Abstract
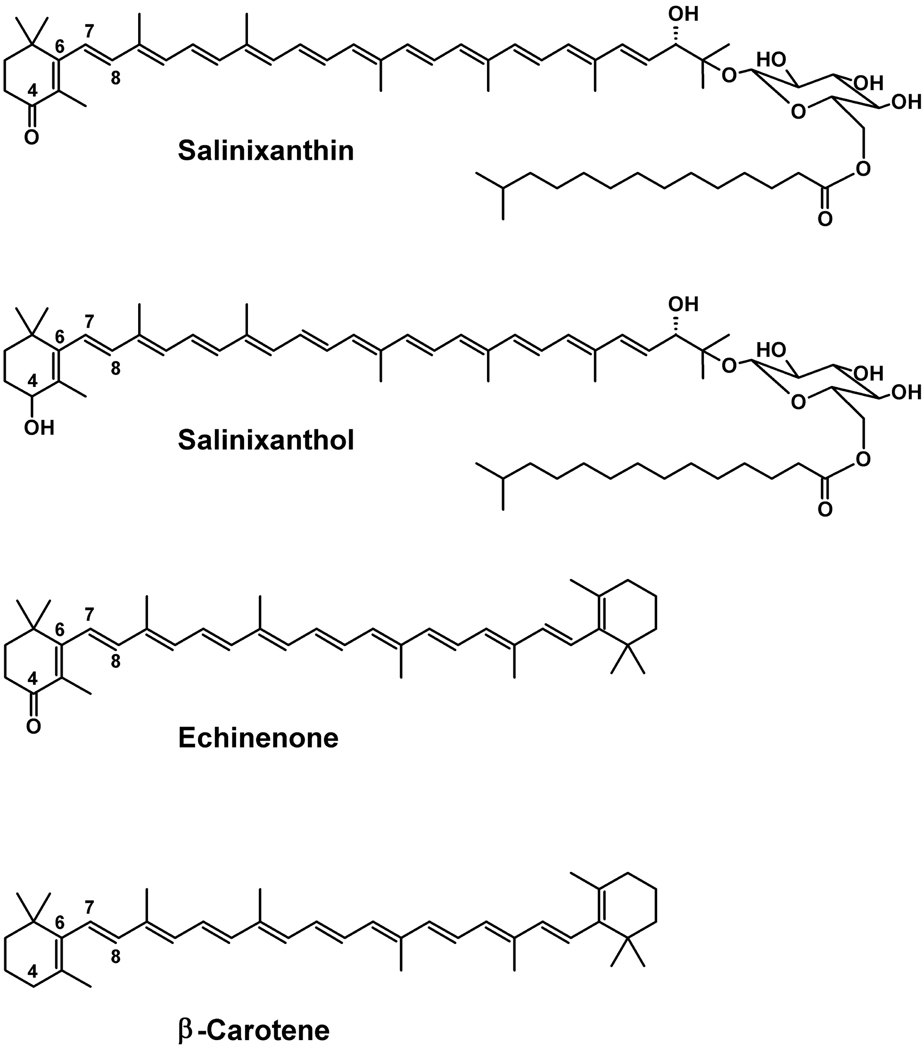
In previous work we reconstituted salinixanthin, the C40-carotenoid acyl glycoside that serves as a light-harvesting antenna to light-driven proton pump xanthorhodopsin, into a different protein, gloeobacter rhodopsin expressed in E. coli, and demonstrated that it transfers energy to the retinal chromophore (Imasheva et al. 2009. Biochemistry 48, 10948). The key to binding of salinixanthin was the accommodation of its ring near the retinal β-ionone ring. Here we examine two questions: do any of the native Gloeobacter carotenoids bind to gloeobacter rhodopsin, and does the 4-keto group of the ring play a role in binding. There is no salinixanthin in Gloeobacter violaceous, but a simpler carotenoid, echinenone, also with a 4-keto group that lacks the acyl glycoside, is present in addition to β-carotene and oscillol. We show that β-carotene does not bind to gloeobacter rhodopsin, but its 4-keto derivative, echinenone, does and functions as a light-harvesting antenna. This indicates that the 4-keto group is critical for the carotenoid binding. Further evidence for this is that salinixanthol, an analogue of salinixanthin in which the 4-keto group is reduced to hydroxyl, does not bind and is not engaged in energy transfer. According to the crystal structure of xanthorhodopsin, the ring of salinixanthin in the binding site is turned out of the plane of the polyene conjugated chain. Similar conformation is expected for echinenone in the gloeobacter rhodopsin. We suggest that the 4-keto group in salinixanthin and echinenone allows for the twisted conformation of the ring around C6-C7 bond and probably is engaged in interaction that locks the carotenoid in the binding site.
Retinal based light-driven ion pumps, like bacteriorhodopsin of the archaea (1, 2) and proteorhodopsin of the marine bacteria (3) are the simplest biological machines that supply cells with energy in the form of electrochemical gradients generated from transmembrane proton transport (4–6). A single protein with the retinal chromophore attached makes the functional unit. The simplicity of design when a single molecule of retinal protein performs several functions (light absorption and transformation of energy into a electrochemical gradient) apparently explains that transducers and sensors (7) of this type are found in many marine and freshwater bacteria, and exist in great varieties as one can judge from the large number of genome sequences in which they have been identified, to date exceeding several thousand, some of them pumps and others sensors (8–12).
Recently, a proton pump, xanthorhodopsin, bearing in addition to retinal a carotenoid light-harvesting antenna was found in the halophilic eubacterium Salinibacter ruber (13). The carotenoid, salinixanthin, a C40-carotenoid acyl glycoside (Figure 1) (14) is a major component of the total carotenoids in cell membrane of S. ruber. It absorbs light in the blue-green spectral region and when bound to xanthorhodopsin transfers 40–45% of the excited state energy to the retinal (13, 15). The light-harvesting antenna approximately doubles the cross section of light absorption for the retinal protein (13, 16). The energy transfer occurs from the S2 state of the carotenoid, whose lifetime is 66 ± 4 fs, to the S1 state of the retinal (15, 17), the lowest singlet excited state of the retinal chromophore (18). Reduction of the retinal Schiff base double bond to a single bond causes a shift in retinal absorption to 360 nm that eliminates energy transfer to the retinal, and results in increase of the S2 lifetime to 110 fs (15, 17), consistent with 40% energy transfer. Energy transfer occurs via Förster-type mechanism (15, 17), but strong coupling of the linear and extended chromophores requires use of sophisticated calculations of the carotenoid-retinal interaction (19). The carotenoid is immobilized in a special binding site of xanthorhodopsin at a distance of 11.7 Å between the centers of the conjugated chains of the two chromophores that lie at a 46° angle (20). Most of the carotenoid conjugated chain is at the protein-lipid boundary of helix F, but the end 4-keto ring is immersed into the protein near the β-ionone ring of the retinal and twisted, as follows from the CD spectra of the bound carotenoid (21, 22) and the X-ray structure of the complex (20). The latter revealed that the 4-keto ring is in the space that in bacteriorhodopsin is occupied by the bulky Trp138, but in xanthorhodopsin it is replaced by a Gly. This gave us a clue for a search of other retinal proteins that may bind a carotenoid light-harvesting antenna similar to salinixanthin.
Figure 1.
Chemical structures of salinixanthin, salinixanthol, echinenone, and β-carotene.
Sequence alignment shows that over a dozen of sequences of retinal proteins, presumably pumps and sensors, from various groups (alpha proteobacteria, actinobacteria, cyanobacteria, flavobacteria, and others) have Gly instead of Trp at this site (23). To demonstrate that at least some of them are indeed capable of binding a carotenoid antenna, we reconstituted salinixanthin into gloeobacter rhodopsin, a proton pump from the cyanobacterium Gloeobacter violaceous (24) expressed in E. coli, and showed that it transfers energy to the retinal (23). Replacing the Gly with a bulky Trp in the G178W mutant abolished carotenoid reconstitution, thus confirming that accommodation of the ring near the retinal is necessary for the carotenoid binding (23).
Gloeobacter violaceous does not contain salinixanthin, but a simpler carotenoid, echinenone, also with a 4-keto group but lacking the acyl glycoside is present as a minor component in addition to β-carotene and oscillol (25).
The questions arise whether any of these carotenoids binds to gloeobacter rhodopsin and whether the 4-keto group plays any role in binding. We address these questions here by comparing binding of β-carotene and echinenone and testing salinixanthol (Figure 1), an analogue of salinixanthin in which the ring keto group (C=O) is reduced to hydroxyl (C-OH). We show that β-carotene and salinixanthol do not bind to gloeobacter rhodopsin, but echinenone does. This finding establishes that a dual-chromophore antenna-system will form in Gloeobacter cells as in the in vitro experiments, and emphasizes the importance of the 4-keto group in the carotenoid binding.
Materials and Methods
Gloeobacter rhodopsin was expressed in E. coli as described in (24). The G178W mutant of the protein was produced earlier (23). Solutions in 0.02% DDM were used in all experiments. Synthetic echinenone was a gift from Roche; β-carotene was from Sigma-Aldrich (> 97% pure, catalog number 22040, Fluka). Absorption spectra were measured on Shimadzu UV-1701 spectrophotometer, CD spectra were taken on Jasco J-720 spectropolarimeter (21) and fluorescence studies were performed on SLM Aminco fluorometer modified by OLIS as described earlier (15).
Echinenone and β-carotene were dissolved in acetone. In reconstitution experiments 10 µl of stock solution were added to 1 ml of solubilized gloeobacter rhodopsin in 0.02% DDM, 25 mM MOPS, pH 7.2, 0.1 M NaCl. The following extinction coefficients were used in calculating concentrations: gloeobacter rhodopsin, 50,000 l/mol cm (23); acetone solution of echinenone, 119,000 l/mol cm; β-carotene, 139,000 l/mol cm (26).
Extraction of salinixanthin from cell membranes and reduction of its keto group to C-OH with borohydride to produce salinixanthol was performed following procedures by Lutnaes et al. (14).
Results and Discussion
Reconstitution with echinenone
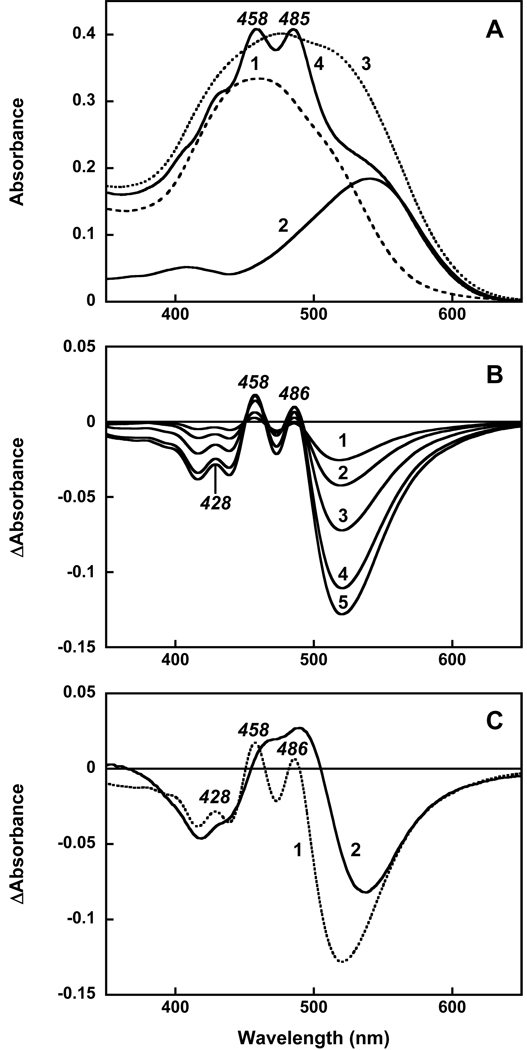
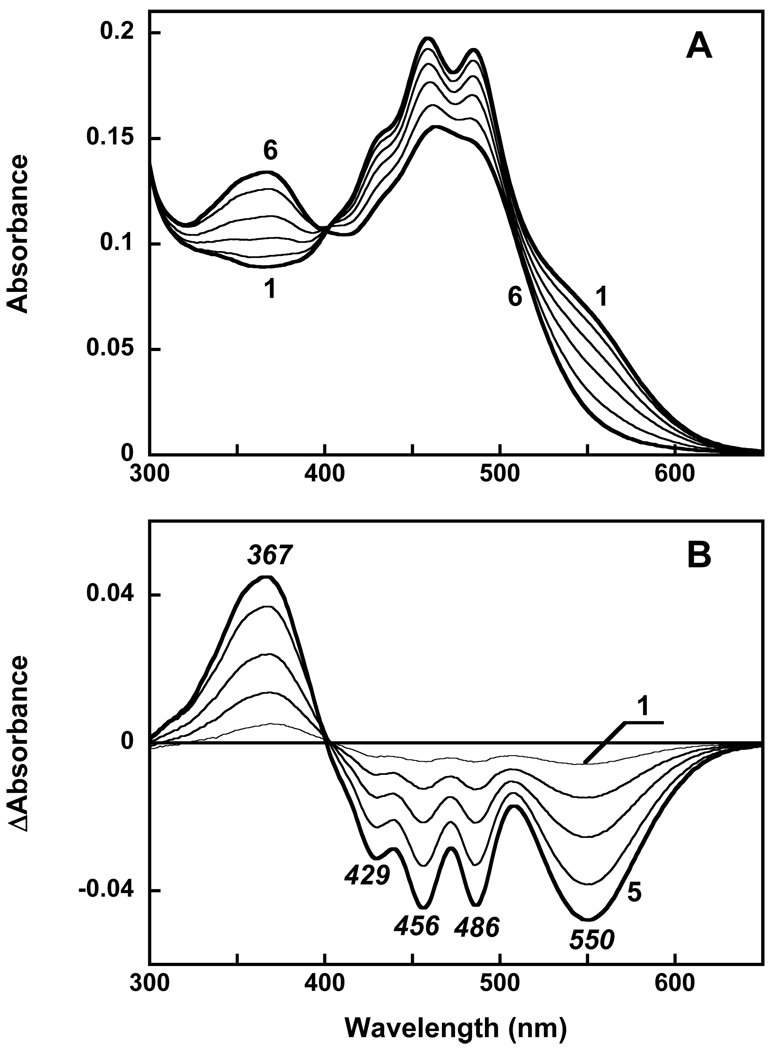
Echinenone in ethanol solution exhibits a broad absorption band with a maximum at 462 nm and poorly resolved vibronic structure (26). Upon addition of echinenone to detergent micelles (0.02% DDM, pH 7.1) this structure becomes even less pronounced and maximum absorbance decreases. The absorption band shifts to 450 nm and a broad shoulder appears at 513 nm (spectrum 1 in Figure 2A). Addition of echinenone to gloeobacter rhodopsin solubilized in the same detergent (spectrum 2) at first produced the structureless spectrum 3. However, long incubation (24 h at room temperature) resulted in dramatic changes in absorption (Figure 2, spectrum 4). The most noticeable of these is the narrowing of the carotenoid vibronic bands, a feature that indicates specific binding of the carotenoid to the protein (13). The difference spectra taken during reconstitution show that the development of the highly structured spectrum with bands at 486, 458 and 428 nm (Figure 2B) occurs very slowly; the time constant is ca. 8 hours. This is different from reconstitution with salinixanthin where sharp vibronic bands of the carotenoid appeared with a time constant of 5 min after mixing (23).
Figure 2.
Reconstitution of gloeobacter rhodopsin expressed in E. coli with echinenone. A. Absorption spectra in 0.02% DM, 25 mM MOPS, pH 7.2, 0.1 M NaCl of: 1, echinenone, 4 µM; 2, gloeobacter rhodopsin, 4 µM; 3, the spectrum taken 3 min after mixing; 4, after 24 h incubation. B. Absorption changes after addition of echinenone to gloeobacter rhodopsin and incubation at room temperature for: 2, 4, 8, 16 and 24 hours (spectra 1 through 5, respectively). C. Difference spectra of absorption changes after addition of 4 µM echinenone and incubation for 24 hours with: 1, gloeobacter rhodopsin, wild type; 2, its G178W mutant.
In order to check whether binding of echinenone occurs to the same binding site near the retinal chromophore as in the xanthorhodopsin crystal structure, we performed experiments with the G178W mutant of gloeobacter rhodopsin. This mutant was specifically designed for the purpose of filling the space that is occupied by the ring of the carotenoid antenna by bulky tryptophan (present in bacteriorhodopsin but replaced by glycine in xanthorhodopsin and gloeobacter rhodopsin). Addition of echinenone to this mutant resulted in slow-developing absorption changes that were somewhat similar to those observed for the wild type rhodopsin (see comparison in Figure 2C), indicating that binding of echinenone to the protein takes place with kinetics similar to that in wild type (no such changes observed in the absence of the protein). However, the spectra show one important difference. The sharp bands of bound echinenone at 486, 457 and 429 nm, seen in the difference spectrum for the wild type, are missing in the mutant. This indicates that the ring does not fit into the space occupied by tryptophan (as expected) and is not immobilized. The lack of ring binding in the mutant has a far-reaching consequence: it eliminates energy transfer to the retinal chromophore (see below).
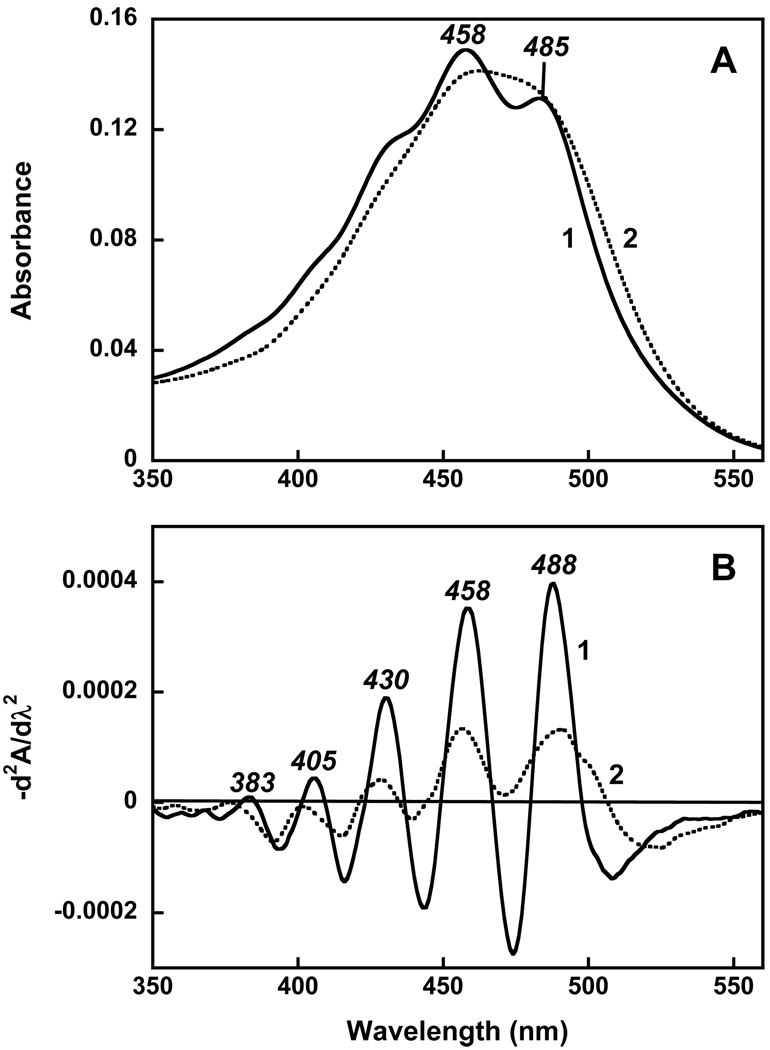
Figure 3A compares the spectrum of echinenone bound to gloeobacter rhodopsin and its spectrum in ethanol. The clear difference is that bound echinenone exhibits fine structure in its spectrum (maxima at 485, 458 and shoulder at 430 nm). In panel B the second derivative of the absorption spectra reveals the overlapping vibronic bands (a sum of the transitions to the C=C and C-C vibrational levels of the S2 excited state). The greater amplitude and the better resolution of the bands of the carotenoid in the protein are from their decreased width since the amplitude of the second derivative is inversely proportional to the square of the half-width.
Figure 3.
A. Absorption spectra of echinenone: 1, bound to the protein (obtained as a difference between spectra 4 and 2 in Figure 2A); 2, in ethanol, normalized at 485 nm maximum. B. Second derivatives of the spectra shown in panel A, d2A/dλ2, multiplied by −1.
The sharper absorption bands of the carotenoid bound to the gloeobacter rhodopsin must originate from restriction of motions in the carotenoid conjugated chain and especially motions around the C6–C7 bond connecting the ring and the conjugated chain by the protein, known to affect the spectral resolution (27, 28). In the crystal structure of xanthorhodopsin, the ring of salinixanthin is rotated from the plane of conjugation and immobilized (20). If similar changes occur in echinenone, one can expect a large Cotton effect in the CD spectrum, from the asymmetric conformation of the bound carotenoid and its interaction with retinal.
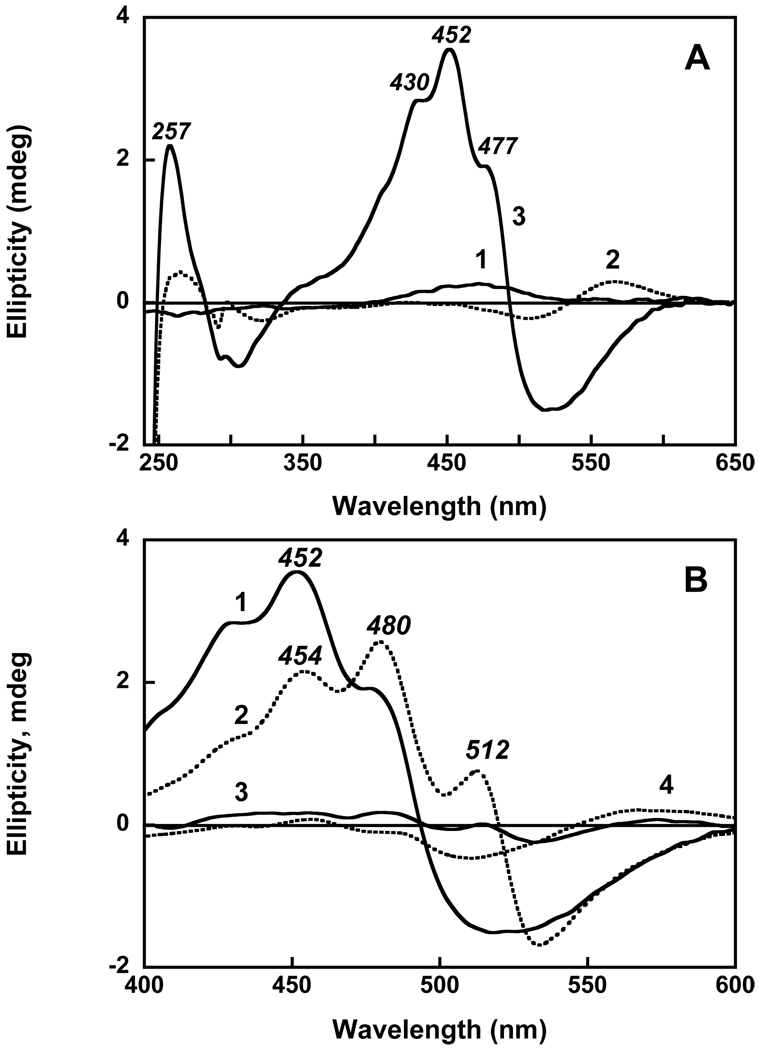
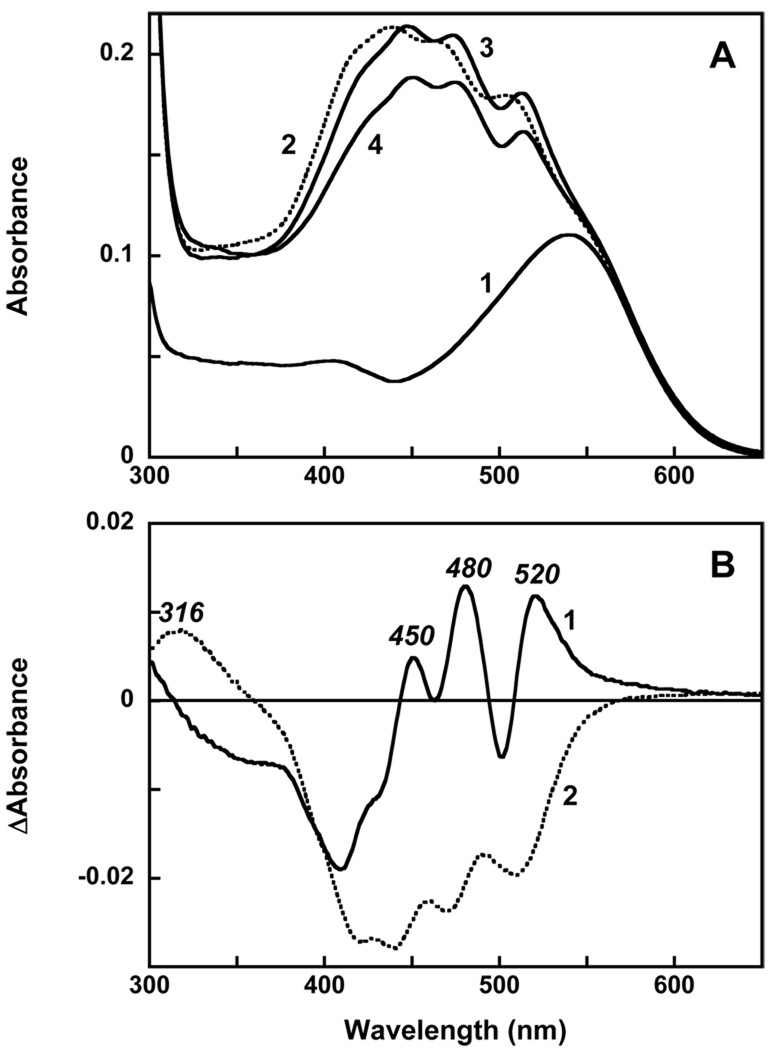
The CD spectrum of gloeobacter rhodopsin, a solution of echinenone in ethanol, and the spectrum of gloeobacter rhodopsin reconstituted with echinenone are shown in Figure 4A. Free echinenone lacks chiral centers (26); in ethanol it shows only a small Cotton effect (spectrum 1). Gloeobacter rhodopsin shows bilobe bands (positive with maximum at 567 nm, negative with minimum at 506 nm and crossover at 536 nm) from the retinal chromophore (spectrum 2), and two bands in UV at 298 and 264 nm, all of relatively low intensity. However, after reconstitution with echinenone, a much greater amplitude of the CD spectrum is observed, with maxima at 477, 452 (main) and 430 nm and UV band at 257 nm (spectrum 3). Similar increase in intensity of the CD signal had been observed upon reconstitution of gloeobacter rhodopsin with salinixanthin (23). It is interesting to compare the two CD spectra, scaled to the same concentration of gloeobacter rhodopsin and shown in Figure 4B. The overall similarity of the shapes is obvious, in spite of the differences in the position of the bands of the echinenone spectrum to shorter wavelengths. The maxima in the CD spectrum of the protein with echinenone at 452 and 477 nm are blue-shifted from similar maxima at 480 and 512 nm in the protein reconstituted with salinixanthin by 28 and 35 nm, respectively. This corresponds to similar shifts in the absorption maxima of the vibronic bands from 0–1 and 0-0 transitions in the two spectra (458 vs. 486 nm 484 vs. 521 nm). The correlation indicates that as in the case of xanthorhodopsin (21), the structure of the CD spectra originates from the bands of bound carotenoid, from its asymmetric conformation, and its steric and electronic interaction with the retinal. In the UV, gloeobacter rhodopsin with bound echinenone exhibits an intense CD band at 257 nm which was not observed upon reconstitution with salinixanthin.
Figure 4.
A. Circular dichroism spectra of: 1, echinenone in ethanol; 2, gloeobacter rhodopsin, 4 µM in 0.02 % DDM, 25 mM MOPS, 0.1 NaCl, pH 7.2; 3; after reconstitution with echinenone. B. Comparison of the CD spectra of gloeobacter rhodopsin reconstituted with: 1, echinenone; 2, salinixanthin; 3, salinixanthol; 4, β-carotene. The spectra were scaled to the same amount of rhodopsin (4 µM). Spectrum 2 was adapted from (23).
Further evidence for interaction of echinenone with retinal was obtained in experiments with removal of retinal from the pigment (by hydrolyzing the retinal Schiff base with hydroxylamine (Figure 5A). Besides the disappearance of the retinal chromophore band at 540 nm (550 nm in the difference spectrum) and the appearance of the retinal oxime band at 367 nm, prominent negative peaks from decrease in extinction and broadening of carotenoid bands are present in the difference spectrum, at 486, 456 and 429 nm (Figure 5B). This indicates that removal of the retinal strongly affects the environment of the carotenoid, presumably decreasing the conformational restrains on its ring that strongly affects resolution of its vibronic bands.
Figure 5.
Absorption changes accompanying hydrolysis of the retinal Schiff base with hydroxylamine, 0.2 M, pH 7.2. A. Spectra of: 1, gloeobacter rhodopsin reconstituted with echinenone immediately after addition of 0.2 M hydroxylamine, 2 through 6, 10, 30, 60, 120, 360 min later; B. Difference “spectrum i minus spectrum 1”.
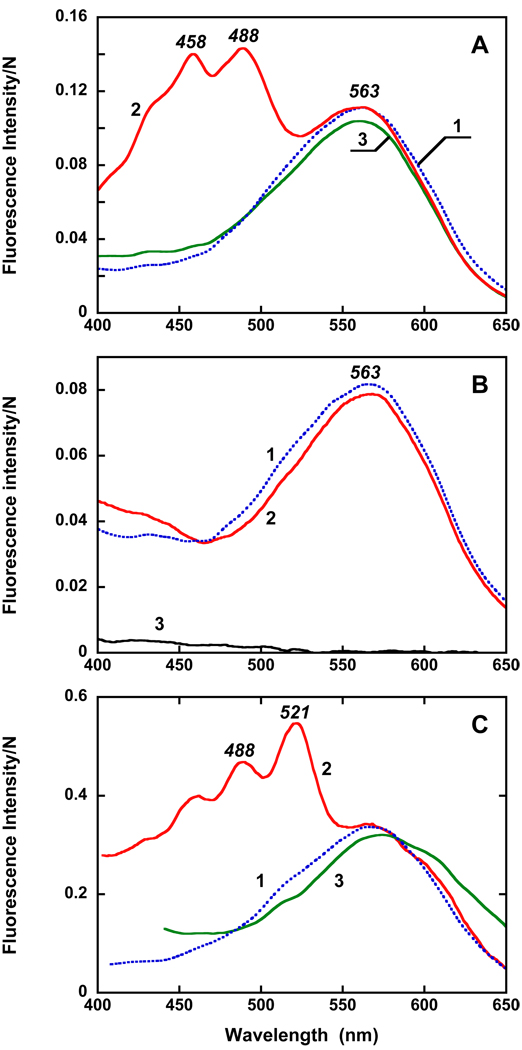
The most important evidence for functional interaction between the echinenone and retinal chromophores was obtained from the excitation spectrum for retinal fluorescence emission. These measurements were performed at two pH values, pH 7.2 and pH 4.2. At pH 4.2 the quantum yield of retinal emission is about 6 times higher than at pH 7.2 since the fraction of the rhodopsin with protonated counter-ion is greater at this pH. As in bacteriorhodopsin (29, 30) and xanthorhodopsin (15), the latter has a much higher quantum yield of fluorescence than the protein with deprotonated counterion (23). This helps to minimize contribution from other fluorescing impurities in the sample that are not pH dependent. The excitation spectrum of retinal chromophore emission of gloeobacter rhodopsin exhibits a single broad band with maximum at 563 nm (Figure 6A). Additional bands at 488, 458 and 430 nm are present in the excitation spectrum of the protein reconstituted with echinenone. The data clearly show that light absorbed by echinenone bound to the protein is transferred to the retinal chromophore, and hence the bound echinenone functions as a light-harvesting antenna. The quantum efficiency is at least 30%. No energy transfer takes place when echinenone was added to the G178W mutant, as the lack of the carotenoid bands in the spectrum taken 28h after addition of echinenone indicates (Figure 6B). Echinenone binds much slower than salinixanthin (time constant 7 hours vs. 14 min when the latter added as solution in ethanol), indicating that the acyl glycoside moiety and/or the 2′-hydroxy group of the latter facilitates binding of the carotenoid to the protein.
Figure 6.
Excitation spectra for fluorescence emission of the retinal chromophore at 720 nm for: A. 1, gloeobacter rhodopsin; 2, as 1 but reconstituted with echinenone; 3, as 1 but with β-carotene added. Carotenoid/retinal ratio was 1:1; concentration of rhodopsin, close to 4 µM. pH 4.2 Absorbance of the samples in the maximum are between 0.1 and 0.2. B. 1, the G178W mutant, pH 4.2; 2, after reconstitution with echinenone (28 hours after addition). 3, Echinenone in 0.02% DDM (same concentration as in 2). C. 1, gloeobacter rhodopsin; 2, after reconstitution of gloeobacter rhodopsin with salinixanthin; 3, after mixing of gloeobacter rhodopsin with salinixanthol. Bandwidth of excitation beam, 8 nm.
Attempt to reconstitute gloeobacter rhodopsin with salinixanthol
Unlike salinixanthin and echinenone, salinixanthol, in which the 4-keto group in the ring is reduced to the C-OH group, exhibits a well-structured spectrum even in a solvent (14). Thus, although addition of salinixanthol to gloeobacter rhodopsin also results in a well resolved spectrum (data not shown), but this cannot be viewed as a sign of binding. Notably, no time dependent changes were observed upon adding of the analogue. Moreover, the mixture did not show any significant CD bands (Figure 4B, spectrum 3). The weak bands at 514 nm and 480 nm are near the bands of salinixanthin and might be from the presence of <10% of salinixanthin, or alternatively from a small fraction of bound salinixanthol. Most important, the excitation spectrum for the emission at 720 nm (close to the maximum of retinal chromophores emission) did not show any bands from salinixanthol absorption (Figure 6C). This is clear evidence that no energy transfer from salinixanthol to the retinal chromophore takes place and that a seemingly minor modification of the carotenoid by reducing its 4-keto group to 4-hydroxy strongly interferes with the binding of the carotenoid, for steric or other reasons.
Reconstitution with β-carotene
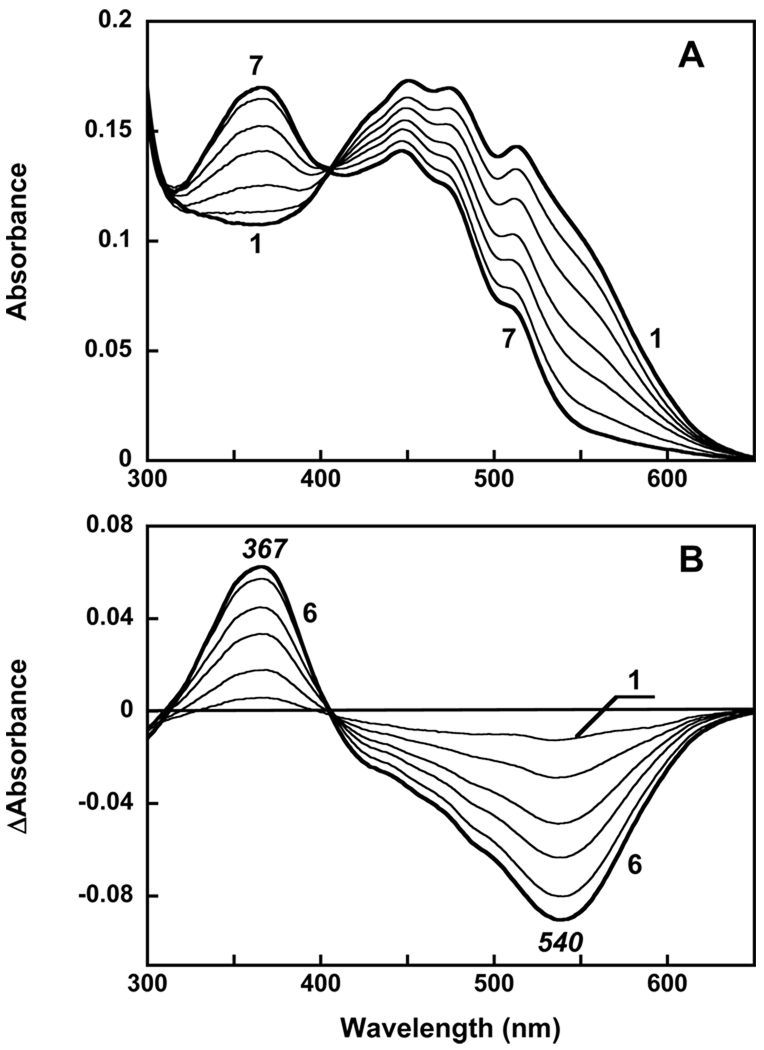
The question arises whether the 4-keto group will affect binding. Addition of β-carotene dissolved in acetone to 0.02% DDM results in spectrum that shows some vibronic structure but less pronounced than in organic solvent. With time the pigment undergoes changes that result in decrease of absorption in the maximum and formation of a red shifted product with a broad band within 1 hour (data not shown). These changes are most likely from aggregation of the carotenoid. However if β-carotene is added when gloeobacter rhodopsin is present, then a red shift of absorption maxima still occurs but no decrease in extinction take place during the first hour (Figure 7). These changes indicate that some kind of binding of β-carotene to the protein takes place. The changes occur with time constant of 10 min and saturate after an hour. Additional incubation results in slow decay (bleaching) of the carotenoid component. The CD spectrum, exhibited only a weak band with maximum at 456 nm (spectrum 4 in Figure 4B). The overall amplitude of the spectrum is about an order of magnitude less than when gloeobacter rhodopsin was reconstituted with echinenone. A small Cotton effect indicates that the conformation of β-carotene is not substantially distorted and no strong interaction with retinal takes place that could produce changes seen when gloeobacter rhodopsin was reconstituted with echinenone and salinixanthin.
Figure 7.
A. Absorption spectra of: 1, gloeobacter rhodopsin, 2 µM in 0.02% DDM, pH 7.2, 0.1 M NaCl; 2, 2 µM of β-carotene added; 3, after 1 hour; 4, 5 hours later. B. Difference spectra: 1, “3 minus 2”; 2, “4 minus 3”.
In order to check whether there is interaction of bound β-carotene with the retinal chromophore, we cleaved the Schiff base with hydroxylamine (Figure 8A). The difference spectra did not show a contribution from the carotenoid (Figure 8B). This indicates that unlike echinenone, β-carotene does not interact sterically with the retinal chromophore.
Figure 8.
Reaction of gloeobacter rhodopsin, with β-carotene added, with 0.2 M of hydroxylamine, pH 7.2 in the dark. A. Absorption spectra; 1, immediately after addition of hydroxylamine; 2 through 7, measured 10, 30, 60, 90, 150 and 240 min after addition; B. 1, through 6, absorption changes “spectrum i minus spectrum 1” where i are spectra 2 through 7 in panel A.
Finally, the excitation spectrum of the retinal chromophores emission for gloeobacter rhodopsin with β-carotene added does not exhibit noticeable carotenoid bands (Figure 6A, spectrum 3). This indicates that no energy transfer from β-carotene to the retinal chromophores takes place. It correlates with lack of CD bands and steric interaction with retinal, and suggests that this carotenoid does not bind, or binds but not to a specific site that would be suitable for efficient energy transfer. β-Carotene acts as an antenna in a number of chlorophyll-binding photosynthetic proteins (31–33) but its inability to bind to a specific site in gloeobacter rhodopsin precludes it from light-harvesting in that protein. Echinenone is not a common light-harvesting carotenoid (33) but as our data indicate it can perform this function in gloeobacter rhodopsin.
The finding that neither β-carotene nor salinixanthol bind to the site at the retinal in gloeobacter rhodopsin is consistent with our observation that salinixanthol does not bind to xanthorhodopsin. It suggests that the 4-keto group plays a key role in binding. Salinixanthin binds to gloeobacter rhodopsin ca. 30 fold faster than echinenone. If one assumes that configuration of the conjugated chains is similar in both carotenoids, then this indicates that the glycoside, the acyl tail and the 2′-hydroxy group, missing in echinenone, strongly accelerate binding of salinixanthin to gloeobacter rhodopsin, but they are not as crucial as the 4-keto group, lack of which completely eliminates binding. Further experiments with carotenoid analogues are needed to dissect the roles of each of these additional groups in salinixanthin. The polar 2′-hydroxy group and glycoside would certainly increase solubility of the carotenoid and facilitate its delivery to the protein in aqueous environment and proper orientation, with the polar glycoside being close to the cytoplasmic surface. In xanthorhodopsin, the glycoside is hydrogen bonded to the side chains of Arg184 and Asn191 (20). The latter residue is conserved in gloeobacter rhodopsin.
According to the crystal structure of xanthorhodopsin, the oxygen of the 4-keto group is not involved in hydrogen bonding. Existence of such a bond could work as a “lock” (34) and would explain its role in binding. An example is the orange carotenoid protein (OCP), that binds 3'-hydroxyechinone (35). The 4-keto group is hydrogen bonded to the side chains of two residues, Trp and Tyr. It is critical for function (36) and affects the spectroscopic properties of the carotenoprotein (37).
The lack of the hydrogen bonding in xanthorhodopsin implies that another mechanism exists by which the 4-keto group could be involved in the carotenoid binding. Two mechanisms involving the 4-keto group might be considered. The first is based on the crystal structure of xanthorhodopsin (20) which shows that the cyclohexanone ring of salinixanthin is rotated 82 degrees from the plane of polyene conjugated chain. The high degree of conservation of residues involved in carotenoid binding and the fact that gloeobacter rhodopsin binds salinixanthin (23) and that binding of echinenone and salinixanthin is abolished by the G178W mutation suggests similar binding sites and conformations of the bound carotenoid in the two proteins. Thus in order to fit the binding site the ring should be able to undergo a large turn around the C6–C7 bond. The presence of the 4-keto group in the ring apparently facilitates this turn because it allows for a broader distribution of dihedral angles around this bond. The evidence for this is the broad absorption spectra of carotenoids with conjugated carbonyl that show poor vibronic structure even in nonpolar solvents (28, 38, 39). Under these conditions the solvent interaction with the carbonyl oxygen is minimal, and broadening is most likely from multiple conformers differing in dihedral angle between the ring and H-8 on the polyene chain. This ability to undergo larger turns around the C6–C7 bond may help to fit the ring into the binding site. Carotenoids with cyclohexene or cyclohexanone β-rings have 6-s-cis conformation in the crystal structures and also in solution (40). This has been shown for β-carotene (41), and 4-keto carotenoids, canthaxanthin (42, 43) and astaxanthin (43). The β-rings are turned -(43–50°) out of the polyene conjugation plane around the C6–C7 single bond, because of steric conflict between the methyl groups of the ring and the hydrogen atoms bonded to the carbon atoms of the chain. In carotenoproteins, the conformation might be very different. Thus, astaxanthin noncovalently bound to β-crustacyanin of lobster shell is in s-trans conformation (44). The ring and the 4-keto group are strongly hydrogen bonded in this protein which is different from salinixanthin in xanthorhodopsin where the ring is in twisted s-cis conformation and nonbound. The ability of the 4-keto carotenoids to undergo large turns around the 6C–7C bond may be rationalized by the presence and polarization of the 4-keto group which produces a partial positive charge on C4 and, through conjugation, on C6. This will facilitate rotation (turning) of the ring around this bond and fitting the ring into its binding site. This twisted conformation of the ring apparently cannot be achieved in β-carotene and salinixanthol, and that could interfere with the binding and immobilization of the polyene chains in the protein. Thus, the twisted ring might work as a lock (or at least a key factor) for optimal positioning of the carotenoid on the protein.
An additional factor to explain the role of the 4-keto group in binding is based on the observation that in the structure of xanthorhodopsin obtained after MD simulation and QM/MM optimization the carotenoid ring is rotated not by 82.8° but by 40.4° (19). In this conformation a nonhydrogen bonding interaction between the carotenoid carbonyl and the Met-233 sulfur atom becomes feasible. Such interaction between carbonyl groups and methionine has been observed in a number of proteins, with an average O to S distance of 3.6 Å (45). In xanthorhodopsin and gloeobacter rhodopsin this interaction might serve the purpose of “locking” the carotenoid into the binding site when it enters it.
In conclusion, we show that gloeobacter rhodopsin can be reconstituted with echinenone, a carotenoid present in the native organism, Gloeobacter violaceus. Bound echinenone functions as light-harvesting antenna. In contrast, β-carotene and salinixanthol do not bind in a specific way that would lead to energy transfer, indicating that the 4-keto group in echinenone and salinixanthin is important for binding. Lack of this group in β-carotene and its modification to hydroxyl group in salinixanthol eliminate functional interaction with the retinal chromophore.
Acknowledgement
We thank Professors Rosalie Crouch (MUSC) and Tomas Polívka (University of South Bohemia) for discussions and helpful suggestions.
Abbreviations
- DDM
n-dodecyl-β-D maltopyranoside
- MOPS
3-[N-morpholino] propanesulfonic acid.
Footnotes
This work was supported in part by grants from the National Institutes of Health (GM29498), the Department of Energy (DEFG03-86ER13525) to J.K.L and the U.S. Army Research Office (W911NF-09-1-0243) to S.P.B., and by a Korea Research Foundation Grant (KRF2004-042-C00113) to K.H.J., the 21C Frontier Microbial Genomics and Application Center Program, Ministry of Education, Science & Technology, Korea to K.H.J., the second stage of Brain Korea 21 graduate Fellowship Program for A.R.C.
References
- 1.Lanyi JK. Proton transfers in the bacteriorhodopsin photocycle. Biochim. Biophys. Acta. 2006;1757:1012–1018. doi: 10.1016/j.bbabio.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Oesterhelt D, Stoeckenius W. Functions of a new photoreceptor membrane. Proc. Natl. Acad. Sci. U.S.A. 1973;70:2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Béjà O, Aravind L, Koonin EV, Suzuki MT, Hadd A, Nguyen LP, Jovanovich SB, Gates CM, Feldman RA, Spudich JL, Spudich EN, DeLong EF. Bacterial rhodopsin: Evidence for a new type of phototrophy in the sea. Science. 2000;289:1902–1906. doi: 10.1126/science.289.5486.1902. [DOI] [PubMed] [Google Scholar]
- 4.Drachev LA, Frolov VN, Kaulen AD, Liberman EA, Ostroumov SA, Plakunova GV, Semenov AY, Skulachev VP. Reconstitution of biological molecular generators of electric current. Bacteriorhodopsin. J. Biol. Chem. 1976;251:7059–7065. [PubMed] [Google Scholar]
- 5.Michel H, Oesterhelt D. Light-induced changes of the pH gradient and the membrane potential in Halobacterium halobium. FEBS Lett. 1980;65:175–178. doi: 10.1016/0014-5793(76)80473-5. [DOI] [PubMed] [Google Scholar]
- 6.Sineshchekov OA, Spudich JL. Light-induced intramolecular charge movements in microbial rhodopsins in intact E. coli cells. Photochem. Photobiol. Sci. 2004;3:548–554. doi: 10.1039/b316207a. [DOI] [PubMed] [Google Scholar]
- 7.Wang WW, Sineshchekov OA, Spudich EN, Spudich JL. Spectroscopic and photochemical characterization of a deep ocean proteorhodopsin. J. Biol. Chem. 2003;278:33985–33991. doi: 10.1074/jbc.M305716200. [DOI] [PubMed] [Google Scholar]
- 8.Béjà O, Spudich EN, Spudich JL, Leclerc M, DeLong EF. Proteorhodopsin phototrophy in the ocean. Nature. 2001;411:786–789. doi: 10.1038/35081051. [DOI] [PubMed] [Google Scholar]
- 9.Sabehi G, Massana R, Bielawski JP, Rosenberg M, Delong EF, Béjà O. Novel Proteorhodopsin variants from the Mediterranean and Red Seas. Environ. Microbiol. 2003;5:842–849. doi: 10.1046/j.1462-2920.2003.00493.x. [DOI] [PubMed] [Google Scholar]
- 10.Venter JC, Remington K, Heidelberg JF, Halpern AL, Rusch D, Eisen JA, Wu D, Paulsen I, Nelson KE, Nelson W, Fouts DE, Levy S, Knap AH, Lomas MW, Nealson K, White O, Peterson J, Hoffman J, Parsons R, Baden-Tillson H, Pfannkoch C, Rogers Y-H, Smith HO. Environmental genome shotgun sequencing of the Sargasso Sea. Science. 2004;304:66–74. doi: 10.1126/science.1093857. [DOI] [PubMed] [Google Scholar]
- 11.Spudich JL, Jung K-H. Microbial Rhodopsins: Phylogenetic and Functional Diversity. In: Briggs WR, Spudich JL, editors. Handbook of photosensory receptors. Darmstadt: Wiley-VCH; 2005. pp. 1–23. [Google Scholar]
- 12.Fuhrman JA, Schwalbach MS, Stingl U. Proteorhodopsins: an array of physiological roles? Nat. Rev. Microbiol. 2008;6:488–494. doi: 10.1038/nrmicro1893. [DOI] [PubMed] [Google Scholar]
- 13.Balashov SP, Imasheva ES, Boichenko VA, Antón J, Wang JM, Lanyi JK. Xanthorhodopsin: A proton pump with a light-harvesting carotenoid antenna. Science. 2005;309:2061–2064. doi: 10.1126/science.1118046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lutnaes BF, Oren A, Liaaen-Jensen S. New C40-carotenoid acyl glycoside as principal carotenoid in Salinibacter ruber, an extremely halophilic eubacterium. J. Nat. Prod. 2002;65:1340–1343. doi: 10.1021/np020125c. [DOI] [PubMed] [Google Scholar]
- 15.Balashov SP, Imasheva ES, Wang JM, Lanyi JK. Excitation energy-transfer and the relative orientation of retinal and carotenoid in xanthorhodopsin. Biophys. J. 2008;95:2402–2414. doi: 10.1529/biophysj.108.132175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boichenko VA, Wang JM, Antón J, Lanyi JK, Balashov SP. Functions of carotenoids in xanthorhodopsin and archaerhodopsin, from action spectra of photoinhibition of cell respiration. Biochim. Biophys. Acta. 2006;1757:1649–1656. doi: 10.1016/j.bbabio.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polívka T, Balashov SP, Chábera P, Imasheva ES, Yartsev A, Sundström V, Lanyi JK. Femtosecond carotenoid to retinal energy transfer in xanthorhodopsin. Biophys. J. 2009;96:2268–2277. doi: 10.1016/j.bpj.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birge RR. Nature of the primary photochemical events in rhodopsin and bacteriorhodopsin. Biochim. Biophys. Acta. 1990;1016:293–327. doi: 10.1016/0005-2728(90)90163-x. [DOI] [PubMed] [Google Scholar]
- 19.Fujimoto KJ, Hayashi S. Electronic Coulombic coupling of excitation-energy transfer in xanthorhodopsin. J. Am. Chem. Soc. 2009;131:14152–14153. doi: 10.1021/ja905697n. [DOI] [PubMed] [Google Scholar]
- 20.Luecke H, Schobert B, Stagno J, Imasheva ES, Wang JM, Balashov SP, Lanyi JK. Crystallographic structure of xanthorhodopsin, the light-driven proton pump with a dual chromophore. Proc. Natl. Acad. Sci. U.S.A. 2008;105:16561–16565. doi: 10.1073/pnas.0807162105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balashov SP, Imasheva ES, Lanyi JK. Induced chirality of the light-harvesting carotenoid salinixanthin and its interaction with the retinal of xanthorhodopsin. Biochemistry. 2006;45:10998–11004. doi: 10.1021/bi061098i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smolensky E, Sheves M. Retinal-salinixanthin interactions in xanthorodopsin: A circular dichroism (CD) spectroscopy study with artificial pigments. Biochemistry. 2009;48:8179–8188. doi: 10.1021/bi900572b. [DOI] [PubMed] [Google Scholar]
- 23.Imasheva ES, Balashov SP, Choi AR, Jung K-H, Lanyi JK. Reconstitution of Gloeobacter violaceus rhodopsin with a light-harvesting carotenoid antenna. Biochemistry. 2009;48:10948–10955. doi: 10.1021/bi901552x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miranda MRM, Choi AR, Shi L, Bezerra AG, Jung K-H, Brown LS. The photocycle and proton translocation pathway in a cyanobacterial ion-pumping rhodopsin. Biophys. J. 2009;96:1471–1481. doi: 10.1016/j.bpj.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takaichi S, Mochimaru M. Carotenoids and carotenogenesis in cyanobacteria: Unique ketocarotenoids and carotenoid glycosides. Cell. Mol. Life Sci. 2007;64:2607–2619. doi: 10.1007/s00018-007-7190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Britton G, Liaaen-Jensen S, Pfander H, editors. Carotenoids Handbook. Basel, Boston, Berlin: Birkhauser Verlag; 2004. [Google Scholar]
- 27.Christensen RL, Kohler BE. Low resolution optical spectroscopy of retinyl polyenes: low lying electronic levels and spectral broadness. Photochem. Photobiol. 1973;1973:293–301. [Google Scholar]
- 28.Britton G. UV/Visible Spectroscopy. In: Britton G, Liaaen-Jensen S, Pfander H, editors. Carotenoids Vol 1B: Spectroscopy. Basel, Boston, Berlin: Birkhäuser Verlag; 1995. pp. 13–62. [Google Scholar]
- 29.Kouyama T, Kinosita K, Ikegami A. Excited-state dynamics of bacteriorhodopsin. Biophys. J. 1985;47:43–54. doi: 10.1016/S0006-3495(85)83875-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balashov SP, Litvin FF, Sineshchekov VA. Photochemical processes of light energy transformation in bacteriorhodopsin. In: Skulachev VP, editor. Physicochemical Biology Reviews. UK: Harwood Academic Publishers GmbH; 1988. pp. 1–61. [Google Scholar]
- 31.De Weerd FL, Kennis JTM, Dekker JP, van Grondelle R. β-Carotene to chlorophyll singlet energy transfer in the Photosystem I core of Synechococcus elongatus proceeds via the β-carotene S2 and S1 states. J. Phys. Chem. B. 2003;107:5995–6002. [Google Scholar]
- 32.Holt NE, Kennis JTM, Fleming GR. Femtosecond fluorescence upconversion studies of light harvesting by β-carotene in oxygenic photosynthetic core proteins. J. Phys. Chem. B. 2004;108:19029–19035. [Google Scholar]
- 33.Polívka T, Frank HA. Molecular factors controlling photosynthetic light harvesting by carotenoids. Acc. Chem. Res. 2010;43:1125–1134. doi: 10.1021/ar100030m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roszak AW, McKendrick K, Gardiner AT, Mitchell IA, Isaacs NW, Cogdell RJ, Hashimoto H, Frank HA. Protein regulation of carotenoid binding: gatekeeper and locking amino acid residues in reaction centers of Rhodobacter sphaeroides. Structure. 2004;12:765–773. doi: 10.1016/j.str.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 35.Kerfeld CA, Sawaya MR, Brahmandam V, Cascio D, Ho KK, Trevithick-Sutton CC, Krogmann DW, Yeates TO. The crystal structure of a cyanobacterial water-soluble carotenoid binding protein. Structure. 2003;11:55–65. doi: 10.1016/s0969-2126(02)00936-x. [DOI] [PubMed] [Google Scholar]
- 36.Punginelli C, Wilson A, Routaboul JM, Kirilovsky D. Influence of zeaxanthin and echinenone binding on the activity of the Orange Carotenoid Protein. Biochim. Biophys. Acta. 2009;1787 doi: 10.1016/j.bbabio.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Polívka T, Kerfeld CA, Pascher T, Sundström V. Spectroscopic properties of the carotenoid 3 '-hydroxyechinenone in the orange carotenoid protein from the cyanobacterium Arthrospira maxima. Biochemistry. 2005;44:3994–4003. doi: 10.1021/bi047473t. [DOI] [PubMed] [Google Scholar]
- 38.Ke B, Imsgard F, Kjosen H, Liaaen-Jensen S. Electronic spectra of carotenoids at 77 degrees K. Biochim. Biophys. Acta. 1970;210:139-&. [PubMed] [Google Scholar]
- 39.Chabera P, Fuciman M, Hribek P, Polívka T. Effect of carotenoid structure on excited-state dynamics of carbonyl carotenoids. Phys. Chem. Chem. Phys. 2009;11:8795–8803. doi: 10.1039/b909924g. [DOI] [PubMed] [Google Scholar]
- 40.Hashimoto H, Yoda T, Kobayashi T, Young AJ. Molecular structures of carotenoids as predicted by MNDO-AM1 molecular orbital calculations. J. Mol. Struct. 2002;604:125–146. [Google Scholar]
- 41.Sterling C. Crystal-structure analysis of β-carotene. Acta Crystallogr. 1964;17:1224–1228. [Google Scholar]
- 42.Bart JC, MacGillavry CH. The crystal and molecular structure of canthaxanthin. Acta Crystallogr. 1968;B24 doi: 10.1107/s056774086800470x. [DOI] [PubMed] [Google Scholar]
- 43.Bartalucci G, Coppin J, Fisher S, Hall G, Helliwell JR, Helliwell M, Liaaen-Jensen S. Unravelling the chemical basis of the bathochromic shift in the lobster carapace; new crystal structures of unbound astaxanthin, canthaxanthin and zeaxanthin. Acta Crystallogr., Sect. B: Struct. Sci. 2007;63:328–337. doi: 10.1107/S0108768106052633. [DOI] [PubMed] [Google Scholar]
- 44.Cianci M, Rizkallah PJ, Olczak A, Raftery J, Chayen NE, Zagalsky PF, Helliwell JR. The molecular basis of the coloration mechanism in lobster shell: β-Crustacyanin at 3.2-angstrom resolution. Proc. Natl. Acad. Sci. U.S.A. 2002;99:9795–9800. doi: 10.1073/pnas.152088999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pal D, Chakrabarti P. Non-hydrogen bond interactions involving the methionine sulfur atom. J. Biomol. Struct. Dyn. 2001;19:115–128. doi: 10.1080/07391102.2001.10506725. [DOI] [PubMed] [Google Scholar]