Abstract
Purpose
To compare pre-cryo data from oocyte cryopreservation (OC) cycles performed for malignancy (MED) vs. elective deferment of reproduction (DR) or oocyte donation (OD).
Methods
All patients were ≤40 y and underwent standard ovarian stimulation and retrieval. Prior to OC, meiotic spindle (MS) and zona pellucida (ZP) retardance was measured using digital polarized light microscopy (DPLM).
Results
Of 130 OC cycles, 49 were for MED, 73 for DR, and 8 for OD. Cycles completed for MED had an average of 9 ± 1 spindle-positive oocytes with a mean MS retardance of 1.2 ± .02 nm and ZP retardance of 2.1 ± .06 nm, which was clinically comparable to the other groups.
Conclusions
Women with malignancy can achieve adequate ovarian response and similar oocyte parameters to those of women undergoing fertility preservation for non-cancer indications. Such information, coupled with the ability to noninvasively study oocyte dynamics, may improve the counseling of cancer patients seeking fertility preservation.
Keywords: Fertility preservation, Malignancy, Oocyte cryopreservation, Meiotic spindle, Zona pellucida
Introduction
The use of oocyte cryopreservation as a fertility preservation measure has been rapidly increasing over the past decade. Coincident has been a steady rise in the incidence of female invasive cancers in women of ages 15–30 over the past quarter century [1]. Modern cancer treatments coupled with earlier detection have led to better quality of life and improved survival; however, radical treatments can leave patients infertile or sterile. Many treatments may even lead to menopause or related complications [2]. The gravity of a new cancer diagnosis coupled with the urgency for fertility preservation creates unique challenges for these patients. In 2006, ASCO published recommendations on fertility preservation and stated that, “surveys of cancer survivors have identified an increased risk of emotional distress in those who become infertile because of their treatment” [2]. In addition, many women may be limited in their fertility preservation options depending on their diagnosis, availability of a partner and cancer treatment recommendations.
In recent publications, noninvasive imaging of both the meiotic spindle (MS) and zona pellucida (ZP) prior to oocyte cryopreservation have been studied as potential markers of oocyte quality. The MS is a critical component within the mature human oocyte, its primary roles being participation in chromosome segregation and extrusion of the second polar body which completes meiosis. The overall structure of the MS depends on precise regulation of tubulin polymerization and depolymerization. Another integral component of the human oocyte is its zona pellucida (ZP), important for controlled and successful fertilization as well as early embryo development. The ZP is also vital for sperm binding, prevention of polyspermy, and protection of the oocyte/embryo from mechanical stress [3]. The initiation of fertilization at the level of the ZP requires highly complex cell-to-cell recognition between sperm and oocyte.
Visualization and evaluation of the MS and ZP could help to improve the selection of viable oocytes and the efficacy of oocyte cryopreservation. The MS and ZP are known to exhibit an intrinsic optical property, known as birefringence, when transilluminated by polarized light [4, 5]. Birefringence is the property of light that causes a beam passed through an anisotropic structure to split into two separate rays which travel at different velocities. The MS and ZP are anisotropic structures because they have two different indices of refraction. Retardance is the phase difference between the fast ray and the slow ray traveling through an anistropic structure and is measured in nanometers. Novel polarized light microscopy allows for visualization of both these critical structures in real-time by digitally amplifying birefringent signals. This allows for quantification and orientation assessment of microtubules within the MS as well as glycoproteins within the ZP. Recent studies suggest that oocyte MS retardance may be associated with embryo quality, and correlation may exist between ZP birefringence and the potential of embryo development [6, 7]. In this study, we sought to assess whether oocytes retrieved for medically-indicated fertility preservation had similar MS and ZP parameters as those harvested to electively defer reproduction or for oocyte donation.
Materials and methods
Source of oocytes
In our laboratory, the majority of oocytes destined for oocyte cryopreservation undergo digital polarized light microscopy (DPLM) using the Oosight™ (CRI Inc., Woburn, MA). Oocytes from women ≤age 40 undergoing oocyte cryopreservation for medical indications (MED) from January 2006 through February 2010 were reported and compared to two other groups of women under age 40 having oocyte cryopreservation to electively defer reproduction (DR) or as an oocyte donor (OD).
In all cycles, DPLM was performed approximately 1.5 h post-oocyte harvest to evaluate for the presence of the MS. Beginning in January 2008, quantitative MS and ZP retardance values were also assessed (Fig. 1). These values were recorded at time of assessment and stored for later analysis. Cumulative data were then compared among the three treatment groups: MED, DR or OD. IRB approval from the NYU School of Medicine Institutional Review Board was obtained to report findings of these cycles.
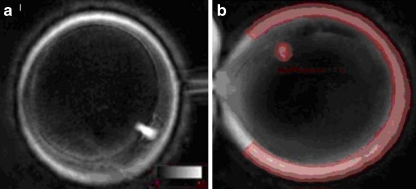
Fig. 1.
a DPLM evaluation of oocyte in telophase prior to cryopreservation; b DPLM retardance measurement of meiotic spindle and zona pellucida of metaphase II oocyte
Oocyte stimulation and cryopreservation
Before initiation of treatment, day 2 serum estradiol and follicle stimulating hormone (FSH) levels were assessed. Patients were then stimulated using injectable gonadotropins (Follitropin beta, Schering Plough, NJ; Serono Pharmaceuticals, Rockland, MA; Menotropins, Parsippany, NJ) with LH suppression being achieved using a GnRH agonist (leuprolide acetate, TAP Pharmaceuticals, Lake Forest, IL) or antagonist (ganirelix acetate, Organon; cetrorelix, Serono). Ovulation trigger was initiated when at least two follicles reached 18 mm in diameter. Approximately 35–35.5 h later, ultrasound-guided transvaginal oocyte retrieval was performed in routine fashion. Oocytes underwent cryopreservation via slow-cooling or vitrification by the same methodology described previously in detail by Grifo and Noyes [8]. Briefly, slow-cooled oocytes were first washed, then transferred into an equilibration solution, followed by placement into loading solution. They were then transferred to cryopreservation straws and placed in a controlled-rate freezer where the temperature was gradually lowered from 20°C to minus 150°C (seeding at minus 7°C) over 2.5 h, then transferred to liquid nitrogen for storage. Cryoprotectants used for slow cooling included propanediol and sucrose. Vitrification was achieved by first passing oocytes through sequential equilibration solutions containing increasing concentrations of the cryoprotectants, ethylene glycol and dimethyl sulfoxide, followed by placement into vitrification solution. Oocytes were then loaded into cryopreservation containers and plunged into liquid nitrogen.
Polarized light microscopy of the meiotic spindle and zona pellucida
Approximately 1 h post-retrieval, oocytes were stripped of surrounding cumulus cells using Cumulase (Medicult, Denmark). Oocytes were then placed in 5-μL drops of HEPES-buffered medium (Irvine Scientific, Santa Ana, CA) covered with mineral oil (Sage IVF, Cooper Surgical, Inc., Trumball CT) in a glass-bottomed culture dish (Will-Co Dish; Intracel, Herts, U.K). MS visualization was performed at 40× magnification using an inverted microscope with liquid crystal (LC) Oosight™ optics and controller combined with a computerized image analysis system using Oosight™ meta software (Oosight™ Meta; CRI Inc.). During MS analysis, temperature was maintained at 37°C using a heated microscope stage. Oocytes were positioned to align the main axis of the MS perpendicular to the light path; this was achieved using a holding pipette while rotating the oocyte until the polar body was aligned with the oocyte’s equatorial plane. Images and raw data were initially saved to minimize the oocyte’s time outside the incubator. At a later time, the MS’s longitudinal axis was measured by drawing a pole-to-pole line. Mean retardance was measured throughout the MS’s longitudinal axis. If no MS was visible, the oocyte was considered “spindle-negative” while if the MS could be seen, it was deemed “spindle-positive”.
To image the ZP, each human oocyte was analyzed using the same initial steps as described for the MS. Alignment of the microscope and calibration of the software were performed before ZP imaging. ZP was imaged 5 times by focusing at an equatorial plane of each oocyte to ensure the accuracy of image and adequate representation of ZP layers. All digital images were saved and best images were selected for subsequent analysis. Briefly, eight points distributing evenly on the ZP (1–12 o’clock positions) were sampled to measure the retardance and thickness of each layer. Chords were then drawn extending from the inner ZP birefringence of the oocyte outward and collected retardance magnitude for the ZP layer was calculated. Quantitative microscopic MS and ZP evaluation took an average of 90 s to perform per oocyte.
Statistical comparisons of cycle data from the MED, DR, and OD groups were carried out on pooled data using chi-squared analysis or t-tests for independent data. A P value < 0.05 was considered significant.
Results
A total of 130 completed oocyte cryopreservation cycles were evaluated. Of these, 49 cycles were for MED, 73 for DR, and 8 for OD. Cumulatively, this represented 2,228 harvested oocytes of which 1,418 (64%) were found to be metaphase II (MII) at time of microscopic evaluation (see Table 1). Diagnoses in the MED group included 21 gynecologic, 10 breast, 9 hematologic, 3 gastrointestinal, 3 central nervous system and 3 other (see Table 2). Ten of the MED women who were in a relationship conducive to childbearing at the time of treatment elected to cryopreserved zygotes as an additional means of fertility preservation. Thus, a total of 124 of their oocytes (100 MII) were used in this way. The MED group were similar in age to the OD group (30 ± 1 vs. 31 ± 1 y, NS), and the DR group was older (37 ± 0.3 y, p ≤ .007).
Table 1.
Cycle demographics and oocyte data
| MED (n = 49) | OD (n = 8) | DR (n = 73) | |
|---|---|---|---|
| Mean age (y) | 30 ± 1a (19–40) | 31 ± 1c (24–37) | 37 ± .3b,d (29–40) |
| Mean estradiol @ ovulation trigger (pg/ml) | 2112 ± 209e | 3396 ± 527f,g | 1974 ± 125f,h |
| Mean no. oocytes retrieved | 20 ± 2i (total 993) | 19 ± 3 (total 179) | 14 ± 1j (total 1056) |
| Mean no. MII oocytes | 16 ± 2k (78%; total 771) | 15 ± 3 (74%; total 132) | 10 ± 1l (49%; total 515) |
| Mean no. spindle-positive oocytes | 9 ± 1 (2–33) | 13 ± 3m (3–26) | 8 ± 1n (0–30) |
| Mean MS retardance | 1.2 ± .02 (0.4–2.3) | 1.4 ± .05 (.6–3.0) | 1.3 ± .01 (.3–3.3) |
| Mean ZP optical density | 2.1 ± .06 (0.6–6.6) | 2.3 ± .1 (.7–4.1) | 1.9 ± .02 (.6–4.5) |
Values are expressed as means ± SEM (range)
MS meiotic spindle; ZP zona pellucida
P < .007 a–b, c–d, e–f, g–h, i–j, k–l; P = .02 m–n
Table 2.
Cancer diagnoses in medical oocyte cryopreservation cases
| Diagnosis | Subdiagnosis |
|---|---|
| Gynecologic | 10 cervical |
| n = 21 | 7 ovarian |
| 4 endometrial | |
| Breast | 3 ductal carcinoma in situ |
| n = 10 | 7 invasive breast |
| Hematologic | 4 Hodgkin’s lymphoma |
| n = 9 | 3 acute myelogenous leukemia |
| 2 non-Hodgkin lymphoma | |
| Gastrointestinal | 2 colon |
| n = 3 | 1 carcinoid |
| Central Nervous System | 2 brain |
| n = 3 | 1 spinal cord |
| Other | 1 rhabdomyosarcoma |
| n = 3 | 1 peritoneal mesothelioma |
| 1 thymic tumor |
Results of meiotic spindle analyses for the three comparison groups are shown in Table 1. Cycles completed for medical indications (MED) had an average of 9 ± 1 spindle-positive oocytes with a mean spindle retardance of 1.2 ± .02 nm and ZP retardance of 2.1 ± .06 nm. Cycles performed for oocyte donation (OD) had a mean of 13 ± 3 spindle-positive oocytes with a mean spindle retardance of 1.4 ± .05 nm and ZP retardance of 2.3 ± .1 nm, and cycles of electively deferred reproduction (DR) had an average of 8 ± 1 spindle-positive oocytes with a mean spindle retardance of 1.5 ± .2 nm and ZP retardance of 1.9 ± .02 nm. MS and ZP results were quantifiably similar among all three groups. Oocytes were evaluated under bright field microscopy and then DPLM. When reevaluated under DPLM (after bright field microscopy), 6% of oocytes were noted to be in telophase rather than metaphase II (5% of MED, 9% of OD and 7% of DR; NS).
Discussion
Females of reproductive age diagnosed with cancer face many unique situations as a consequence of their disease. One of the greatest challenges for these patients is the loss of reproductive potential secondary to their disease process or treatment options. Many find hope in the opportunity to cryopreserve oocytes for future reproductive potential. Current outcomes following oocyte cryopreservation can equal those achieved using conventional IVF making this technology an effective means of fertility preservation [9–11]. In this study, we sought to use MS and ZP retardance values as a measure of oocyte quality, comparing the results of this relatively compromised patient group to that of healthy patients undergoing oocyte cryopreservation for elective deferment of reproduction or oocyte donation.
With the advent of noninvasive imaging techniques and expanded usage of human IVF, the demand for safer and more efficient techniques has increased. Newer technology has allowed for noninvasive imaging of critical structures involved in oocyte development and fertilization. Specifically, DPLM allows for visualization of the MS and ZP without compromising oocyte integrity. Evidence suggests that MS presence, location, and retardance values correlate with embryo quality and fertilization [12]. Specifically, studies suggest that oocytes may have decreased fertilization potential if the MS is absent [13]. In addition, if these oocytes do fertilize, they may be associated with decreased potential to develop into normal embryos [14]. Timing of spindle imaging may be an important factor in the detection of the meiotic spindle, specifically because it has been shown that oocytes imaged ≥38 h after hCG injection were significantly more likely to be spindle‐positive than those imaged at <38 h [15]. In the present analysis, oocyte imaging occurred approximately 36.5 – 37 h post‐ovulation trigger, which may have translated into a falsely elevated number of spindle‐absent oocytes. Recent literature suggests that embryos suitable for day-3 transfer are more likely to be spindle-positive prior to cryopreservation and post-thaw spindle-positive oocytes are more likely to achieve blastocyst formation and be appropriate for day-5 transfer [9]. Other studies have shown that both the quality of embryos and oocytes can be correlated with increased MS retardance [6, 16]. MS retardance values have also been associated with higher pronuclear score and number of ICSI transfer cycles resulting in pregnancy [14]. Additionally, ZP retardance may represent a non-invasive marker for oocyte development potential, specifically a higher retardance value of the inner zona layer has been associated with higher quality embryos that facilitate implantation and stimulate higher blood levels of hCG [17]. In the present analysis, we compared cycles of oocyte cryopreservation with three distinct indications, comparing the MS and ZP.
Interestingly, 6% of all oocytes observed using DPLM that had previously been graded metaphase II under bright field microscopy were noted to be in telophase rather than metaphase II (Fig. 1). Previous studies have shown that oocytes in telophase may have impaired fertilization as compared to those in metaphase II and that telophase may represent an impairment or delay of the completion of meiosis I [18, 19]. Research has suggested that single observation of the meiotic spindle may be misleading, and that multiple images should be performed to assess for the presence of the meiotic spindle [20]. Further research is necessary to better appreciate the clinical significance of telophase findings on DPLM.
Our data suggests that women diagnosed with a medical condition can have a good ovarian response as well as an adequate number of retrieved spindle-positive oocytes. In addition, both MS and ZP measurements were clinically comparable to those of women electively deferring reproduction or having oocytes harvested for donation. In addition, the association between advancing age and declining reproductive potential was demonstrated in our study population. Women donating oocytes were younger (mean age of 31) and had higher estradiol values with more spindle-positive oocytes. In contrast, patients electively deferring reproduction were generally older (mean age of 37), and had a lower estradiol value with fewer oocytes retrieved for cryopreservation.
Women diagnosed with medical diseases can produce good-quality oocytes despite the innumerable physiologic and social stresses occurring at the time of cryopreservation. In addition, MS and ZP retardance measurements were found to be clinically comparable between different demographic groups. DPLM may be a useful tool as it allows for noninvasive study of oocyte dynamics; however more research is needed to determine its value in predicting the ultimate fate of retrieved oocytes.
Conclusions
The increasing incidence of reproductive-age females surviving invasive cancer necessitates better options for fertility preservation among this population. Oocyte cryopreservation, while still labeled experimental, is a hopeful option and may lead to improved quality of life for survivors. Digital polarized light microscopy suggests there is no objective difference in oocytes cryopreserved for medical indications in comparison to other non-cancer patients. This data may help to better counsel patients diagnosed with a malignancy seeking options for fertility preservation.
Acknowledgements
The authors would like to thank the entire NYU Fertility Center staff for their involvement in the oocyte cryopreservation program at New York University and especially, the embryology team for their expertise in oocyte assessment and cryopreservation.
Financial support None
Conflicts of interest None
Footnotes
Capsule
Oocytes from cancer patients seeking fertility preservation are similar to those of healthy women both in quantity and characteristics as viewed by polarized light microscopy.
References
- 1.Bleyer A, Viny A, Barr R. Cancer in 15- to 29-year-olds by primary site. Oncologist. 2006;11:590–601. doi: 10.1634/theoncologist.11-6-590. [DOI] [PubMed] [Google Scholar]
- 2.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 3.Shafie M, Sousa M, Kruger T. An Atlas of the ultrastructure of human oocytes. A guide for assisted reproduction. New York: Parthenon; 2000. [Google Scholar]
- 4.Wang WH, Meng L, Hackett RJ, Keefe DL. Developmental ability of human oocytes with or without birefringent spindles imaged by Polscope before insemination. Hum Reprod. 2001;16:1464–1468. doi: 10.1093/humrep/16.7.1464. [DOI] [PubMed] [Google Scholar]
- 5.Oldenbourg R. Polarized light microscopy of spindles. Methods Cell Biol. 1999;61:175–208. doi: 10.1016/s0091-679x(08)61981-0. [DOI] [PubMed] [Google Scholar]
- 6.Santis L, Cino I, Rabellotti E, Calzi F, Persico P, Borini A, Coticchio G. Polar body morphology and spindle imaging as predictors of oocyte quality. Reprod Biomed Online. 2005;11:36–42. doi: 10.1016/s1472-6483(10)61296-5. [DOI] [PubMed] [Google Scholar]
- 7.Rama Raju GA, Prakash GJ, Krishna KM, Madan K. Meiotic spindle and zona pellucida characteristics as predictors of embryonic development: a preliminary study using PolScope imaging. Reprod Biomed Online. 2007;14:166–174. doi: 10.1016/s1472-6483(10)60784-5. [DOI] [PubMed] [Google Scholar]
- 8.Grifo JA, Noyes N. Delivery rate using cryopreserved oocytes is comparable to conventional in vitro fertilization using fresh oocytes: potential fertility preservation for female cancer patients. Fertil Steril. 2010;93:391–396. doi: 10.1016/j.fertnstert.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 9.Noyes N, Knopman J, Labella P, McCaffrey C, Clark-Williams M, Grifo J. Oocyte cryopreservation outcomes including pre-cryopreservation and post-thaw meiotic spindle evaluation following slow cooling and vitrification of human oocytes. Fertil Steril. 2010 doi: 10.1016/j.fertnstert.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 10.Cobo A, Kuwayama M, Perez S, Ruiz A, Pellicer A, Remohi J. Comparison of concomitant outcome achieved with fresh and cryopreserved donor oocytes vitrified by the CryoTop method. Fertil Steril. 2008;89:1657–1664. doi: 10.1016/j.fertnstert.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 11.Nagy ZP, Chang CC, Shapiro DB, Bernal DP, Elsner CW, Mitchell-Leef D, et al. Clinical evaluation of the efficiency of an oocyte donation program using egg cryo-banking. Fertil Steril. 2009;92:520–526. doi: 10.1016/j.fertnstert.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Shen Y, Betzendahl I, Tinneberg HR, Eichenlaub-Ritter U. Enhanced polarizing microscopy as a new tool in aneuploidy research in oocytes. Mutat Res. 2008;651:131–140. doi: 10.1016/j.mrgentox.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Wang WH, Keefe DL. Prediction of chromosome misalignment among in vitro matured human oocytes by spindle imaging with the PolScope. Fertil Steril. 2001;78:1077–1081. doi: 10.1016/s0015-0282(02)04196-1. [DOI] [PubMed] [Google Scholar]
- 14.Cohen Y, Malcov M, Schwartz T, Mey-Raz N, Carmon A, Cohen T, Lessing JB, Amie A, Azem F. Spindle imaging: a new marker for optimal timing of ICSI? Hum Reprod. 2004;19:649–654. doi: 10.1093/humrep/deh113. [DOI] [PubMed] [Google Scholar]
- 15.Shen Y, Stalf T, Mehnert C, Santis L, Cino I, Tinneberg HR, Eichenlaub-Ritter U. Light retardance by human oocyte spindle is positively related to pronuclear score after ICSI. Reprod Biomed Online. 2006;12:737–751. doi: 10.1016/s1472-6483(10)61086-3. [DOI] [PubMed] [Google Scholar]
- 16.Liu L, Trimarchi JR, Oldenbourg R, Keefe DL. Increased birefringence in the meiotic spindle provides a new marker for the onset of activation in living oocytes. Biol Reprod. 2000;63:251–258. doi: 10.1095/biolreprod63.1.251. [DOI] [PubMed] [Google Scholar]
- 17.Shen Y, Stalf T, Mehnert C, Eichenlaub-Ritter U, Tinneberg HR. High magnitude of light retardation by the zona pellucida is associated with conception cycles. Hum Reprod. 2005;20:1596–1606. doi: 10.1093/humrep/deh811. [DOI] [PubMed] [Google Scholar]
- 18.Hyun CS, Cha JH, Son WY, Yoon SH, Kim KA, Lim JH. Optimal ICSI timing after the first polar body extrusion in in vitro matured human oocytes. Hum Reprod. 2007;22:1991–1995. doi: 10.1093/humrep/dem124. [DOI] [PubMed] [Google Scholar]
- 19.Keefe D, Liu L, Wang W, Silva C. Imaging meiotic spindles by polarization light microscopy: principles and applications to IVF. Reprod Biomed Online. 2003;7:24–29. doi: 10.1016/s1472-6483(10)61724-5. [DOI] [PubMed] [Google Scholar]
- 20.Montag M. Spindle imaging in human oocytes: the impact of the meiotic cell cycle. Reprod Biomed Online. 2006;12:442–446. doi: 10.1016/s1472-6483(10)61996-7. [DOI] [PubMed] [Google Scholar]