Abstract
Objective
Sjögren’s syndrome (SS) is a systemic autoimmune disease with a variety of presenting symptoms which may delay its diagnosis. We previously discovered a number of candidate salivary biomarkers for primary SS (pSS) using both mass spectrometry and expression microarray analysis (Arthritis Rheumatism, 2007;56(11):3588-3600). In this study, we aim to verify these candidate biomarkers in independent patient populations and to evaluate their predictive values for pSS detection.
Methods
In total, 34 patients with pSS, 34 patients with systemic lupus erythematosus (SLE) and 34 healthy individuals were enrolled for the validation studies. Salivary protein biomarkers were measured using either Western blotting or ELISA, and the mRNA biomarkers were measured using quantitative polymerase chain reaction (qPCR). Statistical analysis was performed using R2.9.
Results
Three protein biomarkers, cathepsin D, alpha-enolase and beta-2-microglobulin (B2M), and three mRNA biomarkers, myeloid cell nuclear differentiation antigen (MNDA), Guanylate binding protein 2 (GIP2) and low affinity IIIb receptor for the Fc fragment of IgG (FCGR3B), were significantly elevated in patients with pSS compared to both SLE patients and healthy controls. The combination of three protein biomarkers, cathepsin D, alpha-enolase and B2M, yielded a receiver operating characteristic (ROC) value of 0.99 in distinguishing pSS from healthy controls. The combination of protein biomarkers B2M and two mRNA biomarkers, MNDA and GIP2, reached an ROC of 0.95 in discriminating pSS from SLE.
Conclusion
We have successfully verified a panel of protein and mRNA biomarkers that can discriminate pSS from both SLE and healthy controls. If further validated in pSS patients and those with sicca symptoms but no autoimmune disease, these biomarkers may lead to a simple yet highly discriminatory clinical tool for diagnosis of pSS.
Introduction
Sjögren’s syndrome (SS) is a common autoimmune disease, with an estimated prevalence of 1~4 million patients in the US (1). The syndrome is characterized by progressive inflammation of the exocrine glands, in particular the salivary and lacrimal glands, frequently in combination with extraglandular manifestations. Histopathologically, expression of HLA-DR in glandular epithelial cells, lymphocytic infiltration of glandular tissue and sustained localized cytokine production is present (2). Consequently, patients with SS suffer from irreversible damage of salivary and lacrimal glands and loss of saliva and tear production (dry mouth and eyes). SS primarily affects women, with a ratio of 9:1 over the occurrence in men. The disease may occur alone as primary SS (pSS) or present as secondary SS (sSS), when it is associated with other autoimmune diseases such as rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE). Regarding treatment of SS, gain in knowledge regarding the immunopathogenesis has resulted in new strategies for therapeutic intervention, in particular with the B-lymphocyte depleting drug rituximab (3). Rituximab is a chimeric monoclonal antibody against the B-cell surface antigen CD20. Initial clinical trials suggested that rituximab is an effective agent in the treatment of SS and associated MALT lymphoma (4-10).
Diagnosing SS is complicated by the variety of presenting symptoms a patient may manifest, and the similarity between some symptoms from SS and those caused by other autoimmune disorders. In 2002, an international group reached consensus on a set of US-European criteria for SS classification (11). Classification of pSS requires four of six criteria, including a positive minor salivary gland biopsy or antibody to SS-A/SS-B. Blood tests can determine if a patient has high levels of anti-Ro/SSA and anti-La/SSB. Anti-La/SSB is more specific but its sensitivity is lower; anti-Ro/SSA is more sensitive but associated with various other autoimmune conditions (12). Classification of sSS requires an established connective-tissue disease and one sicca symptom plus two objective tests for dry mouth and eyes at the time of presentation. A minor salivary gland or parotid gland biopsy can reveal lymphocytes clustered around salivary glands, and damage to these glands due to inflammation, and therefore it is a specific diagnostic approach for SS. However, the procedure is invasive, time consuming, requires the evaluation from an expert histopathologist, and may have invertible sequelae (13).
By using mass spectrometry and expression microarray analysis, we previously discovered a set of saliva protein and mRNA biomarkers which are potentially valuable for pSS detection (14). The purpose of this study is to validate the discovered putative biomarkers in independent patient populations using immunoassays and quantitative real-time polymerase chain reaction (qPCR) and to evaluate their predictive values for pSS. Three proteins and three mRNA biomarkers were successfully verified, which may collectively provide a clinical approach for sensitive and specific detection of pSS.
Materials and Methods
Patient and control cohorts
In total, 34 pSS, 34 SLE and 34 healthy control subjects were recruited at the University Medical Center Groningen, the Netherlands, for this study. The three study groups (pSS, SLE and healthy control) were well matched for age, gender, and ethnicity. All the enrolled subjects were Caucasian women because pSS primarily affects women. The mean±SD age was 47 ± 15 years in the pSS patients (n = 34), 46 ± 15 years in the SLE patients (n=34), and 41 ± 9 years in the healthy control subjects (n = 34). Both University of California-Los Angeles and University Medical Center Groningen institutional review board (IRB) committees had approved the use of clinical samples for this project. All patients and controls had given informed consent. The information pertaining to the human samples was recorded in a manner that the subjects could not be identified, directly or through identifiers linked to the subjects. All the patients with pSS were diagnosed in strict accordance with the American-European Consensus Group Criteria for SS, which are commonly used by clinicians to diagnose SS in daily practice. All the patients with SLE were diagnosed in agreement with the current American College of Rheumatology clinical criteria for this disease. Healthy controls used no medication; neither suffered for oral and ocular dryness or had a history of salivary gland pathology. The demographics of patients and healthy controls is shown in Supplemental Table 1. Patients with SS were characterized as early stage (<4 years) or late stage (> 4 years) based on the disease duration since diagnosis (15).
Sample collection
Paraffin-stimulated whole saliva samples were collected from patients with primary SS, patients with SLE and control subjects for comparative analysis. Saliva sample collection was performed at the University Medical Center Groningen, using standardized saliva collection protocols (14). After collection, the saliva samples were immediately processed by centrifugation at 2,600g for 15 minutes at 4°C. The supernatant was removed from the pellet and separated for immediately protein or mRNA stabilization and stored at −80°C.
Immunoassays
Enzyme-linked immunosorbent assay (ELISA, Genway, San Diego, CA) was used to determine the level of beta-2-microglobulin (B2M) in saliva samples from pSS, SLE and control subjects (n=34 for each group). We used 20 μl of saliva sample from each subject and diluted it 10 times for ELISA (100 μl for each well) according to the manufacturer’s instruction manual. Samples were analyzed, in duplicate, and the protein levels were determined according to the calibration curves established from standards.
Western blotting was performed on the same set of saliva samples (n=34 for each of three groups) to measure levels of cathepsin D and alpha-enolase. Proteins (20 μl of each saliva sample) were separated on 12% NuPAGE gels (Invitrogen, Carlsbad, CA) at 150V and then transferred to a polyvinylidene difluoride membrane using an Invitrogen blot transfer cell. After saturating with 5% milk in Tris buffered saline-Tween buffer (overnight at 4°C), the blots were sequentially incubated for 2 hours at room temperature with primary antibodies and then with horseradish peroxidase-conjugated anti-mouse IgG secondary antibody (GE Healthcare, Piscataway, NJ). The bands were detected by enhanced chemiluminescence (Amersham) and quantified using Quantity One software (Bio-Rad, Hercules, CA).
Automatic RNA extraction from human saliva
Salivary RNA from 26 healthy controls, 25 pSS and 26 SLE cases was isolated. We were unable to extract enough amount of total mRNA from the rest cases for validation of mRNA biomarkers. For each sample, 330 μL of saliva supernatant was extracted with the MagMax™ Viral RNA Isolation Kit (Ambion, Austin, TX). This process was performed automatically using KingFisher mL technology (Thermo Fisher Scientific, Waltham, MA), followed by using TURBO™ DNase treatment (Ambion) to eliminate DNA contamination.
Real time quantitative RT-PCR (qPCR)
Eight candidate mRNA biomarkers were tested in patient and control samples by real-time qPCR (Table 1). These candidates were previously discovered using microarray profiling and preliminarily verified on the same set of samples (n=10 for pSS and n=10 for control). All primers used for qPCR were designed with the Primer3 program (http://www.genome.wi.mit.edu.) and synthesized by Sigma (St. Luis, MO). Amplicon lengths were around 100–130 bp for the outer primer pairs used in pre-amplification and 60–80 bp for the inner primer pairs used in qPCR analyses (16). After the salivary RNA was reverse-transcribed using reverse transcriptase and the specific outer primers, qPCR was carried out in reaction volumes of 10 μl using the SYBR-Green Master Mix (Applied Biosystems, Foster City, CA) for 15 min at 95°C for initial denaturing, followed by 40 cycles of 95°C for 30 sec and 60°C for 30 sec with the ABI 7900HT Fast Real Time PCR system (Applied Biosystems). The specificity of the PCR was confirmed according to the melting curve of each gene and the delta CT. The relative expression of each gene was calculated by the comparative CT method, and ΔCt of each biomarker was used for further analysis (14).
Table 1.
The list of candidate mRNA biomarkers
| Gene name | Full name |
|---|---|
| EGR1 | Early growth response 1 |
| B2M | Beta-2-microglobulin |
| BTG2 | BTG family, member 2 |
| GIP2 | Guanylate binding protein 2, interferon-inducible |
| MNDA | Myeloid cell nuclear differentiation antigen |
| FCGR3B | Low affinity IIIb receptor for the Fc fragment of IgG |
| TXNIP | Thioredoxin interacting protein |
| HLA-B | Major histocompatibility complex, class I, B |
Data analysis
Statistical software R was used for computing p values and the receiver operating characteristic (ROC) analysis. The plot area under the curve was computed via numerical integration of the ROC curves. The optimal cutoff point was determined to yield the maximum corresponding sensitivity and specificity. For the mRNA biomarkers, the ΔCt was converted by logistic regression and then the combination ROC of these biomarkers was calculated (17). The biomarker that has the highest AUC value was identified as having the strongest predictive power for detecting pSS. For the combination of protein and mRNA biomarkers, the ROC analysis was performed on the data obtained from 25 pSS, 26 SLE and 26 healthy control subjects.
Results
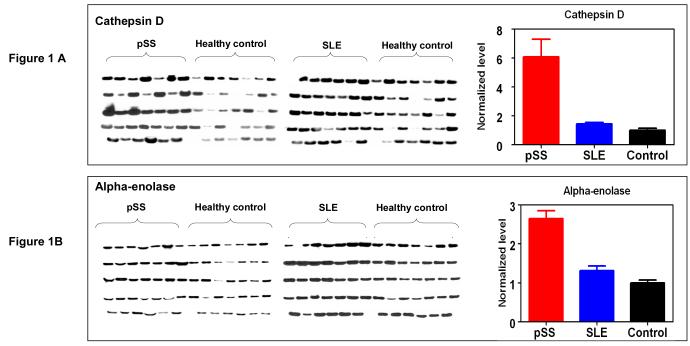
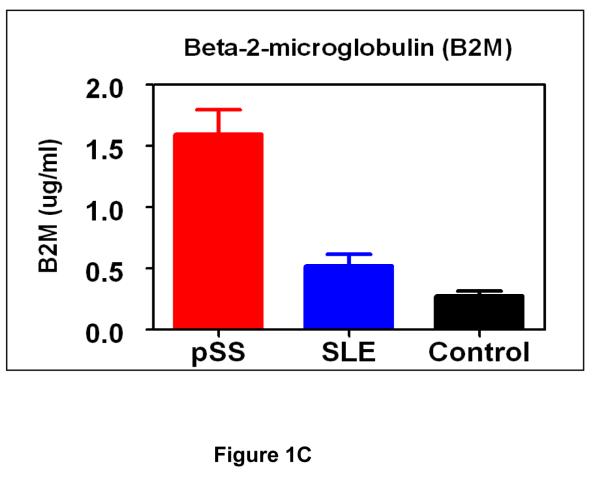
Three protein biomarkers, cathepsin D, alpha-enolase and B2M, were measured in an independent patient/control cohort (34 pSS, 34 SLE and 34 healthy control) to assess their values for pSS detection. These protein biomarkers were previously identified by using 2-D gel electrophoresis/mass spectrometry (2-DE/MS) for a comparative analysis of whole saliva samples from pSS and healthy control subject (14). Figure 1 presents the levels of cathepsin D, alpha-enolase and B2M among the three groups measured by immunoblotting or ELISA. Statistical analysis suggests these proteins are significantly over-expressed in pSS compared to both SLE and healthy control groups (Tables 2 & 3). This panel of protein biomarkers can distinguish pSS patients from healthy control individuals with an ROC of 0.99, sensitivity of 94% and specificity of 97% (n=34 for each group). When used for distinguishing pSS from the SLE group, the three biomarkers reached an ROC of 0.94, sensitivity of 92% and specificity of 88%.
Figure 1.
Validation of protein biomarkers, cathepsin D (A), alpha-enolase (B) and B2M (C), in independent patient (pSS) and control (SLE and healthy control) populations (n=34 for each group). B2M was validated by ELISA and mean±SEM is plotted. Cathepsin D and alpha-enolase were validated by western blotting, and the bar figures indicate the normalized levels of cathepsin D and alpha-enolase among three groups (mean±SEM).
Table 2.
Performance characteristics of salivary biomarkers for distinguishing pSS and healthy controls
| Biomarker | p-value (wilcoxon) | p-value (t-test) | ROC | Sensitivity | Specificity |
|---|---|---|---|---|---|
| B2M (protein) | 1.25E-10 | 1.87E-07 | 0.95 | 0.94 | 0.85 |
| Cathepsin (protein) | 5.33E-08 | 2.02E-04 | 0.88 | 0.76 | 0.88 |
| Enolase (protein) | 5.60E-05 | 5.76E-05 | 0.78 | 0.71 | 0.79 |
| MNDA (mRNA) | 1.49E-05 | 5.01E-04 | 0.84 | 0.88 | 0.77 |
| FCGR3B (mRNA) | 5.88E-05 | 7.19E-03 | 0.82 | 0.80 | 0.81 |
| GIP2 (mRNA) | 3.47E-03 | 6.52E-03 | 0.74 | 0.76 | 0.69 |
| B2M+Cathepsin | 0.99 | 1.00 | 0.92 | ||
| Enolase+B2M+Cathepsin | 0.99 | 0.94 | 0.97 | ||
| MNDA+FCGR3B+GIP2 | 0.86 | 0.88 | 0.81 |
Table 3.
Performance characteristics of salivary biomarkers for distinguishing pSS and SLE
| Biomarker | p value (wilcoxon) | p value (t-test) | ROC | sensitivity | specificity |
|---|---|---|---|---|---|
| B2M(protein) | 1.54E-08 | 1.53E-05 | 0.87 | 0.82 | 0.82 |
| Cathepsin (protein) | 2.54E-06 | 5.66E-04 | 0.82 | 0.76 | 0.88 |
| Enolase (protein) | 6.11E-02 | 6.96E-02 | 0.63 | 0.82 | 0.50 |
| MNDA (mRNA) | 2.23E-04 | 2.34E-04 | 0.79 | 0.92 | 0.65 |
| FCGR3BL (mRNA) | 1.23E-02 | 2.49E-03 | 0.70 | 0.92 | 0.65 |
| GIP2 (mRNA) | 6.11E-03 | 1.76E-03 | 0.72 | 0.80 | 0.73 |
| MNDA+B2M+GIP2 | 0.95 | 0.92 | 0.89 | ||
| Enolase+B2M+Cathepsin | 0.94 | 0.92 | 0.88 | ||
| MNDA+FCGR3B+GIP2 | 0.86 | 0.96 | 0.73 |
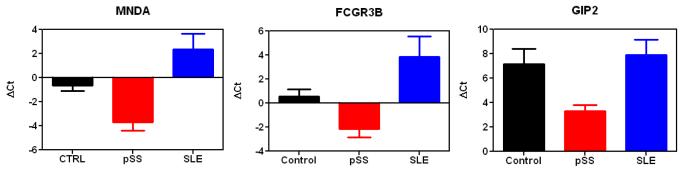
Eight candidate mRNA biomarkers were tested in 25 pSS, 26 SLE and 26 healthy control samples by real-time qPCR (Table 1). These candidates were previously discovered using microarray profiling and preliminarily verified on the same set of samples (n=10 for pSS and n=10 for control) (14). Three mRNA biomarkers, MNDA, FCGR3B and GIP2, were successfully validated and all significantly elevated in pSS patients (lower ΔCt values) compared to healthy controls and SLE patients (higher ΔCt values) (Figure 2), suggesting they are promising validated biomarkers for pSS detection (Tables 2 & 3).
Figure 2.
Validation of mRNA biomarkers, MNDA, FCGR3B, and GIP2, in pSS (n=25), SLE (n=26) and healthy control (n=26) samples. The bar figures indicate the normalized levels of mRNA biomarkers among three groups (mean±SEM). Note that the lower the Ct values, the higher the levels of the mRNA.
Discussion
In this study, we have successfully validated 3 salivary protein and 3 mRNA biomarkers, which can lead to highly sensitive and specific detection for pSS. These biomarkers not only distinguish pSS patients from a healthy control population but more importantly can also differentiate pSS from SLE patients (as an autoimmune disease control). Regarding the performance to discriminate pSS patients and healthy control subjects, the best biomarkers are B2M (ROC=0.95), cathepsin D (ROC=0.88) and MNDA (ROC=0.84). The same three biomarkers also exhibit the best performance to discriminate pSS from SLE patients (B2M, ROC=0.87; cathepsin D, ROC=0.82; MNDA, ROC=0.79). Combining multiple biomarkers indeed improves the sensitivity and specificity for pSS detection. For instance, the combination of B2M and cathepsin D yields an ROC value of 0.99, sensitivity of 100% and specificity of 92%, whereas the combination of all three protein biomarkers can reach an ROC of 0.99, sensitivity of 94% and specificity of 97% (pSS versus healthy control). In contrast, the combination of all three mRNA biomarkers reaches an ROC of 0.86, sensitivity of 88% and specificity of 81% (pSS versus healthy control), which is slightly better than individual mRNA biomarkers. Further addition of mRNA biomarkers to the protein biomarkers does not improve the overall performance (ROC, sensitivity and specificity) to discriminate pSS patients from healthy control subjects. Similarly, the combination of protein biomarkers significantly improves the sensitivity (92%) and specificity (88%) for differentiating pSS from SLE patients (ROC=0.94). Although the combination of mRNA biomarkers performs better than individual mRNA biomarkers, the overall performance appears lower than the combined protein biomarkers (pSS versus SLE). However, it should be noted that GIP2 and FCGR3B are highly sensitive biomarkers (92% sensitivity) in distinguishing pSS from SLE. The best panel of biomarkers for distinguishing pSS from SLE patients includes B2M, MNDA and GIP2 and yields an ROC of 0.95, sensitivity of 92% and specificity of 89%, which is slightly better than the combination of three protein biomarkers (ROC=0.94, sensitivity, 92%, specificity, 88%).
Although cathepsin D and alpha-enolase are novel protein biomarkers, B2M was previously detected in patients with pSS, and the level of salivary (but not serum) B2M was highly related to the salivary gland biopsy focus score (18). The value of salivary B2M was also evaluated for non-invasive confirmation of the diagnosis of SS (19). Besides protein biomarkers, we have also identified and validated novel mRNA biomarkers. As we discussed previously (14), one of the important findings from our initial microarray profiling is that many up-regulated genes in the saliva of pSS patients are involved in the IFN pathway, including the IFN-inducible gene, GIP2. This gene has a function in cell signaling and was reported to be up-regulated at the mRNA level in minor salivary glands from patients with pSS (20).
As a result of this study, we have successfully verified a panel of protein and mRNA biomarkers that are highly sensitive and specific for pSS detection. These biomarkers can discriminate pSS from SLE, which is an autoimmune disease with a similar immunopathologic background. Nevertheless, these markers may only apply to patients with residual salivary function, and it will be important to test if they can distinguish patients with primary SS from those with sicca symptoms but no autoimmune disease. Saliva diagnostics offers a combination of low cost, non-invasiveness, and easy sample collection/processing for disease detection. Testing of these biomarkers in saliva fluids will lead to a simple and non-invasive clinical tool for diagnosis of pSS. Verification of cathepsin D and alpha-enolase was based on Western blot analysis in this study. In reality, we would have to develop quantitative assays to measure the absolute levels of these protein biomarkers for diagnostic screening. Of note is that we are developing point-of-care saliva-based microfluidics-based platform for fast and sensitive measurement of these pSS biomarkers in saliva. This is a worthwhile approach as we recently demonstrated the potential of such a platform for multiplexed measurement of salivary protein and mRNA biomarkers (21). This may lead to a point-of-care diagnostic approach for pSS in a clinical setting.
Supplementary Material
Acknowledgements
This work was supported by the USPHS grant (RO1 DE017593). Shen Hu thanks the support from USPHS grants R21-CA122806 and R03-DE017144.
References
- 1.Fox RI. Sjögren’s syndrome. Lancet. 2005;366(9482):321–331. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 2.Nikolov NP, Illei GG. Pathogenesis of Sjögren’s syndrome. Curr Opin Rheumatol. 2009 doi: 10.1097/BOR.0b013e32832eba21. (1040-8711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meijer J, Pijpe J, Bootsma H, Vissink A, Kallenberg C. The future of biologic agents in the treatment of Sjögren’s syndrome. Clin Rev Allergy Immunol. 2007;32(3):292–297. doi: 10.1007/s12016-007-8005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pijpe J, Meijer JM, Bootsma H, Wal JEvd, Spijkervet FKL, Kallenberg CGM, et al. Clinical and histologic evidence of salivary gland restoration supports the efficacy of rituximab treatment in Sjögren’s syndrome. Arthritis Rheum. 2009;60(11):3251–3256. doi: 10.1002/art.24903. [DOI] [PubMed] [Google Scholar]
- 5.Meijer JM, Pijpe J, Vissink A, Kallenberg CGM, Bootsma H. Treatment of primary Sjögren syndrome with rituximab: extended follow-up, safety and efficacy of retreatment. Ann Rheum Dis. 2009;68(2):284–285. doi: 10.1136/ard.2008.092601. [DOI] [PubMed] [Google Scholar]
- 6.Pijpe J, Imhoff GWv, Spijkervet FKL, Roodenburg JLN, Wolbink GJ, Mansour K, et al. Rituximab treatment in patients with primary Sjögren’s syndrome: An open-label phase II study. Arthritis Rheum. 2005;52(9):2740–2750. doi: 10.1002/art.21260. [DOI] [PubMed] [Google Scholar]
- 7.Dass S, Bowman SJ, Vital EM, Ikeda K, Pease CT, Hamburger J, et al. Reduction of fatigue in Sjögren syndrome with rituximab: results of a randomised, double-blind, placebo-controlled pilot study. Ann Rheum Dis. 2008;67(11):1541–1544. doi: 10.1136/ard.2007.083865. [DOI] [PubMed] [Google Scholar]
- 8.Devauchelle-Pensec a, Pennec Y, Morvan J, Pers J-O, Daridon C, Jousse-Joulin S, et al. Improvement of Sjögren’s syndrome after two infusions of rituximab (anti-CD20) Arthritis Rheum. 2007;57(2):310–317. doi: 10.1002/art.22536. [DOI] [PubMed] [Google Scholar]
- 9.Gottenberg JE, Guillevin L, Lambotte O, Combe B, Allanore Y, Cantagrel A, et al. Tolerance and short term efficacy of rituximab in 43 patients with systemic autoimmune diseases. Ann Rheum Dis. 2005;64(6):913–920. doi: 10.1136/ard.2004.029694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meijer J, Meiners P, Vissink A, Spijkervet F, Abdulahad W, Kamminga N, et al. Effective rituximab treatment in primary Sjögren’s syndrome: a randomised, double-blind, placebo-controlled trial. Arthritis Rheum. 2009 doi: 10.1002/art.27314. in press. [DOI] [PubMed] [Google Scholar]
- 11.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61(6):554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franceschini F, Cavazzana I. Anti-Ro/SSA and La/SSB antibodies. Autoimmunity. 2005;38(1):55–63. doi: 10.1080/08916930400022954. [DOI] [PubMed] [Google Scholar]
- 13.Pijpe J, Kalk WWI, van der Wal JE, Vissink A, Kluin PM, Roodenburg JLN, et al. Parotid gland biopsy compared with labial biopsy in the diagnosis of patients with primary Sjogren’s syndrome. Rheumatol. 2007;46(2):335–341. doi: 10.1093/rheumatology/kel266. [DOI] [PubMed] [Google Scholar]
- 14.Hu S, Wang J, Meijer J, Ieong S, Xie Y, Yu T, et al. Salivary proteomic and genomic biomarkers for primary Sjögren’s syndrome. Arthritis Rheum. 2007;56(11):3588–3600. doi: 10.1002/art.22954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pijpe J, Kalk WW, Bootsma H, Spijkervet FK, Kallenberg CG, Vissink A. Progression of salivary gland dysfunction in patients with Sjogren’s syndrome. Ann Rheum Dis. 2007;66(1):107–12. doi: 10.1136/ard.2006.052647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Z, Zimmermann BG, Zhou H, Wang J, Henson BS, Yu W, et al. Exon-Level expression profiling: A comprehensive transcriptome analysis of oral fluids. Clin Chem. 2008;54(5):824–832. doi: 10.1373/clinchem.2007.096164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Etzioni R, Kooperberg C, Pepe M, Smith R, Gann PH. Combining biomarkers to detect disease with application to prostate cancer. Biostatistics. 2003;4(4):523–38. doi: 10.1093/biostatistics/4.4.523. [DOI] [PubMed] [Google Scholar]
- 18.Swaak A, Visch L, Zonneveld A. Diagnostic significance of salivary levels of beta 2-microglobulin in Sjögren’s syndrome. Clin Rheumatol. 1988;7(1):28–34. doi: 10.1007/BF02284053. [DOI] [PubMed] [Google Scholar]
- 19.Maddali BS, Campana G, D’Agata A, Palermo C, Bianucci G. The diagnosis value of beta 2-microglobulin and immunoglobulins in primary Sjögren’s syndrome. Clin Rheumatol. 1995;14(2):151–156. doi: 10.1007/BF02214934. [DOI] [PubMed] [Google Scholar]
- 20.Hjelmervik TO, Petersen K, Jonassen I, Jonsson R, Bolstad AI. Gene expression profiling of minor salivary glands clearly distinguishes primary Sjögren’s syndrome patients from healthy control subjects. Arthritis Rheum. 2005;52(5):1534–1544. doi: 10.1002/art.21006. [DOI] [PubMed] [Google Scholar]
- 21.Wei F, Patel P, Liao W, Chaudhry K, Zhang L, Arellano-Garcia M, et al. Electrochemical sensor for multiplex biomarkers detection. Clin Cancer Res. 2009;15(13):4446–4452. doi: 10.1158/1078-0432.CCR-09-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.