy | 6 | 4 (66.7%)# | 10.5 ± 0.6 | 2.8 ± 2.8* |
| Cy+LDTAM | 5 | 3 (60%)* | 8.7 ± 1.5 | 8.7 ± 1.5 |
| Cy+HDTAM | 5 | 5 (100%) | 10.2 ± 2.1 | 10.2 ± 2.1 |
Fertility rates and average litter sizes from rats exposed to vehicles (CONT), low dose tamoxifen (LDTAM, 1 mg/kg/d), high dose tamoxifen (HDTAM, 2.5 mg/kg/d), cyclophosphamide (Cy, 50 mg/kg, biweekly), Cy+LDTAM and Cy+HDTAM for 2 weeks. Rats exposed to Cy showed a trend for decreased fertility rate, a defect that was rescued by HDTAM. Cy-treated animals also showed a reduction in average litter size from day 0 to day 10 that is due to cannibalism by the dam. # p = 0.08, * p < 0.05
Oocyte culture The trajectory of these studies next suggested the examination of cyclophosphamide action in vitro. However, the active metabolite of this drug is not available for in vitro study. Doxorubicin was used as an alternative in vitro treatment addressing the same clinical problem (chemotherapy mediated follicle loss). The effects of DOX have been studies extensively in vitro [19] and the same strategy was also used recently to investigate ovoprotective properties of imatinib [20]. Sprague Dawley rats (n = 8, 4 weeks old) were superovulated with 20 IU of pregnant mare serum gonadotropin and human chorionic gonadotropin (hCG) 48 h later. Sixteen hours after hCG injection, cumulus-oocyte complexes were collected from the oviducts. Oocytes were denuded of cumulus cells in 1000 IU/ml hyaluronidase followed by three washes of Modified Rat Embryo Culture Medium HEPES media [21, 22]. Groups of 15–25 oocytes per treatment per replication (n = 9 replication per treatment group) were cultured in 0.1 ml drops of culture medium under sterile mineral oil. Oocytes were incubated with the following treatments: 1) vehicle, 0.1% ethanol, 2) 40 μmol l−1 4hydroxy tamoxifen (4HT, the active metabolite of tamoxifen, in ethanol), 3) 200 nmol l−1 DOX [19, 22] + 0.1% ethanol, or 4) 200 nmol l−1 DOX + 40 μmol l−1 4HT at 37 ºC in a humidified atmosphere with 5% CO2 for 24 h.
Histological and morphological analysis Six-micron serial sections of ovaries were deparaffinized, rehydrated, and stained with hematoxylin and eosin for histological analysis. Follicles were classified as primordial (one layer of flattened pregranulosa cells), primary (one expanded layer of cuboidal granulosa cells), pre-antral (2–5 layers of granulosa cells), early antral, and late antral follicles and counted in every 10th section [23]. The same individual counted the follicles throughout the study and was blinded to the treatment. The number of follicles in each category per ovary is expressed by the sum of counted follicles in the 10th section multiplied by 10. In the oocyte culture experiment, morphological changes indicative of apoptosis or oocyte fragmentation were examined by a light microscope at the end of the treatment period after the method of Tilly and colleagues [22].
Data analysis Data for follicular reserves are presented as the mean ± SEM of follicle loss vs. controls and analyzed for the main effects of DMBA or Cy and TAM, as well as their interaction on numbers of follicles using a one- or two-way ANOVA. Results from oocyte cultures are presented as a percentage of fragmented oocytes and analyzed for the effects of DOX and TAM, using nonparametric Kruskal-Wallis. Bonferroni analysis was used for multiple comparisons. Differences are considered significant when p ≤ 0.05.
Results
Tamoxifen rescues ovarian follicular loss caused by DMBA in vivo
In vivo, total ovarian follicle numbers were quantified in order to assess the effects of tamoxifen on DMBA toxicity. The average number of total follicles per ovary was 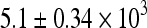 in vehicle-treated animals. Compared to controls, high dose DMBA decreased numbers of total follicles by approximately half (p < 0.05), while the lower dose caused a lesser but still significant loss in total follicle numbers (Fig. 1). The addition of TAM inhibited follicle losses induced by both doses of DMBA (Fig. 1). TAM alone slightly increased total follicle numbers, an effect not seen in our other experiments.
in vehicle-treated animals. Compared to controls, high dose DMBA decreased numbers of total follicles by approximately half (p < 0.05), while the lower dose caused a lesser but still significant loss in total follicle numbers (Fig. 1). The addition of TAM inhibited follicle losses induced by both doses of DMBA (Fig. 1). TAM alone slightly increased total follicle numbers, an effect not seen in our other experiments.
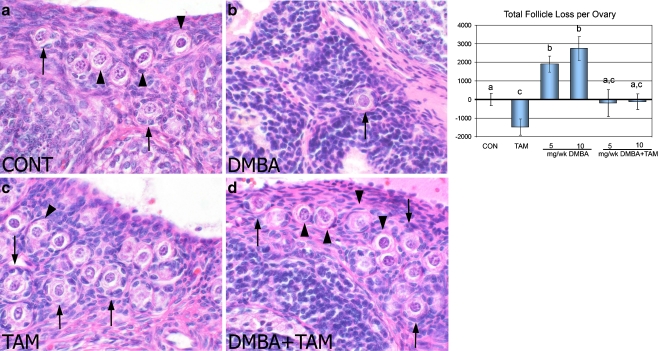
Fig. 1.
H&E sections of the ovary from rats exposed to vehicle (CONT, a), DMBA (10 mg/wk, b), tamoxifen (TAM, c), or DMBA+TAM (d) for 2 weeks. Arrowheads = representative primordial follicles. Arrows = representative primary follicles. DMBA decreased follicular reserves in the ovary (b) compared with vehicle-treated controls (a) and the addition of tamoxifen inhibited this depletion (d). Tamoxifen alone elevated the number of follicles in the ovary compared with vehicle-treated controls (c). Numbers of follicle loss relative to vehicle-treated controls are shown for total (follicles at all developmental stages) follicle populations and are calculated by subtracting the control group mean value. The loss of follicles was expressed by a positive number whereas gain of follicles was expressed as a negative number. Different letters indicate significant differences among treatment groups
Tamoxifen blocks Cy-induced follicular toxicity in vivo
Ovarian follicle numbers (total and by follicle classes) were quantified as described above to examine the effects of tamoxifen on Cy toxicity. The average number of total and primordial follicles was 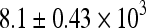 and
and 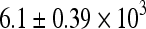 , respectively, per ovary in control animals. Compared to controls, Cy induced significant follicle loss at both doses (p < 0.05, Fig. 2), reducing total follicle numbers by approximately one third. The addition of TAM alleviated this loss (Fig. 2). Neither dose of Cy had an effect on numbers of pre-antral, early antral and late antral follicles but rather seemed to preferentially alter the more plentiful smaller follicle classes.
, respectively, per ovary in control animals. Compared to controls, Cy induced significant follicle loss at both doses (p < 0.05, Fig. 2), reducing total follicle numbers by approximately one third. The addition of TAM alleviated this loss (Fig. 2). Neither dose of Cy had an effect on numbers of pre-antral, early antral and late antral follicles but rather seemed to preferentially alter the more plentiful smaller follicle classes.
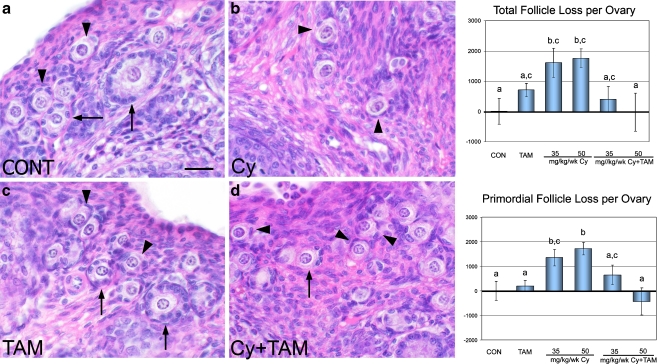
Fig. 2.
H&E sections of the ovary from rats exposed to vehicle (CONT, a), cyclophosphamide (Cy, 50 mg/kg, b), tamoxifen (TAM, c), or Cy+TAM (d) for 4 weeks. Arrowheads = representative primordial follicles. Arrows = representative primary follicles. Cy decreased total numbers of follicles (b) in the ovary compared with vehicle-treated controls (a) and the addition of tamoxifen inhibited this depletion (d). TAM did not affect the number of follicles in the ovary compared with vehicle-treated controls (c). Numbers of follicle loss relative to vehicle-treated controls are shown for total and primordial follicle populations. Cy treatment at 35 and 50 mg/kg/wk induced follicle loss compared with controls and the addition of TAM to both doses of Cy inhibited this depletion. Different letters indicate significant differences among treatment groups
Tamoxifen improves fertility and offspring survival following cyclophosphamide
Follicle counts from ovaries collected at hemiovariectomy confirmed a loss of total and primordial follicles by Cy, and the ability of TAM at both doses to rescue follicles reminiscent of the previous experiment (data not shown). All rats showed normal cyclicity (4–5 day estrous cycle) 1 week prior to the beginning of the mating period. While no differences were found in gestation length or sex ratios of the offspring among treatment groups (data not shown), rats exposed to Cy showed a trend for decreased fertility rate (p = 0.08), a defect that was rescued by HDTAM (Table 1). Interestingly, Cy-treated animals that did become pregnant had reduced numbers of pups by d10, although a portion of this was due to postnatal cannibalism (p < 0.05, Table 1). This reduced pup survival in Cy-treated dams was reversed by tamoxifen. Overall, cyclophosphamide treatment made rats less likely to become pregnant and resulted in fewer surviving pups from each pregnancy giving a markedly reduced reproductive success. Treatment with high dose tamoxifen reversed both the trend towards infertility and decreased numbers of healthy pups.
Tamoxifen prevents oocyte fragmentation from doxorubicin in vitro
To investigate local effects of tamoxifen on chemical-induced ovotoxicity, isolated oocytes were exposed to DOX and/or 4HT in vitro. Results showed that few oocytes exposed to the vehicle and 4HT, respectively, underwent spontaneous fragmentation, a morphological change associated with apoptotic cell death (Fig. 3). Doxorubicin increased (p < 0.05) oocyte fragmentation compared with vehicle-treated controls, and this effect was reversed by 4HT (p < 0.05, Fig. 3).
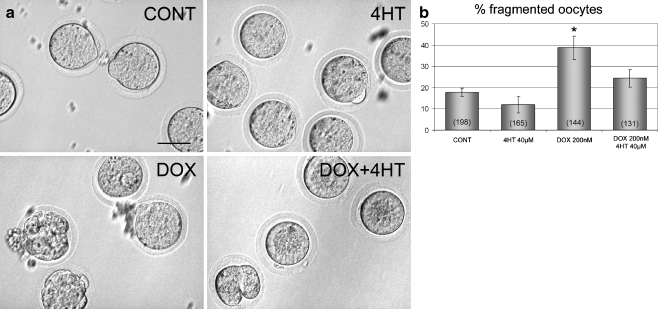
Fig. 3.
a Mature oocytes exposed to vehicle, 4-hydroxytamoxifen (4HT), doxorubicin (DOX), or DOX+4HT for 24 h. Healthy oocytes surround by zona pellucida were observed after vehicle and 4HT treatment. Doxorubicin induces fragmentation in cultured oocytes, an effect that is antagonized by the addition of 4HT. b Quantitative analysis of the percentage of oocytes undergoing fragmentation. Oocytes incubated with doxorubicin showed increased fragmentation rate compared with vehicle-treated controls. This elevated fragmentation rate was inhibited by the addition of 4HT. The total number of oocytes cultured in each treatment group is indicated in parentheses inside the respective bar. * indicates a significant difference in comparison to vehicle-treated controls
Discussion
Tamoxifen protects ovarian reserves in female rats from two widely studied ovotoxicants, DMBA, an experimental agent, and cyclophosphamide, a commonly used cancer drug. Both DMBA and cyclophosphamide reduced numbers of total follicles, and the addition of TAM inhibited this loss mostly by preserving the primordial follicle population. Cyclophosphamide reduced follicular reserves by approximately one third, a decline comparable to that experienced by cancer patients [2]. Reduced fertility was observed in Cy-treated animals (p = 0.08), and tamoxifen restored fertility in these animals. Interestingly, while numbers of healthy pups per litter in dams that did conceive were decreased by Cy and restored by tamoxifen, maternal cannibalism was behind much of this reproductive defect. Factors potentially contributing to maternal cannibalism include abnormalities in the offspring, as well as agalactia and altered maternal behavior [24, 25].
Another ovotoxic chemotherapy drug, doxorubicin, increased oocyte death in vitro when compared to vehicle-treated controls, and the active metabolite of tamoxifen was able to antagonize this toxicity in the current work. Studies have suggested that oocyte fragmentation in vitro occurs through apoptosis [22], therefore, a possible protective role of tamoxifen on DOX-treated oocytes may occur through direct inhibition of apoptosis. These data support a direct action of tamoxifen on the oocyte, but must be interpreted with caution due to use of a different ovotoxicant than other experiments.
This work, while suggestive, has a number of necessary limitations. Data are largely observational and provide minimal direct mechanistic data. The follicular depletion by cyclophosphamide observed here is modest (about one third of total reserves), reflecting the need to avoid systemic toxicities in these experiments. The route of tamoxifen delivery differs from the normal human administration (although doses are in a similar range). The mating study, while laborious and suggestive, has small animal numbers and the induction of infertility by cyclophosphamide here did not reach the predefined level of statistical significance. Additionally, a portion of the benefit observed from tamoxifen when examining reproductive function in dams treated with cyclophosphamide was due to maternal cannibalism (and its correction when tamoxifen is added)—a phenomenon of uncertain relevance to the human perinatal period. Nevertheless, taken together these studies are important because of the great potential benefit if tamoxifen is effective as an ovarian protectant in women.
This begs the question of why such clinical benefit has not been observed previously in women taking tamoxifen. The SERM is a commonly used drug in cancer patients and the subject of many clinical and preclinical trials [26–28]. However, retrospective human studies have overlooked changes in the ovary and have been inappropriate in design, treatment duration, patient age and endpoints, for the assessment of fertility, producing mixed results [26]. In a recent study, Sverrisdottir et al failed to show a positive effect of tamoxifen on the protection of ovary against chemotherapy-induced ovarian failure in breast cancer patients [29]. However, in this study tamoxifen treatment is started at the same time as the chemotherapy (unlike here where tamoxifen pretreatment is used), indicating that the protective effect of tamoxifen may not be optimal against chemotherapy due to the time for tamoxifen to reach steady state [30]. Another study, which examined reproductive endocrinology in premenopausal breast cancer patients receiving cyclophosphamide + methotrexate + 5-fluorouracil chemotherapy, with or without simultaneous prednisone or prednisone + tamoxifen [31], showed results similar to those in our study. Addition of tamoxifen to the regimen was associated with elevated estradiol, decreased FSH concentrations and delayed iatrogenic amenorrhea during 6 and 10 months of treatment [31]. At least one animal study has previously showed rescue of follicular reserves from toxic insult using tamoxifen as well [12].
The cellular mechanisms by which tamoxifen acts to rescue ovarian follicles need further investigation. Systemically, tamoxifen may cause effects similar to GnRH agonist by disrupting the hypothalamic-pituitary-ovarian axis [6], protecting the ovary by keeping it in a quiescent anovulatory state. The local protection afforded by tamoxifen in preventing toxicant-induced follicular loss may be a result of inhibition of follicular apoptosis and disruption of drug transfer into the ovary. Apoptosis-driven ovarian damage has been thought to play a critical role in oocyte destruction and premature ovarian failure caused by environmental toxicants and chemotherapy [32–34]. Interestingly, E2 has been shown to protect against 4-vinylcyclohexene diepoxide-induced follicle loss in the ovary due to anti-oxidant and anti-apoptosis properties, as shown by decreased levels of caspase-3 [34, 35]. Tamoxifen, a SERM, exhibits anti-oxidant and anti-apoptotic properties when exerting its estrogen-like effects [36, 37]. Tamoxifen may also protect the ovary by inhibiting drug transfer to and within the ovary [38, 39]. Finally, a recent study showed chemoprotection from cyclophosphamide-induced follicle loss and infertility in rodents using a different chemotherapy agent – imatinib or Gleevec [20], via the p63 pathway. Elucidation of the cellular mechanism responsible for tamoxifen protection of ovarian follicles will be crucial to development of novel strategies for fertility preservation following chemotherapy in females.
Any fertility preservation strategy in women cancer patients cannot interfere with antitumor effects of other chemotherapy drugs. Tamoxifen has been administered to women for the past 30 years and has an extensive record of safe use including use in combination with other cancer chemotherapy drugs [26–28]. Continuous use of tamoxifen exceeding 5 years is associated with an increased risk of endometrial cancer and blood clots [26]. Side effects of short term tamoxifen use in premenopausal women include menopause-like symptoms and impairment of the hypothalamus-gonadal axis. These are reversible following the discontinuation of treatment [40].
In conclusion, we have shown that tamoxifen decreases loss of ovarian follicular reserves following exposure to the ovotoxic chemical DMBA and the chemotherapy drug Cy in rats. Furthermore, initial work in vitro shows protective actions of tamoxifen against oocyte loss from doxorubicin, another commonly used cancer drug. Current options for fertility preservation during chemotherapy do not prevent ovarian toxicity predisposing to premature menopause. Early menopause is associated with adverse symptoms such as elevated cardiovascular risk, osteoporosis, cognitive impairment and depression, even in women with cancer not seeking fertility preservation. Our results suggest that tamoxifen therapy might be developed to preserve fertility and normal ovarian function in premenopausal women undergoing chemotherapy.
Footnotes
Capsule
Tamoxifen decreases follicle loss and improves reproductive function following exposure to ovarian toxicants, including chemotherapy drugs, in the female rat.
References
- 1.Absolom K, Eiser C, Turner L, Ledger W, Ross R, Davies H, Coleman R, Hancock B, Snowden J, Greenfield D. Ovarian failure following cancer treatment: current management and quality of life. Hum Reprod. 2008;23:2506–2512. doi: 10.1093/humrep/den285. [DOI] [PubMed] [Google Scholar]
- 2.Simon B, Lee SJ, Partridge AH, Runowicz CD. Preserving fertility after cancer. CA Cancer J Clin. 2005;55:211–228. doi: 10.3322/canjclin.55.4.211. [DOI] [PubMed] [Google Scholar]
- 3.Meirow D. Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol. 2000;169:123–131. doi: 10.1016/s0303-7207(00)00365-8. [DOI] [PubMed] [Google Scholar]
- 4.Pacey AA. Fertility issues in survivors from adolescent cancers. Cancer Treat Rev. 2007;33(7):646–655. doi: 10.1016/j.ctrv.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Lobo RA. Potential options for preservation of fertility in women. N Engl J Med. 2005;353(1):64–73. doi: 10.1056/NEJMra043475. [DOI] [PubMed] [Google Scholar]
- 6.Ataya K, Rao LV, Lawrence E, Kimmel R. Luteinizing hormone-releasing hormone agonist inhibits cyclophosphamide-induced ovarian follicular depletion in rhesus monkeys. Biol Reprod. 1995;52(2):365–372. doi: 10.1095/biolreprod52.2.365. [DOI] [PubMed] [Google Scholar]
- 7.Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist. 2007;12(9):1044–1054. doi: 10.1634/theoncologist.12-9-1044. [DOI] [PubMed] [Google Scholar]
- 8.Blumenfeld Z. GnRH-agonists in fertility preservation. Curr Opin Endocrinol Diabetes Obes. 2008;15(6):523–528. doi: 10.1097/MED.0b013e32831a46e9. [DOI] [PubMed] [Google Scholar]
- 9.Blumenfeld Z, Avivi I, Ritter M, Rowe JM. Preservation of fertility and ovarian function and minimizing chemotherapy-induced gonadotoxicity in young women. J Soc Gynecol Investig. 1999;6(5):229–239. doi: 10.1016/s1071-5576(99)00028-3. [DOI] [PubMed] [Google Scholar]
- 10.Oktay K, Sonmezer M, Oktem O, Fox K, Emons G, Bang H. Absence of conclusive evidence for the safety and efficacy of gonadotropin-releasing hormone analogue treatment in protecting against chemotherapy-induced gonadal injury. Oncologist. 2007;12(9):1055–1066. doi: 10.1634/theoncologist.12-9-1055. [DOI] [PubMed] [Google Scholar]
- 11.Ting AY, Kimler BF, Fabian CJ, Petroff BK. Tamoxifen prevents premalignant changes of breast but not ovarian cancer in rats at high risk for both disease. Cancer Prev Res. 2008;1:546–554. doi: 10.1158/1940-6207.CAPR-08-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kossoy G, Ben-Hur H, Elhayany A, Schneider DF, Kossoy N, Zusman I. Effects of tamoxifen and soluble tumor-associated antigens on ovarian structure in mammary tumor-bearing rats. Oncol Rep. 2005;14(5):1317–1321. [PubMed] [Google Scholar]
- 13.Geyer RP, Bleisch VR, Bryant JE, Robbins AN, Saslaw IM, Stare FJ. Tumor production in rats injected intravenously with oil emulsions containing 9,10-dimethyl-1,2-benzanthracene. Cancer Res. 1951;11(6):474–478. [PubMed] [Google Scholar]
- 14.Matikainen T, Perez GI, Jurisicova A, Pru JK, Schlezinger JJ, Ryu HY, Laine J, Sakai T, Korsmeyer SJ, Casper RF, Sherr DH, Tilly JL. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat Genet. 2001;28(4):355–360. doi: 10.1038/ng575. [DOI] [PubMed] [Google Scholar]
- 15.Borman SM, Christian PJ, Sipes IG, Hoyer PB. Ovotoxicity in female Fischer rats and B6 mice induced by low-dose exposure to three polycyclic aromatic hydrocarbons: comparison through calculation of an ovotoxic index. Toxicol Appl Pharmacol. 2000;167(3):191–198. doi: 10.1006/taap.2000.9006. [DOI] [PubMed] [Google Scholar]
- 16.Tsai-Turton M, Nakamura BN, Luderer U. Induction of apoptosis by 9, 10-dimethyl-1, 2-benzanthracene in cultured preovulatory rat follicles is preceded by a rise in reactive oxygen species and is prevented by glutathione. Biol Reprod. 2007;77(3):442–451. doi: 10.1095/biolreprod.107.060368. [DOI] [PubMed] [Google Scholar]
- 17.Shiromizu K, Thorgeirsson SS, Mattison DR. Effect of cyclophosphamide on oocyte and follicle number in Sprague-Dawley rats, C57BL/6 N and DBA/2 N mice. Pediatr Pharmacol (New York) 1984;4(4):213–221. [PubMed] [Google Scholar]
- 18.Ataya KM, McKanna JA, Weintraub AM, Clark MR, LeMaire WJ. A luteinizing hormone-releasing hormone agonist for the prevention of chemotherapy-induced ovarian follicular loss in rats. Cancer Res. 1985;45(8):3651–3656. [PubMed] [Google Scholar]
- 19.Jurisicova A, Lee HJ, D’Estaing SG, Tilly J, Perez GI. Molecular requirements for doxorubicin-mediated death in murine oocytes. Cell Death Differ. 2006;13(9):1466–1474. doi: 10.1038/sj.cdd.4401819. [DOI] [PubMed] [Google Scholar]
- 20.Gonfloni S, Tella L, Caldarola S, Cannata SM, Klinger FG, Bartolomeo C, Mattei M, Candi E, Felici M, Melino G, Cesareni G. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med. 2009;15(10):1179–1185. doi: 10.1038/nm.2033. [DOI] [PubMed] [Google Scholar]
- 21.Oh SH, Miyoshi K, Funahashi H. Rat oocytes fertilized in modified rat 1-cell embryo culture medium containing a high sodium chloride concentration and bovine serum albumin maintain developmental ability to the blastocyst stage. Biol Reprod. 1998;59(4):884–889. doi: 10.1095/biolreprod59.4.884. [DOI] [PubMed] [Google Scholar]
- 22.Perez GI, Tao XJ, Tilly JL. Fragmentation and death (a.k.a. apoptosis) of ovulated oocytes. Mol Hum Reprod. 1999;5(5):414–420. doi: 10.1093/molehr/5.5.414. [DOI] [PubMed] [Google Scholar]
- 23.Tilly JL. Ovarian follicle counts–not as simple as 1, 2, 3. Reprod Biol Endocrinol. 2003;1:11. doi: 10.1186/1477-7827-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stern JM, Strait T. Reproductive success, postpartum maternal behavior, and masculine sexual behavior of neonatally androgenized female hamsters. Horm Behav. 1983;17(2):208–224. doi: 10.1016/0018-506x(83)90008-9. [DOI] [PubMed] [Google Scholar]
- 25.Jakubowski M, Terkel J. Female reproductive function and sexually dimorphic prolactin secretion in rats with lesions in the medial preoptic-anterior hypothalamic continuum. Neuroendocrinology. 1986;43(6):696–705. doi: 10.1159/000124607. [DOI] [PubMed] [Google Scholar]
- 26.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 27.Ezzat AA, Ibrahim EM, Stuart RK, Ajarim D, Bazarbashi S, El-Foudeh MO, Rahal M, Al-Sayed A, Berry J. Adding high-dose tamoxifen to CHOP does not influence response or survival in aggressive non-Hodgkin’s lymphoma: an interim analysis of a randomized phase III trial. Med Oncol. 2000;17(1):39–46. doi: 10.1007/BF02826215. [DOI] [PubMed] [Google Scholar]
- 28.Berman E, McBride M, Lin S, Menedez-Botet C, Tong W. Phase I trial of high-dose tamoxifen as a modulator of drug resistance in combination with daunorubicin in patients with relapsed or refractory acute leukemia. Leukemia. 1995;9(10):1631–1637. [PubMed] [Google Scholar]
- 29.Sverrisdottir A, Nystedt M, Johansson H, Fornander T. Adjuvant goserelin and ovarian preservation in chemotherapy treated patients with early breast cancer: results from a randomized trial. Breast Cancer Res Treat. 2009;117(3):561–567. doi: 10.1007/s10549-009-0313-5. [DOI] [PubMed] [Google Scholar]
- 30.Furlanut M, Franceschi L, Pasqual E, Bacchetti S, Poz D, Giorda G, Cagol P. Tamoxifen and its main metabolites serum and tissue concentrations in breast cancer women. Ther Drug Monit. 2007;29(3):349–352. doi: 10.1097/FTD.0b013e318067ded7. [DOI] [PubMed] [Google Scholar]
- 31.Rose DP, Davis TE. Effects of adjuvant chemohormonal therapy on the ovarian and adrenal function of breast cancer patients. Cancer Res. 1980;40(11):4043–4047. [PubMed] [Google Scholar]
- 32.Hu X, Christian P, Sipes IG, Hoyer PB. Expression and redistribution of cellular Bad, Bax, and Bcl-X(L) protein is associated with VCD-induced ovotoxicity in rats. Biol Reprod. 2001;65(5):1489–1495. doi: 10.1095/biolreprod65.5.1489. [DOI] [PubMed] [Google Scholar]
- 33.Livera G, Petre-Lazar B, Guerquin MJ, Trautmann E, Coffigny H, Habert R. p63 null mutation protects mouse oocytes from radio-induced apoptosis. Reproduction. 2008;135(1):3–12. doi: 10.1530/REP-07-0054. [DOI] [PubMed] [Google Scholar]
- 34.Morita Y, Perez GI, Maravei DV, Tilly KI, Tilly JL. Targeted expression of Bcl-2 in mouse oocytes inhibits ovarian follicle atresia and prevents spontaneous and chemotherapy-induced oocyte apoptosis in vitro. Mol Endocrinol. 1999;13(6):841–850. doi: 10.1210/mend.13.6.0306. [DOI] [PubMed] [Google Scholar]
- 35.Thompson KE, Sipes IG, Greenstein BD, Hoyer PB. 17beta-estradiol affords protection against 4-vinylcyclohexene diepoxide-induced ovarian follicle loss in Fischer-344 rats. Endocrinology. 2002;143(3):1058–1065. doi: 10.1210/endo.143.3.8665. [DOI] [PubMed] [Google Scholar]
- 36.Dubey RK, Tyurina YY, Tyurin VA, Gillespie DG, Branch RA, Jackson EK, Kagan VE. Estrogen and tamoxifen metabolites protect smooth muscle cell membrane phospholipids against peroxidation and inhibit cell growth. Circ Res. 1999;84(2):229–239. doi: 10.1161/01.res.84.2.229. [DOI] [PubMed] [Google Scholar]
- 37.Nathan L, Chaudhuri G. Antioxidant and prooxidant actions of estrogens: potential physiological and clinical implications. Semin Reprod Endocrinol. 1998;16(4):309–314. doi: 10.1055/s-2007-1016289. [DOI] [PubMed] [Google Scholar]
- 38.Darvari R, Boroujerdi M. Investigation of the influence of modulation of P-glycoprotein by a multiple dosing regimen of tamoxifen on the pharmacokinetics and toxicodynamics of doxorubicin. Cancer Chemother Pharmacol. 2005;56(5):497–509. doi: 10.1007/s00280-005-1001-8. [DOI] [PubMed] [Google Scholar]
- 39.Shen LZ, Hua YB, Yu XM, Xu Q, Chen T, Wang JH, Wu WX. Tamoxifen can reverse multidrug resistance of colorectal carcinoma in vivo. World J Gastroenterol. 2005;11(7):1060–1064. doi: 10.3748/wjg.v11.i7.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oktay K, Buyuk E, Davis O, Yermakova I, Veeck L, Rosenwaks Z. Fertility preservation in breast cancer patients: IVF and embryo cryopreservation after ovarian stimulation with tamoxifen. Hum Reprod. 2003;18(1):90–95. doi: 10.1093/humrep/deg045. [DOI] [PubMed] [Google Scholar]