Abstract
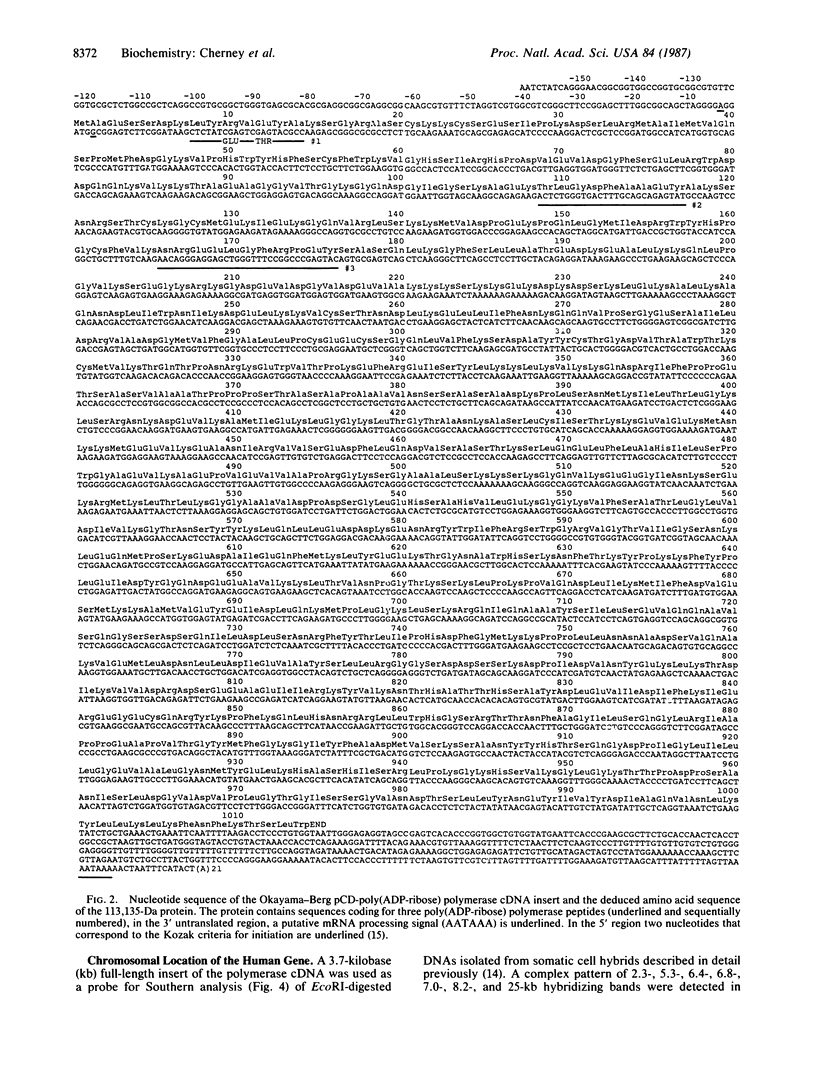
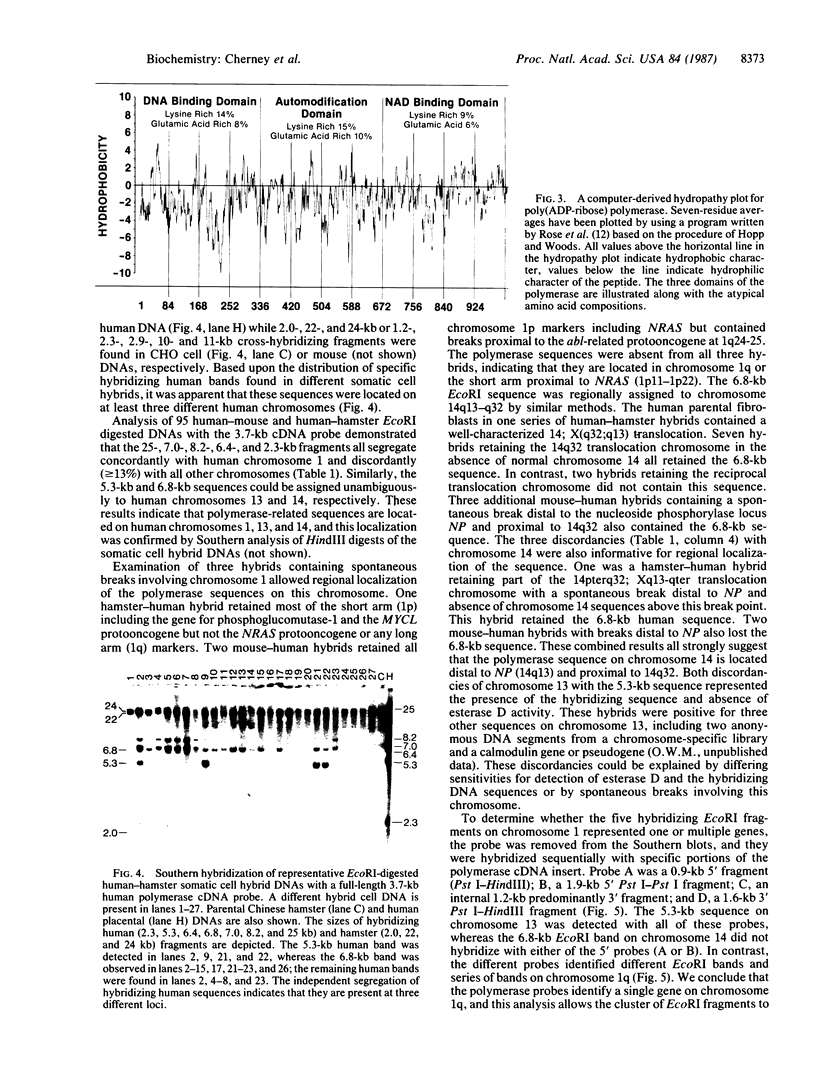
Recently we described a full-length cDNA for the human nuclear enzyme poly(ADP-ribose) polymerase. Here, we report the chromosomal localization and partial map of the human gene for this enzyme as well as the complete coding sequence for this protein. The nucleotide sequence reveals a single 3042-base open reading frame encoding a protein with a predicted Mr of 113,135. A comparison of this deduced amino acid sequence with the amino acid sequence of three peptides derived from human poly(ADP-ribose) polymerase revealed a match of 27 amino acid residues. A computer-derived structural analysis of the enzyme and a search for similarities with other proteins confirmed that the polymerase belongs to a subfamily of DNA/NAD-binding proteins and DNA-repair proteins. Possible Zn2+-binding "fingers," a nucleotide-binding fold, and a nuclear transport signal were noted. Additionally, chromosomal mapping has identified polymerase-hybridizing sequences on human chromosomes 1 (the active gene), 13, and 14 (processed pseudogenes). Using the polymerase cDNA as a probe, we also have detected several DNA restriction fragment length polymorphisms in normal humans.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkhatib H. M., Chen D. F., Cherney B., Bhatia K., Notario V., Giri C., Stein G., Slattery E., Roeder R. G., Smulson M. E. Cloning and expression of cDNA for human poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1224–1228. doi: 10.1073/pnas.84.5.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin R. C., Gill D. M. Poly(ADP-ribose) synthesis in vitro programmed by damaged DNA. A comparison of DNA molecules containing different types of strand breaks. J Biol Chem. 1980 Nov 10;255(21):10502–10508. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Endo Y., Tsurugi K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J Biol Chem. 1987 Jun 15;262(17):8128–8130. [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Williams K. R., Konigsberg W. H., Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated neuronal phosphoprotein. I. Amino acid sequence around the phosphorylated threonine. J Biol Chem. 1984 Dec 10;259(23):14486–14490. [PubMed] [Google Scholar]
- Holtlund J., Kristensen T., Ostvold A. C., Laland S. G. A comparison of purified poly(ADP-ribose) polymerases from Ehrlich ascites tumor cells, pig thymus, and HeLa S3 cells. Eur J Biochem. 1981 Sep;119(1):23–29. doi: 10.1111/j.1432-1033.1981.tb05572.x. [DOI] [PubMed] [Google Scholar]
- Ito S., Shizuta Y., Hayaishi O. Purification and characterization of poly(ADP-ribose) synthetase from calf thymus. J Biol Chem. 1979 May 10;254(9):3647–3651. [PubMed] [Google Scholar]
- Jekel P. A., Weijer W. J., Beintema J. J. Use of endoproteinase Lys-C from Lysobacter enzymogenes in protein sequence analysis. Anal Biochem. 1983 Oct 15;134(2):347–354. doi: 10.1016/0003-2697(83)90308-1. [DOI] [PubMed] [Google Scholar]
- Kameshita I., Matsuda M., Nishikimi M., Ushiro H., Shizuta Y. Reconstitution and poly(ADP-ribosyl)ation of proteolytically fragmented poly(ADP-ribose) synthetase. J Biol Chem. 1986 Mar 15;261(8):3863–3868. [PubMed] [Google Scholar]
- Kawaichi M., Ueda K., Hayaishi O. Multiple autopoly(ADP-ribosyl)ation of rat liver poly(ADP-ribose) synthetase. Mode of modification and properties of automodified synthetase. J Biol Chem. 1981 Sep 25;256(18):9483–9489. [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride O. W., Zmudzka B. Z., Wilson S. H. Chromosomal location of the human gene for DNA polymerase beta. Proc Natl Acad Sci U S A. 1987 Jan;84(2):503–507. doi: 10.1073/pnas.84.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montfort W., Villafranca J. E., Monzingo A. F., Ernst S. R., Katzin B., Rutenber E., Xuong N. H., Hamlin R., Robertus J. D. The three-dimensional structure of ricin at 2.8 A. J Biol Chem. 1987 Apr 15;262(11):5398–5403. [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Rose G. D., Gierasch L. M., Smith J. A. Turns in peptides and proteins. Adv Protein Chem. 1985;37:1–109. doi: 10.1016/s0065-3233(08)60063-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Uchida K., Shima H., Sato T., Okamoto T., Kimura T., Miwa M. Molecular cloning of cDNA for human poly(ADP-ribose) polymerase and expression of its gene during HL-60 cell differentiation. Biochem Biophys Res Commun. 1987 Jul 31;146(2):403–409. doi: 10.1016/0006-291x(87)90543-2. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




