Abstract
Purpose
A laser is commonly used to remove a blastomere from an embryo for genetic testing. The laser uses intense heat which could possibly disrupt embryo development. It is the goal of this study to test the effects of different laser pulse lengths (and consequently heat) on the embryo biopsy procedure and embryo development.
Methods
Each embryo biopsy was performed randomly utilizing laser pulse lengths of 0.604mS (group I), 0.708mS (group II), and 1.010mS (group III).
Results
For groups I, II, and III, 83, 86, and 71 embryos were biopsied, respectively. There was no difference in day 5 embryo quality or lysed blastomeres between groups. Average number of blastomeres biopsied between group I (1.0 ± 0.0), II (1.0 ± 0.2), and III (1.1 ± 0.2) was significant (0.0001).
Conclusion
Our data demonstrates that laser pulse length does not influence the embryo biopsy procedure or embryo development.
Keywords: Embryo biopsy, Laser, Pulse length, Embryo development, IVF
Introduction
The laser has become a critical and integral part to the in vitro fertilization (IVF) laboratory. With its ease of use, the laser has made assisted hatching, polar body biopsy, and embryo biopsy common procedures. The use of the laser has even added to the improvement and advancement of the IVF field with its use in procedures such as laser assisted intracytoplasmic sperm injection (ICSI) [1, 2], laser assisted immobilization of sperm prior to ICSI [3], and most recently, trophectoderm biopsy [4]. The use of the laser for assisted reproductive technologies has become widespread.
The laser uses high energy light and consequently heat, to dissolve or disintegrate the zona pellucida (ZP) thereby allowing the removal of a single blastomere from the embryo. When the light from the laser comes in contact with the media surrounding the embryo, heat is dissipated and transferred through the water of the media creating temperature gradients in increasing concentric circles from the laser beam [5, 6]. These concentric circles represent an increase in temperature around the embryo and blastomeres. The concentric circles can increase in size, diameter, and longevity depending on laser characteristics [7]. It has been estimated that the temperature in the surrounding media can increase to 60 to 80°C [8]. Certainly this increase in temperature would support research that shows blastomeres in the immediate vicinity of the laser beam are damaged [9].
A laser has three different properties that determine its performance and hence its effects on the embryo and surrounding media: power, wavelength, and pulse length. Lasers’ power utilized for IVF are measured in miliwatts (mW) and can range from 20 mW to >400 mW. The power varies depending on the make and model of the laser but is constant for each specific laser. If the power is increased the hole diameter is increased. Conversely, if the power is decreased the hole diameter is decreased. Each increase or decrease in power also influences the temperature gradient through the media and embryo.
Laser wavelength refers to which section of the light spectrum the particular laser falls. A wide variety of laser wavelengths have been used on both the mouse and human model. In the beginning lasers used ultraviolet (UV) radiation to create holes in the zona pellucida [10]. The main problem to using UV radiation is the susceptibility of DNA to this type of wavelength which resulted in limited applications in a routine IVF setting. To become applicable to IVF lasers now mainly utilize near infrared light (2,500 nm to 750 nm). This type of light still allows for successful applications yet minimizes the damage to the DNA within each blastomere [5].
Pulse length refers to the amount of time or duration of the laser beam. Pulse lengths range anywhere from 20 ms (Fertilase) to >1,000 μs (Saturn Zilos). The shorter duration of time and decreased energy results in less heat and decreases the temperature gradient within the media. Most laser systems allow for the adjustment of pulse length to increase or decrease the diameter of the beam and resultant hole.
As previously discussed, an increase or decrease in pulse length or power will cause a corresponding increase or decrease in the temperature gradient within the media. If the media is exposed to an increase in temperature the embryo is also exposed which could compromise development. By adjusting these settings the laser hole size also changes and may have an effect on the biopsy procedure. For example, multiple small laser holes are required in order to breach the ZP and allow for the extraction of a blastomere for preimplantation genetic diagnosis (PGD). Whereas fewer larger laser holes are needed to achieve the same effect.
A laser with a high power and short pulse lengths will produce smaller temperature gradients than a laser with low power and longer pulse lengths. A smaller temperature gradient will less likely compromise embryo development. Smaller laser holes may also make it more difficult to remove blastomeres for PGD. It is the aim of this study to determine if different pulse lengths have an effect on the embryo biopsy procedure and subsequent embryo development to the blastocysts stage.
Materials and methods
Study design
This was a prospective, randomized study involving 23 patients between September 2008 and April 2010. Only those patients whom elected for PGD for aneuploidy for advanced maternal age (AMA) were included in the study.
Since ovarian stimulation, semen preparation, conventional and ICSI insemination, and fertilization were the same for each group, the details of these procedures are discussed elsewhere [11].
Embryo assessment
Embryo assessment took place 42 h to 46 h (day 2) and 66 h to 70 h (day 3) post insemination. The evaluation consisted of the number and symmetry of blastomeres, percentage of fragmentation, and presence of multi-nucleated blastomeres. Day 3 embryos were given a letter score of “A” though “D”, where “A” was the highest and “D” the lowest quality embryo.
Embryo biopsy, randomization and blastocyst culture
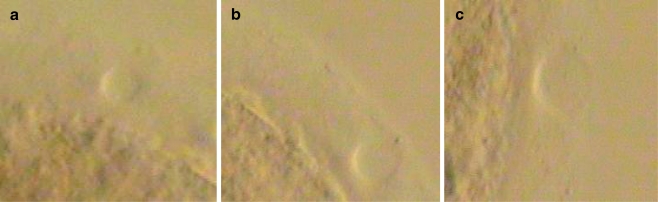
All biopsies were performed by one technician to eliminate inter-operator variability. Embryos were biopsied on day 3 by placing each embryo into a 20 μL drop of Ca/Mg free medium (Cooper/Sage, Bedminster, New Jersey, US) supplemented with 5% SPS and overlayed with oil. Each embryo was placed in its own drop and numbered appropriately. Pulse lengths were chosen for each embryo biopsy at random. Biopsy was performed with the SaturnActive Laser™ (Research Instruments, Cornwall, United Kingdom) utilizing laser pulse lengths of 0.604mS (group I), 0.708mS (group II), and 1.010mS (group III) which correspond to hole sizes of 10.5 nm, 13.5 nm, and 16.5 nm respectively (Fig. 1). After opening ZP a single blastomere was aspirated and removed from the embryo. The embryos were then placed back into individual 50 μL drop of blastocyst media (Cooper/Sage, Bedminster, New Jersey, US) supplemented with 10% SPS under oil and incubated until day 5.
Fig. 1.
a Laser shot utilizing a pulse length of 0.604mS. b A laser shot utilizing a pulse length of 0.708mS. c A laser shot utilizing a pulse length of 1.010mS
Blastocyst assessment took place 120 to 124 hours (day 5) and 144 to 148 hours (day 6) post insemination. The evaluation consisted of the score of inner cell mass and trophectoderm. Day 5/6 embryos were given a letter score of “A” through “D”, where “A” was the highest and “D” the lowest quality embryo. Blastocysts were further divided into “good” embryos which were frozen or transferred and “poor” embryos which were discarded.
Statistical analysis
One-way ANOVA or chi-square tests were applied where appropriate and statistical significance was set at P < 0.05.
Results
A total of 240 embryos (10.4 ± 5.1 embryos) from 23 patients (38.4 ± 4.8 years old) were biopsied on day 3 and included in this study. Three different pulse lengths were utilized during the embryo biopsy procedure, group I (0.604 mS), group II (0.708 mS), and group III (1.010 mS). A total of 83, 86, and 71 embryos were biopsied with each pulse length, respectively (Table 1). A total of 114 patients were included in the control group. The control group consisted of patients diagnosed AMA and between 38-39 years old proceeding with IVF but not opting for PGD at the same time as this study was being conducted. Differences in embryo quality and cell number on day 3 were not significant between groups (Table 1).
Table 1.
Embryo development based on the different laser pulse lengths used during embryo biopsy
| Group I (0.604 mS) | Group II (0.708 mS) | Group III (1.010mS) | No PGD (control) | P value | |
|---|---|---|---|---|---|
| No. patients | 23 | 23 | 23 | 114 | |
| No. embryos | 83 | 86 | 71 | 549 | |
| Avg. age | 38.4 ± 4.8 | 38.4 ± 4.8 | 38.4 ± 4.8 | 38.5 ± 1.5 | 0.9971a |
| Avg. cell no. day 3 | 6.3 ± 1.8 | 6.2 ± 1.7 | 6.5 ± 1.6 | 6.2 ± 1.5 | 0.4769a |
| Day 3 embryo quality | |||||
| #A (%) | 29 (34.9%) | 32 (37.2%) | 27 (38.0%) | 205 (37.3%) | 0.9662b |
| #B (%) | 24 (28.9%) | 21 (24.4%) | 20 (28.2%) | 160 (29.1%) | |
| #C (%) | 21 (25.3%) | 24 (27.9%) | 20 (28.2%) | 131 (23.9%) | |
| #D (%) | 9 (10.8%) | 9 (10.5%) | 4 (5.6%) | 53 (9.7%) | |
| Day 5 embryo quality | |||||
| #A (%) | 11 (13.3%) | 17 (19.8%) | 8 (11.3%) | 29 (11.8%) | 0.4723b |
| #B (%) | 16 (19.3%) | 11 (12.8%) | 15 (21.1%) | 48 (19.4%) | |
| #C (%) | 19 (22.9%) | 28 (32.6%) | 23 (32.4%) | 79 (32.0%) | |
| #D (%) | 37 (44.6%) | 30 (34.9%) | 25 (35.2%) | 91 (36.8%) | |
| Good quality (%) | 27 (32.5%) | 28 (32.6%) | 23 (32.4%) | 77 (31.2%) | 0.9919b |
| Poor quality (%) | 56 (67.5%) | 58 (67.4%) | 48 (67.6%) | 170 (68.8%) |
aone-way ANOVA
bchi-square test for independence
Blastocyst quality among the groups was not significant (Table 1). To increase the statistical power, we combined our blastocyst quality groups to “good” quality blastocyst (those that would either be frozen or transferred) and “poor” quality blastocyst (those that would discard). Twenty seven of 83 (32.5%) from group I were “good” quality blastocyst, along with 28 of 86 (32.6%), 23 of 71 (32.4%), and 77 of 247 (31.2%) from group II, III, and control group respectively (P = 0.9919; chi-square test; Table 1).
The average number of blastomeres removed from each group was as follows, group I (1.0 ± 0.0 blastomeres), group II (1.0 ± 0.2), and group III (1.1 ± 0.2) (P = 0.0001; one-way ANOVA; Table 2). A total of 8 blastomeres were lysed during this trial, 3, 3, and 2 from groups I, II, and III, respectively (P = 0.9402; chi-square test; Table 2)
Table 2.
The effect of different laser pulse lengths on the embryo biopsy procedure
| Group I (0.604 mS) | Group II (0.708 mS) | Group III (1.010mS) | P Value | |
|---|---|---|---|---|
| No. embryos biopsied | 83 | 86 | 71 | |
| No. cells biopsied | 83 | 88 | 75 | |
| Avg. no. cells biopsied per embryo | 1.0 ± 0.0 | 1.0 ± 0.2 | 1.1 ± 0.2 | 0.0001a |
| No. lysed (%) | 3 (3.6%) | 3 (3.4%) | 2 (2.8%) | 0.9402b |
aone-way ANOVA
bChi-square test for independence
Discussion
The laser has been utilized successfully in IVF for more than a decade but the effects of the laser on embryo development and embryo biopsy have largely been ignored. Previous research used the rate of lysed blastomeres to describe the efficiency of the embryo biopsy procedure [12, 13]. In the current study, the variation of the pulse length and the resultant hole did not influence the rate of lysed blastomeres. Three blastomeres from groups I and II, and two from group III lysed during the biopsy procedure. These results are surprising considering the larger pulse length correlates to a larger laser beam. We would expect that the larger pulse length would cause more damage to the blastomere because it produces a larger diameter hole. This increase in damage could cause the blastomere to more easily lyse during the embryo biopsy. This was not the case as the same number of lysed blastomeres occurred in the large pulse length group as the small pulse length group. An isolated laser shot may not be enough to lyse the blastomere.
Another way to correlate the effectiveness of the embryo biopsy procedure is to look at how many blastomeres were removed. The removal of two blastomeres has a detrimental effect on embryo development and should be avoided [14]. When chosen, the embryologist needs to be certain that the blastomere contains a nucleus, although sometimes fragmentation or cytoplasmic abnormalities prevent the direct viewing of a nucleus. In the present study, the number of blastomeres removed was significantly different among the three groups. No additional blastomeres were removed from group I. Two embryos from group II needed to have an additional blastomere removed and a total of 4 embryos from group III had an additional blastomere removed (Table 2). Of those six, three were from the same patient. This patient presented with extremely poor embryo quality making the embryo biopsy more difficult as the embryos had to rebiospied due to no nucleus. Pulse length may not influence the embryo biopsy procedure as the data suggests.
When using the laser, one aspect that is largely overlooked is the distance from the embryo to the laser objective. The energy of the laser is directly proportional to the distance it must travel. This is evident during embryo biopsy if the embryo is not placed directly on the bottom of the dish. For example, if the embryo is held in the middle of the droplet and the laser is fired the hole produced would be significantly smaller than if the embryo were placed on the bottom of the droplet at the same power and pulse length. The smaller hole is directly proportional to energy and residual heat. To control for this factor we made sure that all embryos were held at the bottom of the dish prior to the laser being used.
Ideally the study should have controlled for the number of shots in each group which would effectively control for the heat distributed within the media. For example, only allow ten shots from the smaller pulse length (no more or less), five shots from the medium pulse length (no more or less), and two from the larger pulse length (no more or less). Unfortunately this could not be completed due to the differences in ZP thickness between each embryo. Standardizing the number of shots could compromise patient care by allowing more or less laser shots than necessary causing either a greater temperature increase or a more difficult embryo biopsy due to incomplete ablation of ZP.
Embryo development to the blastocyst stage was not compromised by the different pulse lengths. The pulse length is only one determining factor that influences the size of the laser beam. The laser power also plays a large role. In our study, we were unable to compare laser power because our laser is set to 400 mW and this value is unable to be changed. Newer lasers utilize higher power (such as 400 mW) as opposed to the older lasers that utilize less power. Less power requires a much larger pulse length to have the same effect as a high power and small pulse length laser beam. Even though our data shows no difference in blastocyst development from different pulse lengths, utilizing a less powerful laser and large pulse length may influence blastocyst development as that combination will also have a different effect on the surrounding media (i.e., heating the media up and the media staying warmer for longer). We would expect the different laser pulse lengths to heat the surrounding media to different degrees, causing possible heat damage to the developing embryo. A study by Hartshorn et al. [15] showed no increase in hsp70i (heat shock protein) in embryos subjected to the laser. Typically we should expect an increase in hsp70i expression if temperature were a factor during embryological development. Research has shown that if an embryo is heated above 37°C then hsp70i transcription is also increased which could potentially cause damage to the developing embryo [16, 17]. In the current study, we did not see compromised embryo development between the three groups, signifying that the laser does not heat surrounding media enough to result in damage to the embryo. This supports previous research that shows no structural damage to mouse embryos during the assisted hatching procedure [8, 18].
This is the first study that we are aware that addresses the optimal settings when utilizing the laser for embryo biopsy. Our data shows that a larger pulse length can be safely used during embryo biopsy thereby making the procedure more efficient and convenient for the embryologist. It is for this reason we recommend utilizing a larger pulse length while performing embryo biopsy. In conclusion, the laser seems to be an effective and safe tool for use with embryo biopsy and does not compromise embryo development to the blastocyst stage.
Conclusions
Different laser pulse lengths used during the embryo biopsy procedure do not influence the embryo biopsy procedure or embryo development to the blastocyst stage.
Footnotes
Capsule
Different laser pulse lengths used during the embryo biopsy procedure do not influence the procedure or embryo development to the blastocyst stage
References
- 1.Rienzi L, Greco E, Ubaldi F, Iacobelli M, Martinez F, Tesarik J. Laser-assisted intracytoplasmic sperm injection. Fertil Steril. 2001;76:1045–7. doi: 10.1016/s0015-0282(01)02861-8. [DOI] [PubMed] [Google Scholar]
- 2.Nagy ZP, Oliveira SA, Abdelmassih V, Abdelmassih R. Novel use of laser to assist ICSI for patients with fragile oocytes: a case report. Reprod Biomed Online. 2002;4:27–31. doi: 10.1016/s1472-6483(10)61911-6. [DOI] [PubMed] [Google Scholar]
- 3.Ebner T, Yaman C, Moser M, Sommergruber M, Hartl J, Tews G. Laser assisted immobilization of spermatozoa prior to intracytoplasmic sperm injection in humans. Hum Reprod. 2001;16:2628–31. doi: 10.1093/humrep/16.12.2628. [DOI] [PubMed] [Google Scholar]
- 4.Veiga A, Sandalinas M, Benkhalifa M, Boada M, Carrera M, Santalo J, et al. Laser blastocyst biopsy for preimplantation diagnosis in the human. Zygote. 1997;5:351–4. doi: 10.1017/s0967199400003920. [DOI] [PubMed] [Google Scholar]
- 5.Douglas-Hamilton DH, Conia J. Thermal effects in laser-assisted pre-embryo zona drilling. J Biomed Opt. 2001;6:205–13. doi: 10.1117/1.1353796. [DOI] [PubMed] [Google Scholar]
- 6.Tadir Y, Doughlas-Hamilton DH. Laser effects in the manipulation of human eggs and embryos for in vitro fertilization. Methods Cell Biol. 2007;82:409–31. doi: 10.1016/S0091-679X(06)82014-5. [DOI] [PubMed] [Google Scholar]
- 7.Tucker MJ, Ball GD. Assisted hatching as a technique for use in human in vitro fertilization and embryo transfer is long overdue for careful and appropriate study. J Clin Embr. 2009;12:9–14. [Google Scholar]
- 8.Rink K, Delacretaz G, Salathe RP, Senn A, Nocera D, Germond M, et al. Non contact microdrilling of mouse zona pellucida with an objective-delivered 1.48 um diode laser. Lasers Surg Med. 1996;18:52–62. doi: 10.1002/(SICI)1096-9101(1996)18:1<52::AID-LSM7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 9.Chatzimeletiou K, Picton HM, Handyside AH. Use of non-contact, infrared laser for zona drilling of mouse embryos: assessment of immediate effects on blastomeres viability. Reprod Biomed Online. 2001;2:178–87. doi: 10.1016/s1472-6483(10)90002-3. [DOI] [PubMed] [Google Scholar]
- 10.Blanchet GB, Russell JB, Fincher CR, Portmann M. Laser micromanipulation in the mouse embryo: a novel approach to zona drilling. Fertil Steril. 1992;57:1337–41. doi: 10.1016/s0015-0282(16)55097-3. [DOI] [PubMed] [Google Scholar]
- 11.Taylor TH, Wright G, Jones-Colon S, Mitchell-Leef D, Kort HI, Nagy ZP. ICSI and IVF fertilization in patients with increased oocyte immaturity. Reprod Biomed Online. 2008;17:46–52. doi: 10.1016/s1472-6483(10)60292-1. [DOI] [PubMed] [Google Scholar]
- 12.Jones AE, Wright G, Kort HI, Straub RJ, Nagy ZP. Comparison of laser-assisted hatching and acidified Tyrode’s hatching by evaluation of blastocyst development rates in sibling embryos: a prospective randomized trial. Fertil Steril. 2006;85:487–91. doi: 10.1016/j.fertnstert.2005.07.1314. [DOI] [PubMed] [Google Scholar]
- 13.Joris H, Vos A, Janssens R, Devroey P, Liebaers I, Steirteghem A. Comparison of the results of human embryo biopsy and outcome of PGD after zona drilling using acid Tyrode medium or a laser. Hum Reprod. 2003;18:1896–902. doi: 10.1093/humrep/deg355. [DOI] [PubMed] [Google Scholar]
- 14.Cohen J, Wells D, Munne S. Removal of 2 cells from cleavage stage embryos is likely to reduce the efficacy of chromosomal tests that are used to enhance implantation rates. Fertil Steril. 2007;87:496–503. doi: 10.1016/j.fertnstert.2006.07.1516. [DOI] [PubMed] [Google Scholar]
- 15.Hartshorn C, Anshelevich A, Wangh LJ. Laser zona drilling does not induce hsp70i transcription in blastomeres of eight-cell mouse embryos. Fertil Steril. 2005;84:1547–50. doi: 10.1016/j.fertnstert.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 16.Hartshorn C, Anshelevich A, Wangh LJ. Rapid, single-tube method for quantitative preparation and analysis of RNA and DNA in samples as small as one cell. BMC Biotechnol. 2005;5:2. doi: 10.1186/1472-6750-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Katanani YM, Hansen PJ. Induced thermotolerance in bovine two-cell embryos and the role of heat shock protein 70 in embryonic development. Mol Reprod Dev. 2002;62:174–80. doi: 10.1002/mrd.10122. [DOI] [PubMed] [Google Scholar]
- 18.Germons M, Nocera D, Senn A, Rink K, Delacretaz G, Fakan S. Microdissection of mouse and human zona pellucida using a 1.48 um diode laser beam: efficacy and safety of the procedure. Fertil Steril. 1995;64:604–11. doi: 10.1016/s0015-0282(16)57800-5. [DOI] [PubMed] [Google Scholar]