Summary
Patients with T-cell (TCL) and natural killer-cell lymphomas (NKCL) have poor outcomes. This study examined the role of allogeneic haematopoietic cell transplantation (HCT) after non-myeloablative conditioning in this setting. Seventeen patients with TCL or NKCL, including three patients in first complete remission, received allogeneic HCT after 2 Gy total-body irradiation and fludarabine. The median age was 57 (range, 18–73) years. The median number of prior therapies was 3 (range, 1–7), six patients (35%) had failed prior autologous HCT, and five patients (29%) had refractory disease at the time of allograft. Postgrafting immunosuppression was provided with mycophenolate mofetil with cyclosporine or tacrolimus. After a median follow-up of 3.3 (range, 0.3–8.0) years among surviving patients, the estimated probabilities of 3-year overall and progression-free survival were 59% and 53%, respectively, while the estimated probabilities of non-relapse mortality and relapse at three years were 19% and 26%, respectively. Sixty-five percent of patients developed grades 2–4 acute graft-versus-host disease and 53% of patients developed chronic graft-versus-host disease. Allogeneic HCT after non-myeloablative conditioning is a promising salvage option for selected patients TCL and NKCL. These results suggest that graft-versus-T-cell lymphoma activity is responsible for long-term disease control.
Introduction
T-cell and natural killer (NK)-cell neoplasms are a heterogeneous group of lymphoid malignancies that represent approximately 5% of all lymphomas in North America (Morton et al, 2006), 7% worldwide (The Non-Hodgkin’s Lymphoma Classification Project, 1997), and up to 20% in Southeast Asia (Rudiger et al, 2002). Among World Health Organization recognized subtypes, peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS); anaplastic large cell lymphoma (ALCL); angioimmunoblastic T-cell lymphoma (AITL); and mycosis fungoides/Sezary syndrome (MF/SS) account for the majority of diagnoses (Armitage et al, 2008). With the prominent exception of anaplastic lymphoma kinase (ALK)-protein-expressing ALCL(Savage et al, 2008) and MF (Kim et al, 2003), the T-cell and NK-cell phenotype has been associated with poor outcomes after conventional anthracycline-based therapeutic approaches, with 5-year overall survival rates of 30–40% in most studies (Melnyk et al, 1997; Savage et al, 2004; Gisselbrecht et al, 1998; Lopez-Guillermo et al, 1998). Intensification of primary therapy with high-dose therapy (HDT) and autologous haematopoietic cell transplantation (HCT) has not shown significant benefit over standard treatments (Jantunen & D’Amore, 2004). One exception may be patients with angioimmunoblastic lymphoma who achieved complete remission after initial therapy (Rodriguez et al, 2007; Kyriakou et al, 2008).
Relapsed and refractory T-cell lymphoma (TCL) and NK- cell lymphoma (NKCL) are considered incurable with conventional approaches. HDT with autologous HCT may provide long-term remission in 30–40% of patients with chemotherapy-sensitive disease (Kewalramani et al, 2006; Vose et al, 1990; Rodriguez et al, 2001). However, the majority of the patients who relapsed after autologous HCT or who were unable to receive autologous HCT due to failure of stem cell collection, prohibitive comorbidities, age, or chemotherapy refractoriness of the disease have a very poor prognosis and short survival.
Allogeneic haematopoietic stem cell transplantation may overcome chemotherapy resistance via graft-versus-lymphoma effects and result in long-term disease control, even in poor-risk and chemotherapy-refractory patients (Ratanatharathorn et al, 1994; Bernard et al, 1999). However, the use of myeloablative conditioning regimens, while providing additional cytotoxic anti-tumour effects, is associated with a high transplant-related mortality (TRM) rate of 25 – 50% (Dhedin et al, 1999; Aksentijevich et al, 2006; Jones et al, 1991). Even higher TRM rates were reported in patients who had failed prior autologous HCT (Tsai et al, 1997), limiting this approach to medically fit, younger patients.
Nonmyeloablative conditioning regimens allow allogeneic engraftment with reduced morbidity and mortality, even in older and heavily pretreated patients (Khouri et al, 1998; McSweeney et al, 2001; Robinson et al, 2002; Corradini et al, 2004). For patients with disease controlled at the time of HCT, the relapse rates after allograft are comparable for myeloablative and nonmyeloablative approaches, suggesting that graft-versus-tumour effect might be more important than conditioning intensity in long-term disease control (Sorror et al, 2008; Scott et al, 2006; Sorror et al, 2004). Nonmyeloablative conditioning allows allogeneic transplantation to be considered in patients with pre-transplant comorbidities and advanced age who would otherwise have a high risk of treatment-related complications.
Considering the poor outcome in patients with relapsed, refractory and high-risk newly diagnosed TCL and NKCL, the search for novel treatment approaches is warranted. In this report, we evaluated the outcomes after nonmyeloablative allogeneic HCT for patients with advanced T-cell and NK-cell lymphomas.
Patients and Methods
Eligibility criteria
This analysis includes data from 17 patients with relapsed/refractory (n=14) or poor-risk newly diagnosed (n=3) TCL and NKCL who underwent allogeneic HCT after nonmyeloablative conditioning on Fred Hutchinson Cancer Research Center (FHCRC; Seattle, WA) multi-institutional protocols for patients with haematological malignancies between December 16, 1997 and March 2008. Poor risk for newly diagnosed patients was defined by histology historically associated with dismal prognosis (1 patient with NK-cell leukaemia/lymphoma and 1 patient with adult T-cell leukaemia/lymphoma) or a high-risk International Prognostic Index score of >3 (1 patient with PTCL-NOS).
Patients were treated at five centres, with the FHCRC functioning as the coordinating centre. Protocols were approved by the institutional review boards of the FHCRC and collaborating centres. All patients signed informed consent forms approved by the local institutional review boards. Results are reported as of April 1, 2009.
Patients with any subtype of mature (peripheral) TCL or NKCL, including T-cell prolymphocytic leukaemia or advanced/transformed cutaneous T-cell lymphoma, that failed prior systemic therapies or had poor risk features at initial diagnosis were included in this analysis (Table I). Other inclusion criteria were age ≥50 years or age < 50 years but at high risk for non-relapse mortality (NRM) with myeloablative preparative regimens as a result of prior treatment or other comorbidities.
Table I.
Patients’ Clinical Characteristics
| Patient No. | Histological Subtype | Age (years) | Sex | Donor | Prior Tx (n) | Failed Prior ASCT | Disease Status Prior to Tx | Time from Dx to Tx (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | AITL | 51 | F | MR | 5 | Yes | SD | 22 |
| 2 | PTCL-NOS | 61 | M | MUR | 4 | Yes | CR | 29 |
| 3 | PTCL-NOS | 55 | M | MUR | 3 | Yes | CR | 27 |
| 4 | T-PLL | 59 | M | MUR | 3 | No | PD | 25 |
| 5 | AITL | 51 | M | MUR | 6 | Yes | CR | 25 |
| 6 | PTCL-NOS | 61 | F | MR | 8 | Yes | PR | 144 |
| 7 | PTCL-NOS | 25 | M | MUR | 1 | No† | CR | 8 |
| 8 | CTCL-SS | 61 | M | MUR | 7 | No | PD | 20 |
| 9 | AITL | 72 | M | MR | 3 | No | PR | 6 |
| 10 | PTCL-NOS | 57 | F | MUR | 4 | No | PR | 21 |
| 11 | PTCL-NOS | 58 | M | MR | 3 | No | SD | 22 |
| 12* | NKC-L/L | 63 | M | MR | 1 | No | CR | 9 |
| 13 | T-PLL | 62 | F | MR | 2 | No | PR | 9 |
| 14 | T-PLL | 59 | M | MUR | 3 | No | PR | 50 |
| 15* | AITL | 57 | F | MUR | 1 | No | CR | 6 |
| 16 | ALCL | 45 | M | MR | 4 | Yes | CR | 48 |
| 17* | PTCL-NOS | 73 | M | MUR | 2 | No | CR | 6 |
Abbreviations: AITL=angioimmunoblastic T-cell lymphoma; ALCL=anaplastic large cell lymphoma; ASCT=autologous stem cell transplantation; CR=complete remission; MR=matched related; MRD=minimal residual disease; MUR=matched unrelated; NK-L/L=NK-cell leukaemia/lymphoma; PD=progressive disease; PR=partial remission; PTCL-NOS=peripheral T-cell lymphoma, not otherwise specified; CTCL-SS = Cutaneous T-cell Lymphoma-Sezary Syndrome; SD=stable disease; T-PLL=T-cell prolymphocytic leukaemia; Dx=diagnosis; Tx=therapy.
Received NMA HCT as part of the primary therapy due to poor-risk disease characteristics or histology.
ASCT was performed as part of planned tandem autologous-NMA allogeneic transplantation.
Patients with the diagnosis of T-cell lymphoblastic lymphoma/leukaemia were excluded. Additional exclusion criteria were pregnancy, cardiac ejection fraction of less than 35%, pulmonary diffusion capacity of less than 35% of predicted value, decompensated liver disease (fulminant hepatic failure or hepatic cirrhosis with portal hypertension), Karnofsky performance status of less than 60%, or serological evidence of infection with human immunodeficiency virus. Patients with refractory, rapidly progressive disease after last therapy had to obtain at least partial remission with salvage chemotherapy prior to allogeneic transplantation.
Pretransplantation Characteristics
Chemotherapy-sensitive disease was defined by attainment of complete (CR) or partial remission (PR) according to standard criteria (Cheson et al, 1999), to the chemotherapy regimen immediately preceding HCT, including HDT and autologous HCT as part of the treatment plan in two patients. CR was defined as disappearance of all clinical, biological, and radiographic signs and symptoms related to lymphoma. PR was defined as more than 50% reduction in tumour burden. Progressive disease (PD) was defined as more than 25% increase in tumour burden. Other cases were defined as stable disease (SD). Pre-transplantation comorbidities were scored using the HCT-specific comorbidity index for allogeneic transplantation (HCT-CI) (Charlson et al, 1987; Sorror et al, 2005a).
Human leucocyte antigen (HLA) Typing and Matching
All patients and their donors were matched for HLA-A, HLA-B, and HLA-C by at least intermediate-resolution DNA typing and for HLA-DRB1 and HLA-DQB1 by high resolution techniques (Petersdorf et al, 1998).
Conditioning Regimen and Postgrafting Immunosuppression
Patients were conditioned with 2 Gy of total-body irradiation (TBI) on day 0 and three doses of fludarabine 30 mg/m2/d on days −4 to −2 before HCT. Postgrafting immunosuppression included ciclosporin and mycophenolate mofetil (MMF) or tacrolimus (FK506), as described previously (Maris et al, 2003; Maloney et al, 2003). Initially, all patients received MMF 15 mg/kg orally every 12 h for 28 days; subsequently, the protocols were altered such that recipients of unrelated-donor allografts were administered MMF 15 mg/kg every 8 h until day +28 to reduce the risk of graft-versus-host disease (GVHD) and graft rejection. The taper of MMF was completed by day +96.
Post-HCT monitoring
Patients underwent bone marrow aspiration on days +28, +56 and +84 after HCT to assess chimerism. A unilateral bone marrow biopsy was obtained on day +84 to assess for lymphoma. Patients underwent computed tomography scans of the chest, abdomen and pelvis on day +56 after HCT (if abnormal before transplantation), on day +84; at 6, 12, 18 and 24 months after HCT; and annually thereafter up to 5 years after HCT. Responses were assessed according standard criteria, as above.
Toxicities occurring within the first 100 days after HCT were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf). Acute or chronic GVHD was graded according to the international procedure: grades 0, I, II, III, IV or absent, limited or extensive, respectively (Diaconescu et al, 2004; Flowers et al, 1999). Any death occurring after HCT in the absence of documented disease progression was considered NRM.
Collection of Haematopoietic Cells and Supportive Care
All patients received granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells (Maris et al, 2003; Maloney et al, 2003). The median CD34+ cell dose was 9.2 × 106 (range, 3.3–15.9 × 106) cells/kg. Anti-microbial and cytomegalovirus (CMV) prophylaxis and blood product support were administered as described previously (Maris et al, 2003). Growth factors were administered for persistent neutropenia only after day +28.
Statistical Analysis
Overall survival (OS) and progression-free survival (PFS) were estimated using the Kaplan-Meier method. Cumulative incidence estimates were used to summarize the probabilities of relapse and NRM, where NRM was considered a competing risk for relapse and relapse a competing risk for NRM. Progression and NRM were considered as failure events for PFS. Patients with progressive disease after HCT were categorized as relapsed for the purpose of PFS, even if they subsequently became disease-free in response to post-transplant relapse treatment.
Results
Patient Characteristics
A total of 17 patients were identified. Fourteen patients had relapsed disease or failed to achieve CR after initial therapy and three patients were in first CR. Ten patients received unrelated and seven patients received related HLA-matched donor grafts (Table I). Median age at transplantation was 57 (range, 18–73) years with seven patients over the age of 60 years. The median time from diagnosis to transplantation was 1.8 (range, 0.5–12) years. Median HCT-CI score was 2; four out of 17 patients had scores > 3. Median number of prior treatments was 3 (range, 1–7), and 7 patients (41%) received prior HDT and autologous stem cell transplantation (ASCT). Of these, 6 patients (35%) progressed after autologous HCT and 1 underwent autologous HCT as part of a planned tandem autologous-allogeneic transplantation protocol. Eight (47%) patients were in CR, 4 (24%) patients were in PR at the time of HCT and 5 (29%) patients had refractory disease after the last treatment.
Engraftment
All patients engrafted. Chimerism analysis at day +28 after HCT showed median peripheral blood CD3, peripheral blood CD33 and marrow donor chimerism levels of 97%, 99% and 97%, respectively, for both related and unrelated allograft recipients. None of the 17 patients experienced graft failure or graft rejection.
The median neutrophil nadir was 0.243 (range, 0.02–2.38) × 109 cells/l and median duration of neutropenia (< 0.5 × 109 cells/l) was 6 (range, 0–24) days. The median time from HCT to neutrophil nadir was 9 (range, 5–70) days. Median platelet nadir was 33(range, 8.0–161.) × 109/l with median duration of platelet count < 20 × 109 /l of 0 days (range, 0–10 days). Eleven out of 17 patients (65%) did not develop severe thrombocytopenia (< 20 × 109 /l).
Data on platelet and packed red blood cell (PRBC) transfusions were available for 13 of 17 patients. Six (46%) of 13 patients required platelet transfusion, and the median number of units transfused was 1.5 (range, 1–31). In patients requiring platelet transfusions, the median number of days with a platelet count < 20 × 109 /l was 3 (range, 1–10) days. Nine (69%) of 13 required PRBC transfusions, and the median number of PRBC units transfused was 4 (range, 2–32).
GVHD and Toxicities
All 17 patients were assessable for acute GVHD. Eleven out of 17 patients (65%) developed grade II-IV acute GVHD, with 5 (29%) having grade III acute GVHD. Extensive chronic GVHD developed in 9 patients (53%). Two patients died of complications of extensive chronic GVHD of the gastro-intestinal tract. The median times from HCT to development of acute and chronic GVHD were 40 (range, 22–97) days and 147 (range, 84–467) days among patients who developed GVHD, respectively.
Data on toxicities were available on all patients. Grade 4 haematological toxicities were common and included thrombocytopenia (< 25 × 109/l) in 7 (47%) and neutropenia (< 0.5 × 109/l) in 12 (80%) of 17 patients. Grade 4 non-haematological toxicity was uncommon, observed in 1 patient (6%; Table II). The most common non-haematological toxicity was renal, observed in 9 patients with 2 patients having grade 3 events. Despite aggressive infectious prophylaxis, fungal, bacterial and viral infections were observed in 5, 10 and 8 patients, respectively, including CMV reactivation in one patient. There was one infection-related death at 1,407 days post-transplantation (see NRM below). The most common identified infection was coagulase-negative staphylococcal bacteraemia.
Table II.
Non-haematological Toxicities*
| Toxicity (n=17) | All Grades | Grade 3 | Grade 4 |
|---|---|---|---|
| Cardiovascular | 3 | 3 | – |
| Pulmonary | 1 | 1 | – |
| Gastrointestinal | 5 | 4 | – |
| Hepatic | 5 | 1 | 1 |
| Renal | 9 | 2 | – |
| Neurological | 5 | 2 | 1 |
| Other | 3 | 3 | – |
All toxicities are graded according to NCI Common Terminology Criteria for Adverse Events 3.0 (NCI-CTCAE-3.0).
Disease Response
Sixteen out of 17 patients were evaluable for disease response. One patient (#6) died at day 34 post-transplant before response could be assessed. Of 8 patients in CR at the time of HCT, only 1 experienced disease relapse after transplantation. Of 9 patients with measurable disease at the time of HCT, CR was achieved in 5 patients after allograft (Table III). Of note, one patient with measurable disease at the time of allograft (#10) achieved CR after transplantation that was maintained at last follow-up 96.8 months post-transplant.
Table III.
Patient Outcomes after Allogeneic Transplantation
| Patient No. | Histological Subtype | Acute GVHD Grade | Chronic GVHD (Extensive) | Disease Status/Survival | Last Follow-up (months) |
|---|---|---|---|---|---|
| 1 | AITL | 3 | Yes | CR (died of GVHD) | 10.8 |
| 2 | PTCL-NOS | 2 | No | CR | 42.4 |
| 3 | PTCL-NOS | 0 | No | PD (died of disease) | 17.1 |
| 4 | T-PLL | 3 | Yes | PD (died of disease) | 14.2 |
| 5 | AITL | 3 | Yes | CR (died of NRM) | 5.3 |
| 6 | PTCL-NOS | 3 | No | NE (died of NRM) | 1.1 |
| 7 | PTCL-NOS | 2 | Yes | CR | 52.2 |
| 8 | CTCL-SS | 2 | Yes | CR | 39.3 |
| 9 | AITL | 2 | Yes | CR (died of NRM) | 46.3 |
| 10 | PTCL-NOS | 2 | Yes | CR | 95.4 |
| 11 | PTCL-NOS | 0 | Yes | PD (died of disease) | 14.5 |
| 12 | NKC-L/L | 0 | No | CR | 49.1 |
| 13 | T-PLL | 0 | No | PD (alive last follow-up) | 13.7 |
| 14 | T-PLL | 2 | No | CR | 8.8 |
| 15 | AITL | 0 | No | CR | 25.2 |
| 16 | ALCL | 3 | Yes | CR (died of GVHD) | 45.8 |
| 17 | PTCL-NOS | 0 | No | CR | 3.4 |
Abbreviations: AITL=angioimmunoblastic T-cell lymphoma; ALCL=anaplastic large cell lymphoma; PTCL-NOS=peripheral T-cell lymphoma, not otherwise specified; T-PLL=T-cell prolymphocytic leukaemia; CTCL-SS = Cutaneous T-cell Lymphoma-Sezary Syndrome; NK-L/L=NK-cell leukaemia/lymphoma; GVHD=graft-versus-host disease; PD=progressive disease; CR=complete remission; NE=not evaluable; NRM=non=relapse mortality.
Disease Progression
Four patients experienced disease progression or relapse (at days 17, 173, 369 and 423) after HCT leading to an estimated probability of progression/relapse at 3 years of 26%. Among the patients with disease progression after HCT, two had chemotherapy-resistant disease, one patient was in PR, and one was in CR prior to transplantation. Among 7 patients with PTCL-NOS, only 2 patients experienced disease progression/relapse after the allograft. None of the three patients with AITL experienced disease progression after the transplantation. Noteworthy, 2 out of these 3 patients had evidence of measurable disease prior to transplantation.
Survival, Progression-free Survival, and NRM
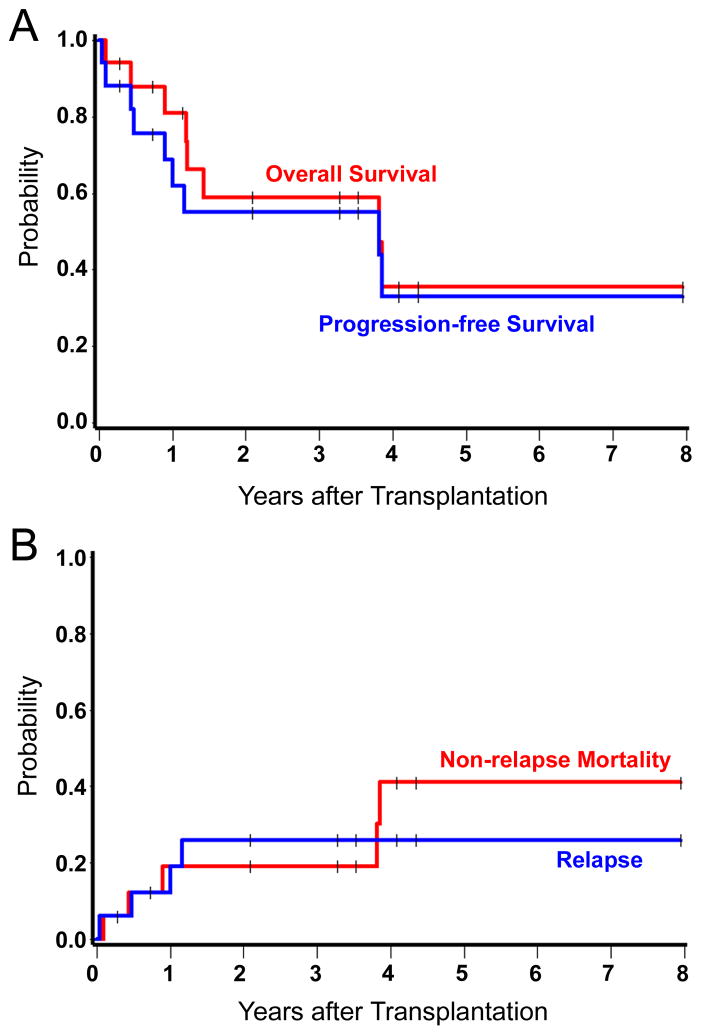
Median follow-up among the survivors was 3.3 (range 0.3–8.0) years. At the time of the last follow-up, 9 of 17 patients were alive and 8 were in CR. One patient relapsed but was alive at last contact. Deaths occurred at day 34, 161, 327, 433, 440, 521, 1394, and 1407, leading to an estimated 3-year OS and PFS of 59% and 53%, respectively (Figure 1A). The survival of patients with different TCL and NKCL histologies is listed in Table III. Five patients died from non-relapse causes at days 34, 161, 327, 1394, and 1407, leading to an estimated NRM at 3 years of 19% (Figure 1B). Causes of NRM included GVHD (n=2), progressive encephalopathy (n=1), Epstein-Barr virus-related multi-system organ failure (n=1) and coagulase-negative staphylococcal sepsis (n=1). Out of 7 patients aged 60 years or older, two died of NRM and five patients were alive with no evidence of lymphoma at last follow-up.
Figure 1. Kaplan-Meier estimates of overall survival, progression-free survival, non-relapse mortality and probability of relapse.
The probabilities of overall survival and progression-free survival at 3 years were 59% and 53%, respectively (A). The estimated probabilities of non-relapse mortality and relapse at 3 years were 19% and 26%, respectively (B).
Discussion
Relapsed, refractory, and high-risk newly diagnosed T-cell and NK-cell malignancies have poor outcomes with current therapies. HDT-ASCT can provide benefit for a minority of patients who demonstrate response to salvage therapy and can tolerate the dose intensity of myeloablative conditioning. However, the ability to collect a tumour-free autologous stem cell product may be hampered by bone marrow involvement and the cumulative myelotoxicity of prior treatments. Several studies have reported 3-year OS rates after ASCT in relapsed/refractory patients of 30–40% (Kewalramani et al, 2006; Vose et al, 1990; Rodriguez et al, 2001). Survival was inferior in patients with refractory disease. Despite ASCT, the majority of patients will eventually relapse or will not achieve a CR. In one study (Kewalramani et al 2006), while 5-year OS for PTCL patients transplanted for relapsed and refractory disease was 39%, only 17% of patients remained progression-free after HDT. In addition, high failure rates were observed after ASCT in patients with specific lymphoma subtypes, including hepato-splenic T-cell lymphoma, systemic nasal-type NK-cell lymphoma, NK-cell leukaemia/lymphoma and human T cell lymphotropic virus-1 associated T-cell leukaemia/lymphoma.
Several recent studies have shown promising results in patients with various types of T-cell and NK-cell lymphomas after allogeneic stem cell transplantation (Dhedin et al, 1999; Corradini et al, 2004; Le Gouill et al, 2008; Fukushima et al, 2005). The long-term disease control in these studies is probably due to graft-versus lymphoma (GVL) immunological responses. The role for GVL is supported by observations of the ability to achieve CR after allografting in patients with PR and minimal residual disease prior to treatment, as well as the attainment of CR in relapsed patients after withdrawal of immunosuppression and donor lymphocyte infusion (DLI). While no patients in our study received DLI, five out of nine patients who had evidence of disease prior to stem cell infusion achieved CR after transplantation. It is unlikely that attainment of CRs was due to fludarabine and low-dose TBI only. Similar conversion from PR to CR after nonmyeloablative conditioning and allografting was observed in studies by Corradini et al, (2004) and Le Gouill et al, (2008) in patients with PTCL.
The high treatment-associated toxicity and mortality of myeloablative conditioning precludes wide application of allogeneic HCT in haematological malignancies in general and in TCL and NKCL in particular. Dhedin et al. (1999) reported on 73 patients with aggressive B-cell (n=57) or T-cell (n=16) lymphomas from the Societe Francaise de Greffe de Moelle (SFGM) database. While 5-year OS and PFS were encouraging (41% and 40%, respectively), TRM was considerable at 44% despite the relatively young median age of study patients (35 years). In a retrospective analysis of 7 patients with PTCL treated with myeloablative conditioning and an allograft, 4 died in CR from treatment-related complications (Rodriguez et al, 2001). We and others have reported meaningful reductions in toxicity of treatment with comparable outcomes after nonmyeloablative conditioning prior to allografting in various lymphoid malignancies (Robinson et al, 2002; Corradini et al, 2004; Sorror et al, 2005b; Rezvani et al, 2008). In agreement with these earlier reports, the 3-year NRM in our study was 19% (although discounting follow-up information, 29% of patients had NRM by last contact). The observed NRM in this small study is encouraging due to the nature of the disease being treated as well as the advanced age of the patients (median age 57 years) and the presence in some patients of advanced comorbidities. These results suggest that the applicability of NMA-conditioning protocols allows treatment of older patients and those with comorbidities who otherwise would not be eligible for conventional approaches.
Corradini et al. (2004) recently reported on 17 patients with several subtypes of relapsed PTCL after reduced-intensity conditioning consisting of cyclophosphamide, thiotepa and fludarabine. In this prospective study of younger patients (median age 47 years), 2-year OS and PFS were 80% and 75%, respectively. Two-year cumulative treatment-associated mortality was only 6%. Disparate outcomes in our study could be explained by several important differences in patient population and treatments between the two studies: 1) patients in our study were older (59 years vs. 47 years); 2) a higher proportion of patients in our study had treatment refractory disease prior to allografting (5/17 vs. 2/17); 3) a higher number of patients received an unrelated allograft in our study (10/15 vs. 1/17); as well as 4) a longer duration of follow-up (a median of 3 years vs. 2 years). Despite these differences, long-term disease control in both studies and sustained CR in patients with otherwise incurable T-cell and NK-cell lymphomas are promising.
Patients with highly aggressive NK-cell leukaemia/lymphoma (NKC-L/L) and disseminated adult T-cell leukaemia/lymphoma (ATLL) are considered incurable with conventional approaches, and disease in most cases follows an aggressive fulminant clinical course resulting in fatal outcome within months of initial presentation (Chan, 1998; Ryder et al, 2007; Song et al, 2002; Shimoyama, 1991). Hence, it is encouraging that one patient with NKC-L/L and one patient with ATLL in our study were alive and in continuous CR at 50 and 25 months after transplantation, respectively.
In conclusion, relapsed/refractory and high-risk-histology newly diagnosed TCL and NKCL present significant therapeutic challenges. While novel chemotherapeutic and targeted biological agents are being developed, allogeneic HCT provides a chance of long-term disease control even for patients with relapsed and refractory disease. This is probably due to graft-versus-tumour effects. Our study suggests that nonmyeloablative conditioning with allogeneic HCT is an effective treatment with acceptable NRM rates and extends the applicability of this approach to an older patient population and to those with higher co-morbidity scores. Further multi-institutional studies are warranted to better define the role of allogeneic transplantation in patients with these rare T-cell and NK-cell malignancies.
Acknowledgments
This research was supported by grant Nos. CA78902, CA92058, CA18029, CA49605, CA15704 and HL088021 from the National Institute of Health, Department of Health and Human Services, Bethesda, MD and Leukemia and Lymphoma Society Specialized Center of Research grant 7040.
The authors wish to thank the transplant teams, physicians, nurses, and support personnel for their care of the patients on this study. The authors would also like to thank research nurses Michelle Bouvier and Hsien-Tzu Chen, and data manager Gresford Thomas for their invaluable help in making the study possible. The authors are grateful to Helen Crawford, Bonnie Larson and Sue Carbonneau for manuscript preparation.
Footnotes
Presented in part at the 10th International Conference on Malignant Lymphoma (10-ICML), June 3-8, 2008, Lugano, Switzerland.
Conflicts of Interest Disclosure
The authors have no conflicts of interest to disclose.
References
- Aksentijevich I, Jones RJ, Ambinder RF, Garrett-Mayer E, Flinn IW. Clinical outcome following autologous and allogeneic blood and marrow transplantation for relapsed diffuse large-cell non-Hodgkin’s lymphoma. Biology of Blood and Marrow Transplantation. 2006;12:965–972. doi: 10.1016/j.bbmt.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Armitage J, Vose J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. Journal of Clinical Oncology. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- Bernard M, Dauriac C, Drenou B, Leberre C, Branger B, Fauchet R, Le Prise PY, Lamy T. Long-term follow-up of allogeneic bone marrow transplantation in patients with poor prognosis non-Hodgkin’s lymphoma. Bone Marrow Transplantation. 1999;23:329–333. doi: 10.1038/sj.bmt.1701587. [DOI] [PubMed] [Google Scholar]
- Chan JK. Natural killer cell neoplasms (Review) Anatomic Pathology. 1998;3:77–145. [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group (Review) [erratum appears in J Clin Oncol 2000 Jun;18(11):2351] Journal of Clinical Oncology. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- Corradini P, Dodero A, Zallio F, Caracciolo D, Casini M, Bregni M, Narni F, Patriarca F, Boccadoro M, Benedetti F, Rambaldi A, Gianni AM, Tarella C. Graft-versus-lymphoma effect in relapsed peripheral T-cell non-Hodgkin’s lymphomas after reduced-intensity conditioning followed by allogeneic transplantation of hematopoietic cells. Journal of Clinical Oncology. 2004;22:2172–2176. doi: 10.1200/JCO.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Dhedin N, Giraudier S, Gaulard P, Esperou H, Ifrah N, Michallet M, Milpied N, Rio B, Cahn JY, Molina L, Laporte JL, Guilhot F, Kuentz M. Allogeneic bone marrow transplantation in aggressive non-Hodgkin’s lymphoma (excluding Burkitt and lymphoblastic lymphoma): a series of 73 patients from the SFGM database. British Journal of Haematology. 1999;107:154–161. doi: 10.1046/j.1365-2141.1999.01666.x. [DOI] [PubMed] [Google Scholar]
- Diaconescu R, Flowers CR, Storer B, Sorror ML, Maris MB, Maloney DG, Sandmaier BM, Storb R. Morbidity and mortality with nonmyeloablative compared to myeloablative conditioning before hematopoietic cell transplantation from HLA matched related donors. Blood. 2004;104:1550–1558. doi: 10.1182/blood-2004-03-0804. [DOI] [PubMed] [Google Scholar]
- Flowers MED, Kansu E, Sullivan KM. Pathophysiology and treatment of graft-versus-host disease. Hematology - Oncology Clinics of North America. 1999;13:1091–1112. doi: 10.1016/s0889-8588(05)70111-8. [DOI] [PubMed] [Google Scholar]
- Fukushima T, Miyazaki Y, Honda S, Kawano F, Moriuchi Y, Masuda M, Tanosaki R, Utsunomiya A, Uike N, Yoshida S, Okamura J, Tomonaga M. Allogeneic hematopoietic stem cell transplantation provides sustained long-term survival for patients with adult T-cell leukemia/lymphoma. Leukemia. 2005;19:829–834. doi: 10.1038/sj.leu.2403682. [DOI] [PubMed] [Google Scholar]
- Gisselbrecht C, Gaulard P, Lepage E, Coiffier B, Briere J, Haioun C, Cazals-Hatem D, Bosly A, Xerri L, Tilly H, Berger F, Bouhabdallah R, Diebold J. Prognostic significance of T-cell phenotype in aggressive non-Hodgkin’s lymphomas. Groupe d’Etudes des Lymphomes de l’Adulte (GELA) Blood. 1998;92:76–82. [PubMed] [Google Scholar]
- Jantunen E, D’Amore F. Stem cell transplantation for peripheral T-cell lymphomas (Review) Leukemia and Lymphoma. 2004;45:441–446. doi: 10.1080/10428190310001597955. [DOI] [PubMed] [Google Scholar]
- Jones RJ, Ambinder RF, Piantadosi S, Santos GW. Evidence of graft-versus-lymphoma effect associated with allogeneic bone marrow transplantation. Blood. 1991;77:649–653. [PubMed] [Google Scholar]
- Kewalramani T, Zelenetz AD, Teruya-Feldstein J, Hamlin P, Yahalom J, Horwitz S, Nimer SD, Moskowitz CH. Autologous transplantation for relapsed or primary refractory peripheral T-cell lymphoma. British Journal of Haematology. 2006;134:202–207. doi: 10.1111/j.1365-2141.2006.06164.x. [DOI] [PubMed] [Google Scholar]
- Khouri IF, Keating M, Körbling M, Przepiorka D, Anderlini P, O’Brien S, Giralt S, Ippoliti C, von Wolff B, Gajewski J, Donato M, Claxton D, Ueno N, Andersson B, Gee A, Champlin R. Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. Journal of Clinical Oncology. 1998;16:2817–2824. doi: 10.1200/JCO.1998.16.8.2817. [DOI] [PubMed] [Google Scholar]
- Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Archives of Dermatology. 2003;139:857–866. doi: 10.1001/archderm.139.7.857. [DOI] [PubMed] [Google Scholar]
- Kyriakou C, Canals C, Goldstone A, Caballero D, Metzner B, Kobbe G, Kolb HJ, Kienast J, Reimer P, Finke J, Oberg G, Hunter A, Theorin N, Sureda A, Schmitz N. High-dose therapy and autologous stem-cell transplantation in angioimmunoblastic lymphoma: complete remission at transplantation is the major determinant of Outcome-Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Journal of Clinical Oncology. 2008;26:218–224. doi: 10.1200/JCO.2008.12.6219. [DOI] [PubMed] [Google Scholar]
- Le Gouill S, Milpied N, Buzyn A, de Latour RP, Vernant JP, Mohty M, Moles MP, Bouabdallah K, Bulabois CE, Dupuis J, Rio B, Gratecos N, Yakoub-Agha I, Attal M, Tournilhac O, Decaudin D, Bourhis JH, Blaise D, Volteau C, Michallet M. Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. Journal of Clinical Oncology. 2008;26:2264–2271. doi: 10.1200/JCO.2007.14.1366. [DOI] [PubMed] [Google Scholar]
- Lopez-Guillermo A, Cid J, Salar A, Lopez A, Montalban C, Castrillo JM, Gonzalez M, Ribera JM, Brunet S, Garcia-Conde J, Fernandez DS, Bosch F, Montserrat E. Peripheral T-cell lymphomas: initial features, natural history, and prognostic factors in a series of 174 patients diagnosed according to the R.E.A.L. Classification (Review) Annals of Oncology. 1998;9:849–855. doi: 10.1023/a:1008418727472. [DOI] [PubMed] [Google Scholar]
- Maloney DG, Molina AJ, Sahebi F, Stockerl-Goldstein KE, Sandmaier BM, Bensinger W, Storer B, Hegenbart U, Somlo G, Chauncey T, Bruno B, Appelbaum FR, Blume KG, Forman SJ, McSweeney P, Storb R. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood. 2003;102:3447–3454. doi: 10.1182/blood-2002-09-2955. [DOI] [PubMed] [Google Scholar]
- Maris MB, Niederwieser D, Sandmaier BM, Storer B, Stuart M, Maloney D, Petersdorf E, McSweeney P, Pulsipher M, Woolfrey A, Chauncey T, Agura E, Heimfeld S, Slattery J, Hegenbart U, Anasetti C, Blume K, Storb R. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, Chauncey TR, Gooley TA, Hegenbart U, Nash RA, Radich J, Wagner JL, Minor S, Appelbaum FR, Bensinger WI, Bryant E, Flowers MED, Georges GE, Grumet FC, Kiem HP, Torok-Storb B, Yu C, Blume KG, Storb RF. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- Melnyk A, Rodriguez A, Pugh WC, Cabannillas F. Evaluation of the Revised European-American Lymphoma classification confirms the clinical relevance of immunophenotype in 560 cases of aggressive non-Hodgkin’s lymphoma. Blood. 1997;89:4514–4520. [PubMed] [Google Scholar]
- Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersdorf EW, Gooley TA, Anasetti C, Martin PJ, Smith AG, Mickelson EM, Woolfrey AE, Hansen JA. Optimizing outcome after unrelated marrow transplantation by comprehensive matching of HLA class I and II alleles in the donor and recipient. Blood. 1998;92:3515–3520. [PubMed] [Google Scholar]
- Ratanatharathorn V, Uberti J, Karanes C, Abella E, Lum LG, Momin F, Cummings G, Sensenbrenner LL. Prospective comparative trial of autologous versus allogeneic bone marrow transplantation in patients with non-Hodgkin’s lymphoma. Blood. 1994;84:1050–1055. [PubMed] [Google Scholar]
- Rezvani AR, Storer B, Maris M, Sorror ML, Agura E, Maziarz RT, Wade JC, Chauncey T, Forman SJ, Lange T, Shizuru J, Langston A, Pulsipher MA, Sandmaier BM, Storb R, Maloney DG. Nonmyeloablative allogeneic hematopoietic cell transplantation in relapsed, refractory, and transformed indolent non-Hodgkin lymphoma. Journal of Clinical Oncology. 2008;28:211–217. doi: 10.1200/JCO.2007.11.5477. [DOI] [PubMed] [Google Scholar]
- Robinson SP, Goldstone AH, Mackinnon S, Carella A, Russell N, de Elvira CR, Taghipour G, Schmitz N. Chemoresistant or aggressive lymphoma predicts for a poor outcome following reduced-intensity allogeneic progenitor cell transplantation: an analysis from the Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Blood. 2002;100:4310–4316. doi: 10.1182/blood-2001-11-0107. [DOI] [PubMed] [Google Scholar]
- Rodriguez J, Munsell M, Yazji S, Hagemeister FB, Younes A, Andersson B, Giralt S, Gajewski J, de Lima M, Couriel D, Romaguera J, Cabanillas FF, Champlin RE, Khouri IF. Impact of high-dose chemotherapy on peripheral T-cell lymphomas. Journal of Clinical Oncology. 2001;19:3766–3770. doi: 10.1200/JCO.2001.19.17.3766. [DOI] [PubMed] [Google Scholar]
- Rodriguez J, Conde E, Gutierrez A, Arranz R, Gandarillas M, Leon A, Ojanguren J, Sureda A, Carrera D, Bendandi M, Moraleda J, Ribera JM, Albo C, Morales A, Garcia JC, Fernandez P, Canigral G, Bergua J, Caballero MD. Prolonged survival of patients with angioimmunoblastic T-cell lymphoma after high-dose chemotherapy and autologous stem cell transplantation: the GELTAMO experience. European Journal of Haematology. 2007;78:290–296. doi: 10.1111/j.1600-0609.2007.00815.x. [DOI] [PubMed] [Google Scholar]
- Rudiger T, Weisenburger DD, Anderson JR, Armitage JO, Diebold J, MacLennan KA, Nathwani BN, Ullrich F, Muller-Hermelink HK Non-Hodgkin’s Lymphoma Classification Project. Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the Non-Hodgkin’s Lymphoma Classification Project. Annals of Oncology. 2002;13:140–149. doi: 10.1093/annonc/mdf033. [DOI] [PubMed] [Google Scholar]
- Ryder J, Wang X, Bao L, Gross SA, Hua F, Irons RD. Aggressive natural killer cell leukemia: report of a Chinese series and review of the literature. International Journal of Hematology. 2007;85:18–25. doi: 10.1532/IJH97.A10612. [DOI] [PubMed] [Google Scholar]
- Savage KJ, Chhanabhai M, Gascoyne RD, Connors JM. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Annals of Oncology. 2004;15:1467–1475. doi: 10.1093/annonc/mdh392. [DOI] [PubMed] [Google Scholar]
- Savage KJ, Harris NL, Vose JM, Ullrich F, Jaffe ES, Connors JM, Rimsza L, Pileri SA, Chhanabhai M, Gascoyne RD, Armitage JO, Weisenburger DD, International PTC. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111:5496–5504. doi: 10.1182/blood-2008-01-134270. [DOI] [PubMed] [Google Scholar]
- Scott BL, Sandmaier BM, Storer B, Maris MB, Sorror ML, Maloney DG, Chauncey TR, Storb R, Deeg HJ. Myeloablative vs nonmyeloablative allogeneic transplantation for patients with myelodysplastic syndrome or acute myelogenous leukemia with multilineage dysplasia: a retrospective analysis. Leukemia. 2006;20:128–135. doi: 10.1038/sj.leu.2404010. [DOI] [PubMed] [Google Scholar]
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–87) British Journal of Haematology. 1991;79:428–437. doi: 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- Song SY, Kim WS, Ko YH, Kim K, Lee MH, Park K. Aggressive natural killer cell leukemia: clinical features and treatment outcome. Haematologica. 2002;87:1343–1345. [PubMed] [Google Scholar]
- Sorror ML, Maris MB, Storer B, Sandmaier BM, Diaconescu R, Flowers C, Maloney DG, Storb R. Comparing morbidity and mortality of HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative and myeloablative conditioning: influence of pretransplant comorbidities. Blood. 2004;104:961–968. doi: 10.1182/blood-2004-02-0545. [DOI] [PubMed] [Google Scholar]
- Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, Storer B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005a;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorror ML, Maris MB, Sandmaier BM, Storer BE, Stuart MJ, Hegenbart U, Agura E, Chauncey TR, Leis J, Pulsipher M, McSweeney P, Radich JP, Bredeson C, Bruno B, Langston A, Loken MR, Al-Ali H, Blume KG, Storb R, Maloney DG. Hematopoietic cell transplantation after nonmyeloablative conditioning for advanced chronic lymphocytic leukemia. Journal of Clinical Oncology. 2005b;23:3819–3829. doi: 10.1200/JCO.2005.04.569. [DOI] [PubMed] [Google Scholar]
- Sorror ML, Storer BE, Maloney DG, Sandmaier BM, Martin PJ, Storb R. Outcomes after allogeneic hematopoietic cell transplantation with nonmyeloablative or myeloablative regimens for treatment of lymphoma and chronic lymphocytic leukemia. Blood. 2008;111:446–452. doi: 10.1182/blood-2007-07-098483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Non-Hodgkin’s Lymphoma Classification Project. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- Tsai T, Goodman S, Saez R, Schiller G, Adkins D, Callander N, Wolff S, Freytes CO. Allogeneic bone marrow transplantation in patients who relapse after autologous transplantation. Bone Marrow Transplantation. 1997;20:859–863. doi: 10.1038/sj.bmt.1700989. [DOI] [PubMed] [Google Scholar]
- Vose JM, Peterson C, Bierman PJ, Weisenburger DD, Linder J, Harrington D, Vaughan WP, Kessinger A, Armitage JO. Comparison of high-dose therapy and autologous bone marrow transplantation for T-cell and B-cell non-Hodgkin’s lymphomas. Blood. 1990;76:424–431. [PubMed] [Google Scholar]