Abstract
Background
In the post intervention period of the Women’s Health Initiative (WHI) clinical trial, estrogen plus progestin increased total cancer incidence and an adverse influence on lung cancer mortality was suggested.
Methods
We conducted post hoc analyses over the full follow-up period of the WHI randomized, placebo-controlled clinical trial evaluating daily conjugated equine estrogen (CEE, 0.625 mg) plus medroxyprogesterone acetate (MPA, 2.5 mg) influence on lung cancer incidence and mortality in 16,608 postmenopausal women.
Findings
After 5.6 years intervention and 2.4 years additional follow-up (mean), there were 109 lung cancers in the hormone group and 85 in the placebo group (hazard ratio (HR) 1.23, 95% confidence interval (CI), 0.92, 1.63, P=0.16). While the difference was not statistically significant, for non-small cell lung cancer a possible divergence emerged over time, with more diagnoses in the CEE plus MPA group (96 vs 72 cases, respectively, HR 1.28, 95% CI 0.94, 1.73, P=0.12) and these cancers were more commonly poorly differentiated and more commonly had distant metastasis. Deaths from lung cancer were significantly increased in the CEE plus MPA group (73 vs 40 deaths, respectively, HR 1.71, 95% CI 1.16, 2.52, P=0.01) as were deaths from non-small cell lung cancer (62 vs 31 deaths, respectively, HR 1.87, 95% CI 1.22, 2.88, P=0.004). Small cell lung cancer incidence and mortality was comparable between randomization groups.
Interpretation
Use of estrogen plus progestin did not increase lung cancer incidence but significantly increased deaths from lung cancer. The effect may primarily be through influence on non-small cell lung cancer outcome.
Intervention in the Women’s Health Initiative (WHI) clinical trial of estrogen plus progestin ended when more risks than benefits were identified for hormone use. 1 Through 5.6 years mean follow-up, women in the combined hormone therapy group had higher risk of cardiovascular disease, coronary heart disease, stroke, venous thromboembolic, and breast cancer and lower risk of fractures and colorectal cancers with no adverse mortality influence. 2–8 After the intervention ended, during an additional 2.4 years (mean) follow-up, an apparent excess in mortality in the combined hormone therapy group along with a greater risk of cancers was seen in the post intervention period. The excess mortality was not explained by deaths associated with breast, colorectal, endometrial, or ovarian cancers, the protocol specified cancer outcomes and there were somewhat more lung cancer deaths seen. 9
In this regard, a substantial body of preclinical evidence supports a role for hormonal influence on lung cancer. 10, 11 Among other factors, estrogen receptors can be expressed in normal lung, 12 non-small cell lung cancer cell lines and lung cancers 12–14 and estradiol modulates proliferation gene expression and growth of non-small cell lung cancer cell lines. 13,15 Although clinical evidence is limited, higher estradiol levels has been associated with higher mortality in both men and women with advanced non-small cell lung cancer.16, 17
Our clinical findings in the WHI trial and the evidence suggesting estrogen may influence lung cancer led to the question of whether combined hormone therapy increases lung cancer mortality. To address this issue, we conducted analyses of the centrally adjudicated lung cancers diagnosed in the WHI trial of estrogen plus progestin over the entire follow-up period.
Methods
The WHI combined estrogen plus progestin clinical trial randomized 16,608 predominantly healthy postmenopausal women aged 50 to 79 years with no prior hysterectomy at 40 clinical centers from 1993 to 1998 as previously described. 1, 18 Women with prior breast cancer, anticipated survival less than 3 years or other prior cancer within the last 10 years except for non-melanoma skin cancer were excluded. Prior menopausal hormone therapy users had a 3 month wash out before entry. The trial was approved by institutional review boards at each clinical center and all participants provided written informed consent. Baseline characteristics were collected by interview (for hormone use) or by using standardized questionnaires for other variables including tobacco use.
Women were randomly assigned to receive daily conjugated equine estrogen (CEE, 0.625 mg) and medroxyprogesterone acetate (MPA, 2.5 mg) (Prempro ®, Wyeth Ayerst, Philadelphia, PA) or an identical appearing placebo. Study medications were discontinued after a breast cancer diagnosis, certain endometrial pathologies, venous thromboembolic events, malignant melanoma, substantially elevated triglycerides, or use of non-study estrogens or progestins or any use of androgen, tamoxifen or raloxifene. 18
Participants were contacted for clinical outcomes at 6 month intervals and had yearly clinic visits. The main study outcomes used for trial monitoring included coronary heart disease, invasive breast cancer, stroke, pulmonary embolism, colorectal cancer, endometrial cancer, hip fracture, and death. Initial self reports of outcomes (including lung cancers) were confirmed by physician adjudicators at the local clinic. All cases were subsequently adjudicated centrally using the Surveillance, Epidemiology and End Results coding system. Attribution of cause of death was also based on medical record review. All reviewers were blinded to randomization allocation.
The intervention was stopped after 5.6 years (mean, SD 1.3 years) when overall risks exceeded benefits for the main study outcomes. All participants were instructed to stop study pills on July 8, 2002 simultaneously with the publication of the initial results. 1 After the intervention ended participants continued to be followed for limited data collection and health outcomes on the same schedule in the post-intervention phase. The originally specified trial completion date of March 31, 2005 was the end date for outcomes included in this report. The flow of participants in this trial throughout the study period has been previously described in detail. 9
The described analyses were not protocol pre-specified as lung cancer was not a predefined study outcome of the WHI estrogen plus progestin clinical trial. Chest radiology or other chest imaging was not protocol mandated either at entry or serially. Medical decisions regarding workup of chest findings were directed by community physicians. Detailed lung cancer analyses on centrally adjudicated cases have not been previously reported. Based on literature review and emerging information from this trial a prospective, written analyses plan was developed and reviewed and approved by the WHI Publications and Presentations Committee on September 4, 2008. The major objective of the analysis plan was to determine the influence of combined hormone therapy use on lung cancer mortality.
Role of the Funding Source
The National Institutes of Health had input into the design and conduct of the study and participated in the review of this article but did not participate in the manuscript’s preparation. The corresponding author (RTC) has full access to the data and made the final decision where to submit the paper for publication.
Statistical Analysis
Baseline characteristics of participants were compared between randomization groups using chi square tests of association. Age at menopause was defined as previously described; in most cases by the last age of any menstrual bleeding, date of bilateral oophorectomy or when menopausal hormone therapy began. 19 At baseline, participants were classified as “never”, “past”, or “current’ smokers based on whether they had ever smoked more than 100 cigarettes in their lifetime and if they were smoking at the present time.
Results of CEE plus MPA influence on overall, small cell lung cancer and non-small lung cancer incidence and mortality were assessed using time-to-event methods, based on the intent-to-treat principle. Hazard ratios (HRs) and 95% confidence intervals (CIs) are reported from Cox proportional hazards regression analyses stratified by age, prior lung cancer and dietary modification trial randomization assignment. Event times are defined relative to date of randomization. 20 P values from Wald Z statistics are reported from Cox proportional hazards regression analyses stratified by age and randomization status in the dietary modification trial of the WHI. Kaplan-Meier method plots describe lung cancer event rates by time from randomization for incidence and mortality and lung cancer survival by time since diagnosis. Parallel analyses were performed on the influence of CEE plus MPA on incidence and mortality of small cell lung cancer and non-small cell lung cancer separately.
Subgroup analyses: based on the strong relationship between smoking and lung cancer, analyses were conducted separately for women based on smoking status (never smoker, past smoker, current smoker) at study entry. Four other subgroup comparisons were examined, less than one would be expected to be significant at the 0.05 level by chance alone. Potential interactions between baseline characteristics and randomization group were assessed in Cox proportional hazards regression models that included the risk factor in question and randomization group as main effects. P-values for assessing interactions are from Wald chi-square tests. SAS for Windows, version 9.1.3 (SAS Institute Inc., Cary, NC) and S-Plus for Windows, version 8.0 (Insightful Corp.) were used for all analyses.
Results
Baseline clinical and demographic characteristics were comparable in the two randomization groups including prior hormonal exposure, age, education, self-reported health and race/ethnicity. Tobacco exposure was also closely comparable with about 50% never smokers, 40% past smokers and 10% current smokers in each group. Also balanced were cigarettes per day and years smoked (Table 1).
Table 1.
Descriptive Characteristics by Randomization Group
| CEE+MPA | Placebo | |||
|---|---|---|---|---|
| N | % | N | % | |
| Number participants randomized | 85061 | 100.0 | 8102 | 100.0 |
| Age at screening, y | ||||
| 50–59 | 2837 | 33.4 | 2683 | 33.1 |
| 60–69 | 3854 | 45.3 | 3655 | 45.1 |
| 70–79 | 1815 | 21.3 | 1764 | 21.8 |
| Race/ethnicity | ||||
| White | 7141 | 84.0 | 6805 | 84.0 |
| Black | 548 | 6.4 | 574 | 7.1 |
| Hispanic | 471 | 5.5 | 415 | 5.1 |
| American Indian | 25 | 0.3 | 30 | 0.4 |
| Asian/Pacific Islander | 194 | 2.3 | 169 | 2.1 |
| Unknown | 127 | 1.5 | 109 | 1.3 |
| Hormone use | ||||
| Never | 6277 | 73.8 | 6022 | 74.4 |
| Past | 1671 | 19.7 | 1587 | 19.6 |
| Current2 | 554 | 6.5 | 490 | 6.1 |
| Duration of prior hormone use, y | ||||
| No prior use | 6277 | 73.8 | 6022 | 74.4 |
| <5 | 1534 | 18.0 | 1467 | 18.1 |
| 5–10 | 427 | 5.0 | 355 | 4.4 |
| ± 10 | 263 | 3.1 | 255 | 3.1 |
| Oral contraceptive use ever | 3695 | 43.4 | 3447 | 42.5 |
| Tobacco exposure | ||||
| Smoking Status | ||||
| Never | 4178 | 49.6 | 3999 | 50.0 |
| Past | 3362 | 39.9 | 3157 | 39.5 |
| Current | 880 | 10.5 | 838 | 10.5 |
| Cigarettes/day3 | ||||
| <25 | 3345 | 81.6 | 3175 | 82.0 |
| 25+ | 752 | 18.4 | 698 | 18.0 |
| Years Smoked, y | ||||
| <30 | 2563 | 62.4 | 2422 | 61.9 |
| 30+ | 1546 | 37.6 | 1490 | 38.1 |
| Pack years of smoking | ||||
| Never-smoker | 4178 | 50.8 | 3999 | 51.1 |
| <5 | 1119 | 13.6 | 1004 | 12.8 |
| 5–<20 | 1168 | 14.2 | 1140 | 14.6 |
| ≥20 | 1763 | 21.4 | 1679 | 21.5 |
| General health status | ||||
| Excellent | 1593 | 18.8 | 1513 | 18.8 |
| Very good | 3678 | 43.5 | 3539 | 44.0 |
| Good | 2656 | 31.4 | 2441 | 30.3 |
| Fair | 497 | 5.9 | 528 | 6.6 |
| History of lung cancer 4 | 3 | <0.1 | 2 | <0.1 |
| Prior breast biopsy | ||||
| No | 6340 | 83.6 | 6278 | 83.3 |
| Yes, 1 biopsy | 956 | 12.6 | 972 | 12.9 |
| Yes, ≥ 2 biopsies | 290 | 3.8 | 288 | 3.8 |
| Physical activity, metabolic equivalents (METS), wk | ||||
| None | 1427 | 18.6 | 1356 | 17.9 |
| > 0–3.75 | 1501 | 19.6 | 1519 | 20.0 |
| > 3.75–8.75 | 1356 | 17.7 | 1352 | 17.8 |
| > 8.75–17.5 | 1648 | 21.5 | 1634 | 21.5 |
| > 17.5 | 1739 | 22.7 | 1735 | 22.8 |
P-values from chi-squared tests of association demonstrated no significant differences for descriptive characteristics between randomization group.
Because of rounding, percentages may not all total 100.
Includes 331 women previously randomized to an estrogen alone group who were reassigned to the estrogen plus progestin group following a protocol change as previously described.
Three months “wash out” required before entry
Current and previous smokers were combined when estimating the total number of cigarettes/day, years smoked, and past years of smoking.
Lung cancer diagnosed > 10 years previously.
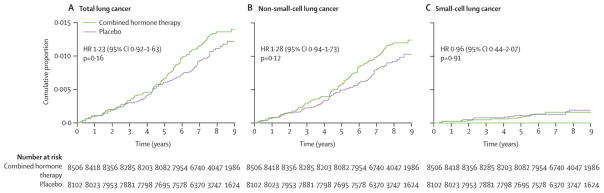
In intent-to-treat analyses considering all 16,608 participants over the entire study period, there were somewhat more lung cancers diagnosed in the combined hormone therapy group but the difference was not statistically significant (109 vs 85 cases, respectively (HR) 1.23 95% CI 0.92, 1.63, P=0.16) (Table 2, Figure 1). The influence of CEE plus MPA on non-small cell lung cancer incidence also was not statistically significant. However, a possible divergence emerged after about five years of intervention with more diagnoses in the CEE plus MPA group (96 vs 72 cases, respectively, HR 1.28 95% CI 0.94, 1.73, P=0.12) (Figure 1). The non-small cell lung cancer cases were generally of similar histology in the two randomization groups. Use of CEE plus MPA was associated with higher incidence rates for non-small cell lung cancers with distant metastases (HR 1.71; 95% CI: 1.02–2.88) and for poorly differentiated tumors (HR 2.01, 95% CI: 1.06–3.81) (Table 2). Only 26 small cell lung cancers were diagnosed in this trial. Combined hormone therapy had no effect on small cell lung cancer incidence (Table 2, Figure 1).
Table 2.
Lung Cancer Incidence (Annualized %) and Characteristics by Randomization Group
| CEE plus MPA | Placebo | HR1 | (95% CI) | P-value | |||
|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | ||||
| Lung cancer Incidence | 109 | (0.16%) | 85 | (0.13%) | 1.23 | (0.92, 1.63) | 0.16 |
| Non-small cell lung cancer (NSCLC) | 96 | (0.14%) | 72 | (0.11%) | 1.28 | (0.94, 1.73) | 0.12 |
| Small cell lung cancer (SCLC) | 13 | (0.02%) | 13 | (0.02%) | 0.96 | (0.44, 2.07) | 0.91 |
| Non-small cell lung cancer histology | |||||||
| Adenocarcinoma | 46 | (0.07%) | 36 | (0.06%) | 1.23 | (0.80, 1.91) | 0.35 |
| Squamous cell | 14 | (0.02%) | 17 | (0.03%) | 0.78 | (0.39, 1.59) | 0.50 |
| Large cell/neuroendocrine | 8 | (0.01%) | 6 | (0.01%) | 1.34 | (0.46, 3.85) | 0.59 |
| Unspecified | 28 | (0.04%) | 13 | (0.02%) | 2.00 | (1.04, 3.87) | 0.04 |
| Non-small cell lung cancer stage | |||||||
| Local | 17 | (0.03%) | 18 | (0.03%) | 0.91 | (0.47, 1.77) | 0.78 |
| Regional | 21 | (0.03%) | 18 | (0.03%) | 1.12 | (0.60, 2.10) | 0.72 |
| Distant metastases | 40 | (0.06%) | 22 | (0.03%) | 1.71 | (1.02, 2.88) | 0.04 |
| Non-small cell lung cancer grade | |||||||
| Well differentiated | 8 | (0.01%) | 6 | (0.01%) | 1.34 | (0.46, 3.86) | 0.59 |
| Moderately differentiated | 14 | (0.02%) | 17 | (0.03%) | 0.77 | (0.38, 1.57) | 0.48 |
| Poorly differentiated | 29 | (0.04%) | 14 | (0.02%) | 2.01 | (1.06, 3.81) | 0.03 |
| Anaplastic | 3 | (<0.01%) | 4 | (0.01%) | 0.69 | (0.15, 3.09) | 0.63 |
Small cell lung cancer characteristics not included given the limited number of total cases.
HRs, 95% CIs and p-values are from Cox proportional hazards models stratified according to age, prior lung cancer, and DM trial randomization assignment.
Figure 1.
Kaplan-Meier cumulative hazards for incidence from lung cancer by category, randomization group and by time in the trial. Hazard ratios (HR), 95% confidence interval (CI), and P-values are from Cox proportional hazards regression models, stratified by age and dietary modification randomization group.
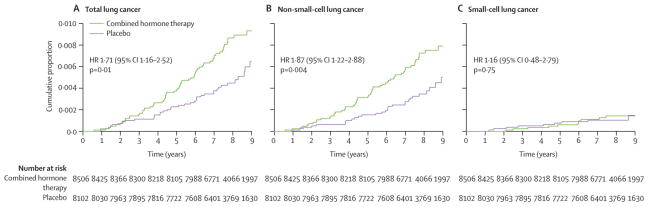
For women in the combined hormone therapy group there was a statistically significant, adverse influence of CEE plus MPA use on deaths from lung cancer (deaths attributed to lung cancer) (73 vs 40 deaths, respectively, HR 1.71, 95% CI 1.16, 2.52, P=0.01) (Table 3, Figure 2). Considering all deaths which occurred following a lung cancer diagnosis, the cause of death was directly attributed to lung cancer by chart review in 73 of 78 cases (94%) in the CEE plus MPA group and 40 of 49 cases (82%) in the placebo group. Other deaths were attributed to cardiovascular disease (2 placebo, 2 hormone), unknown other causes (4 placebo, 1 hormone), cerebrovascular events (2 hormone) and colon cancer (1 placebo). A similar pattern regarding hormonal influence on lung cancer deaths was seen when all deaths following a lung cancer diagnosis are considered (78 vs 49 deaths in hormone group vs placebo, respectively, HR 1.50, 95% CI 1.05, 2.14, P=0.03). Deaths from lung cancer could account for 43% of the excess in total deaths seen in the post intervention period for women in the combined hormone therapy group (37 excess total deaths and 16 excess deaths from lung cancer).
Table 3.
Lung Cancer Mortality (Annualized %) by Randomization Group
| CEE plus MPA | Placebo | HR1 | (95% CI) | P-value | |||
|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | ||||
| Death from lung cancer2 | 73 | (0.11%) | 40 | (0.06%) | 1.71 | (1.16, 2.52) | 0.01 |
| Death from non-small cell lung cancer | 62 | (0.09%) | 31 | (0.05%) | 1.87 | (1.22, 2.88) | 0.004 |
| Death from small cell lung cancer | 11 | (0.02%) | 9 | (0.01%) | 1.16 | (0.48, 2.79) | 0.75 |
| Death after lung cancer diagnosis3 | 78 | (0.12%) | 49 | (0.08%) | 1.50 | (1.05, 2.14) | 0.03 |
| Death after non-small cell lung cancer | 67 | (0.10%) | 39 | (0.06%) | 1.61 | (1.09, 2.39) | 0.02 |
| Death after small cell lung cancer | 11 | (0.02%) | 10 | (0.02%) | 1.04 | (0.44, 2.46) | 0.92 |
HRs, 95% CIs and p-values are from Cox proportional hazards models stratified according to age, prior lung cancer, and DM trial randomization assignment.
Includes deaths after lung cancer diagnosis attributed to lung cancer
Follow-up starts at randomization and denominator includes all participants; includes all deaths after lung cancer diagnosis regardless of attributed etiology.
Figure 2.
Kaplan-Meier cumulative hazard for death from lung cancer by category, randomization group and time in the trial. Hazard ratios (HR), 95% confidence interval (CI), and P-values are from Cox proportional hazards regression models, stratified by age and dietary modification randomization group.
Use of CEE plus MPA significantly increased deaths from non-small cell lung cancer (deaths attributed to non-small cell lung cancer) with 62 vs 31 deaths, respectively HR 1.87, 95% CI 1.22, 2.88, P=0.004 (Table 3, Figure 2). Results were similar when all deaths after a non-small cell lung diagnosis are considered (67 vs 39 deaths, respectively for CEE plus MPA vs placebo, HR 1.59, 95% CI 1.03, 2.46, P=0.04). In an exploratory analysis, mortality after a non-small cell lung cancer diagnosis was significantly higher for women in the combined hormone therapy group (9.4 vs 16.1 months median survival and 70% vs 54% mortality after 4 years from diagnosis for CEE plus MPA vs placebo groups, respectively, HR 1.59, 95% CI 1.03, 2.46, P=0.04). There was no effect of combined hormone therapy on deaths from small cell lung cancer (Table 3, Figure 2).
Of subgroup comparisons, no significant interaction was seen among randomization group, death from lung cancer and the variables: age at screening, years since menopause, prior hormone therapy use, prior estrogen plus progestin use or smoking status (Table 4). The absolute increase in risk of death from lung cancer for never smokers in the CEE plus MPA compared to placebo groups was extremely low (9 deaths, cumulative risk 0.2% vs 4 deaths, cumulative risk, 0.1%, respectively, HR 2.11 95% CI 0.65–6.84). However, the absolute risk of lung cancer death was substantially higher in women who entered as current smokers (30 deaths, cumulative risk 3.4% vs 20 deaths, cumulative risk 2.4% for CEE plus MPA vs placebo groups, respectively, HR 1.46 95% CI 0.83,2.57) (Table 4). Past smokers had an intermediate increase in risk with combined hormone use (32 deaths, cumulative risk 1.0% vs 16 deaths, cumulative risk 0.5%, respectively, HR 1.81 95% CI 0.99, 3.30). The relative hazards were all elevated regardless of smoking category, although none were individually statistically significant (Table 4).
Table 4.
Death from Lung Cancer (Cumulative %) by Randomization Group and Selected Baseline Characteristics
| CEE+MPA | Placebo | HR | (95% CI) | P-value for Interaction | |||
|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | ||||
|
Entire WHI Study Period | |||||||
| Age at screening, y | 0.23 | ||||||
| 50–59 | 13 | (0.46%) | 12 | (0.45%) | 1.02 | (0.47, 2.24) | |
| 60–69 | 35 | (0.91%) | 16 | (0.44%) | 2.00 | (1.11, 3.62) | |
| 70–79 | 25 | (1.38%) | 12 | (0.68%) | 2.00 | (1.01, 3.99) | |
| Prior hormone use | 0.65 | ||||||
| Never | 56 | (0.89%) | 29 | (0.48%) | 1.83 | (1.17, 2.86) | |
| Past | 14 | (0.84%) | 8 | (0.50%) | 1.53 | (0.64, 3.69) | |
| Current | 3 | (0.54%) | 3 | (0.61%) | 1.07 | (0.22, 5.34) | |
| Prior E+P use | 0.54 | ||||||
| Never | 65 | (0.93%) | 33 | (0.49%) | 1.86 | (1.22, 2.82) | |
| Past | 5 | (0.49%) | 4 | (0.42%) | 0.89 | (0.22, 3.61) | |
| Current | 3 | (0.60%) | 3 | (0.68%) | 1.10 | (0.22, 5.46) | |
| Smoking | 0.77 | ||||||
| Never | 9 | (0.22%) | 4 | (0.10%) | 2.09 | (0.64, 6.81) | |
| Past | 32 | (0.95%) | 16 | (0.51%) | 1.89 | (1.04, 3.45) | |
| Current | 30 | (3.41%) | 20 | (2.39%) | 1.50 | (0.85, 2.64) | |
Cumulative % = % cumulative risk for death from lung cancer over entire 7.9 year (mean) study period HRs, 95% CIs and p-values are from Cox proportional hazards models stratified according to age, prior lung cancer, and DM trial randomization assignment.
P-value is from a Wald Chi-square test for the interaction between the given characteristic and E+P trial randomization arm.
Discussion
In secondary analyses of a randomized, placebo-controlled clinical trial, use of CEE plus MPA did not increase lung cancer incidence but significantly increased deaths from lung cancer, the most common cause of cancer death in women. 21 The evidence was most consistent with combined hormone therapy influencing primarily non-small cell lung cancer with the absolute increase in risk of death from this disease of special relevance to women already at elevated risk due to smoking.
In the initial report from the trial, no effect of combined hormone therapy on lung cancer incidence was seen during the intervention phase based on the distribution of 104 lung cancer cases. 1 Now with additional follow-up and central coding, a total of 194 incident lung cancer cases have been identified and hormonal influences on lung cancer mortality and separate influence on non-small cell lung cancer and small cell lung cancer incidence and mortality can be described.
Considering the entire study duration, no effect of CEE plus MPA on small cell lung cancer incidence or mortality was seen. Given the few cases seen, this result should be cautiously interpreted. For non-small cell lung cancer, a possible divergence in the incidence curve emerged after several years on trial with non-small cell lung cancers diagnosed more frequently in the CEE plus MPA group. In addition, there were significantly more poorly differentiated cancers and cancers with metastatic spread diagnosed in the CEE plus MPA group. These findings, together with the significant increase in deaths after a non-small cell lung cancer diagnosis, suggest combined hormone therapy’s major influence may be on stimulating growth of already established non-small cell lung cancers.
Considering all participants, risk of death from lung cancer was significantly higher for women in the CEE plus MPA group. The estimated effect of CEE plus MPA use on death from lung cancer was not modified by smoking status (P for interaction = 0.78). Hence, among never smokers, where the absolute risk is very low, the attributable risk for CEE plus MPA was similarly small. However, among women with elevated risk associated with smoking, the number of lung cancer deaths attributable to CEE plus MPA rises substantially. Based on these findings, women at elevated risk for lung cancer from smoking should be made aware of this additional hazard if considering beginning or continuing combined hormone therapy.
Precise allocation of the cause of death following a lung cancer diagnosis by medical record review is challenging given the common underlying co-morbidities and potential for interaction with cancer therapies. Nonetheless, the vast majority of deaths after lung cancer in this trial were attributed to lung cancer. In addition, we report comparable findings regarding total lung cancer mortality and non-small cell lung cancer mortality regardless of attributed cause of death. The current findings provide a plausible explanation for at least part of the trend for an increase in overall cancer mortality seen in the combined hormone therapy group during the postintervention period of the WHI clinical trial of estrogen plus progestin. 9
Strong evidence suggests estrogen simulates angiogenesis 22, 23 and increased angiogenesis is linked to tumor metastatic potential in both breast and lung cancer. 24, 25, 26 In addition, angiogenesis inhibition has proven an effective intervention strategy in both diseases. 27, 28 Thus, the stimulatory influence of estrogen on angiogenesis provides one potential mechanism for a differential mortality effect on lung cancer by facilitating early metastatic spread of established lung cancers. As combined hormone therapy had been shown to interfere with mammographic breast imaging, 2, 29 alternatively, a delay in lung cancer diagnosis through interference with imaging detection of lesions limited to the lung could be considered. The significant increase in lung cancers diagnosed with distant metastases in the combined hormone group in this report is consistent with either potential mechanism.
Gender differences in lung cancer outcome suggest a role for hormonal influences. Women have higher survival rates then men following lung cancer diagnoses 30–32 with most reports focusing on non-small cell lung cancer outcomes. While only providing indirect evidence of a role of estrogen based on the assumption that age can be a surrogate for menopausal status, multivariate analyses of pooled data from two large non-small cell lung cancer clinical trial databases have reported that older women (> 60 years old) had a survival advantage compared to younger women, but a similar age effect was not seen in men. 16, 33 More direct evidence comes from studies in men and women being treated for advanced non-small cell lung cancer where high estradiol levels were associated with higher mortality risk. 16,17 Based on such results, non-small cell lung cancer treatment strategies targeting estrogen receptor expression have been proposed. 11 The current clinical findings provide support for such efforts.
Prior observational studies on postmenopausal hormone therapy and lung cancer incidence have been mixed with some showing lower risk, 14, 34, 35 and others no influence 36, 37 and higher risk 38, 39 associated with hormone use. For influence on lung cancer mortality, only two retrospective studies have been reported. In one, women diagnosed while using unspecified menopausal hormone therapy had significantly shorter survival than non-users. 40 Lung cancer survival was not associated with menopausal hormone therapy use in a second report. 41 Such observational studies have not provided details of the hormone therapy regimen and do not commonly separate findings for small cell lung cancer and non-small cell lung cancer. Future studies should specify category of therapy used (combined estrogen plus progestin vs estrogen alone), duration of use and category of impacted disease (non-small cell lung cancer vs small cell lung cancer).
The Heart and Estrogen/Progestin Replacement Study (HERS) is a randomized clinical trial that entered women at risk for or with prior coronary heart disease and evaluated the same hormone therapy used in the WHI trial of estrogen plus progestin. In HERS, with fewer participants and somewhat shorter follow-up, more lung cancers were seen in the hormone group (37 vs 27 cases, respectively HR 1.39 95% CI 0.84, 2.28) but the difference was not statistically significant. 42
Strengths of the study include the randomized double-blind study design, the large ethnically diverse study population, the balanced tobacco exposure, and the central adjudication of the lung cancer outcomes. Limitations include the modest number of lung cancers, the absence of information on therapy after diagnosis and the increased possibility of a chance finding based on the nature of post hoc analyses. This trial evaluated one regimen given for about five and a half-years and cannot provide information on other oral or topical hormone therapies or other durations of use. Similar analyses on lung cancer will be conducted in a parallel Women’s Health Initiative trial evaluating CEE alone in women with prior hysterectomy.
In summary, in a post hoc analysis in a randomized clinical trial, use of CEE plus MPA did not significantly increase the incidence of lung cancer but was associated with a significantly increased risk of death from lung cancer in postmenopausal women. The effect may primarily be through influence on non-small cell lung cancer outcome. Postmenopausal women, especially those at elevated baseline risk of lung cancer, such as current smokers or long term past smokers, should consider this additional hazard before beginning, or continuing, combined menopausal hormone therapy.
Acknowledgments
We gratefully acknowledge the dedicated efforts of investigators and staff at the WHI clinical centers, the WHI Clinical Coordinating Center, and the NHLBI program office (listing available at http://www.whi.org). Most importantly, we recognize the WHI participants for their extraordinary commitment to the WHI program.
Funding/Support: The Women’s Health Initiative program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.
The following investigators participated in the Women’s Health Initiative studies
Program Office
(National Heart, Lung, and Blood Institute, Bethesda, Maryland) Elizabeth Nabel, Jacques Rossouw, Shari Ludlam, Joan McGowan, Nancy Geller, Leslie Ford.
Clinical Coordinating Center
(Fred Hutchinson Cancer Research Center, Seattle, WA) Ross Prentice, Garnet Anderson, Andrea LaCroix, Ruth Patterson, Anne McTiernan, Barbara Cochrane, Julie Hunt, Lesley Tinker, Charles Kooperberg, Martin McIntosh, C. Y. Wang, Chu Chen, Deborah Bowen, Alan Kristal, Janet Stanford, Nicole Urban, Noel Weiss, Emily White; (Medical Research Laboratories, Highland Heights, KY) Evan Stein, Peter Laskarzewski; (San Francisco Coordinating Center, San Francisco, CA) Steven R. Cummings, Michael Nevitt, Lisa Palermo; (University of Minnesota, Minneapolis, MN) Lisa Harnack; (Fisher BioServices, Rockville, MD) Frank Cammarata, Steve Lindenfelser; (University of Washington, Seattle, WA) Bruce Psaty, Susan Heckbert.
Clinical Centers
(Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller, William Frishman, Judith Wylie-Rosett, David Barad, Ruth Freeman; (Baylor College of Medicine, Houston, TX) Aleksandar Rajkovic, Jennifer Hays, Ronald Young, Haleh Sangi-Haghpeykar; (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson, Kathryn M. Rexrode, Brian Walsh, J. Michael Gaziano, Maria Bueche; (Brown University, Providence, RI) Charles B. Eaton, Michele Cyr, Gretchen Sloane; (Emory University, Atlanta, GA) Lawrence Phillips, Vicki Butler, Vivian Porter; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley A.A. Beresford, Vicky M. Taylor, Nancy F. Woods, Maureen Henderson, Robyn Andersen; (George Washington University, Washington, DC) Lisa Martin, Judith Hsia, Nancy Gaba, Richard Katz; (Los Angeles Biomedical Research Institute at Harbor-UCLA Research and Education Institute, Torrance, CA) Rowan Chlebowski, Robert Detrano, Anita Nelson, Michele Geller; (Kaiser Permanente Center for Health Research, Portland, OR) Yvonne Michael, Evelyn Whitlock, Victor Stevens, Njeri Karanja; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan, Stephen Sidney, Geri Bailey Jane Hirata; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen, Vanessa Barnabei, Theodore A. Kotchen, Mary Ann C. Gilligan, Joan Neuner; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard, Lucile Adams-Campbell, Lawrence Lessin, Cheryl Iglesia, Linda K Mickel; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn, Philip Greenland, Janardan Khandekar, Kiang Liu, Carol Rosenberg; (Rush University Medical Center, Chicago, IL) Henry Black, Lynda Powell, Ellen Mason; Martha Gulati; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick, Mark A. Hlatky, Bertha Chen, Randall S. Stafford, Sally Mackey; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane, Iris Granek, William Lawson, Catherine Messina, Gabriel San Roman; (The Ohio State University, Columbus, OH) Rebecca Jackson, Randall Harris, Electra Paskett, W. Jerry Mysiw, Michael Blumenfeld; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis, Albert Oberman, James M. Shikany, Monika Safford; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A Thomson, Tamsen Bassford, Cheryl Ritenbaugh, Zhao Chen, Marcia Ko; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende, Maurizio Trevisan, Ellen Smit, Susan Graham, June Chang; (University of California at Davis, Sacramento, CA) John Robbins, S. Yasmeen; (University of California at Irvine, CA) F. Allan Hubbell, Gail Frank, Nathan Wong, Nancy Greep, Bradley Monk; (University of California at Los Angeles, Los Angeles, CA) Lauren Nathan, David Heber, Robert Elashoff, Simin Liu; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer, Michael H. Criqui, Gregory T. Talavera, Cedric F. Garland, Matthew A. Allison; (University of Cincinnati, Cincinnati, OH) Margery Gass, Nelson Watts; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher, Michael Perri, Andrew Kaunitz, R. Stan Williams, Yvonne Brinson; (University of Hawaii, Honolulu, HI) J. David Curb, Helen Petrovitch, Beatriz Rodriguez, Kamal Masaki, Patricia Blanchette; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace, James Torner, Susan Johnson, Linda Snetselaar, Jennifer Robinson; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene, Milagros Rosal, Ira Ockene, Robert Yood, Patricia Aronson; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser, Baljinder Singh, Vera Lasser, John Kostis, Peter McGovern; (University of Miami, Miami, FL) Mary Jo O’Sullivan, Linda Parker, JoNell Potter, Diann Fernandez, Pat Caralis; (University of Minnesota, Minneapolis, MN) Karen L. Margolis, Richard H. Grimm, Mary F. Perron, Cynthia Bjerk, Sarah Kempainen; (University of Nevada, Reno, NV) Robert Brunner, William Graettinger, Vicki Oujevolk, Michael Bloch; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss, Pamela Haines, David Ontjes, Carla Sueta, Ellen Wells; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller, Jane Cauley, N. Carole Milas; (University of Tennessee Health Science Center, Memphis, TN) Karen C. Johnson, Suzanne Satterfield, Rongling Li, Stephanie Connelly, Fran Tylavsky; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski, Robert Schenken; (University of Wisconsin, Madison, WI) Gloria E. Sarto, Douglas Laube, Patrick McBride, Julie Mares, Barbara Loevinger; (Wake Forest University School of Medicine, Winston-Salem, NC) Mara Vitolins, Greg Burke, Robin Crouse, Scott Washburn; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Michael Simon.
Former Principal Investigators and Project Officers
(Baylor College of Medicine, Houston, TX) Jennifer Hays, John Foreyt; (Brown University, Providence, RI) Annlouise R. Assaf; (Emory University, Atlanta, GA) Dallas Hall; (George Washington University, Washington, DC) Valery Miller; (Kaiser Permanente Center for Health Research, Portland, OR) Barbara Valanis; (Kaiser Permanente Division of Research, Oakland, CA) Robert Hiatt; (National Cancer Institute, Bethesda, MD) Carolyn Clifford1; (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Linda Pottern; (University of California at Irvine, CA) Frank Meyskens, Jr.; (University of California at Los Angeles, CA) Howard Judd1; (University of Cincinnati, Cincinnati, OH) James Liu, Nelson Watts; (University of Miami, Miami, FL) Marianna Baum, (University of Minnesota, Minneapolis, MN) Richard Grimm; (University of Nevada, Reno, NV) Sandra Daugherty1; (University of North Carolina, Chapel Hill, NC) David Sheps, Barbara Hulka; (University of Tennessee Health Science Center, Memphis, TN) William Applegate; (University of Wisconsin, Madison, WI) Catherine Allen1; (Wake Forest University School of Medicine, Winston-Salem, NC) Denise Bonds.
Footnotes
Contributors: RTC wrote the analysis proposal and initial draft of the report. He accepts full responsibility for the overall content of the report. All authors contributed to the interpretation of data and the approval of the final report. RTC, GLA, MLS, JEM, JW, JMK, KCJ, MJD, JKO, and FAH additionally collected the data and obtained study funding. GLA and RJR additionally performed the statistical analyses. GLA, RJR, MLS, JEM and JW additionally contributed to the study design. RTC, GLA, AGS, HW, JWC, JW, MJO, FAH and CC additionally contributed to the manuscript writing.
Conflicts of Interest
RTC has received speaker’s fee and honorarium for advisory boards and consulting from AstraZeneca, Novartis; honorarium for advisory boards and consulting for Lilly, Amgen and Pfizer and grant support from Amgen. RTC, GLA, MLS. JEM, JW, MG, JMK, KCJ, MJO, JKO and FAH have received grant support from NIH; KCJ and RTC additionally have received grant support from the National Cancer Institute of Canada. MG has received grant support from Wyeth. AGS, HW, RJR, JWC and CC have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Deceased.
References
- 1.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 2.Chlebowski RT, Hendrix SL, Langer RD, et al. Estrogen plus progestin influence on breast cancer and mammography in healthy postmenopausal women: The Women’s Health Initiative randomized trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 3.Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA. 2003;290:1729–1738. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 4.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 5.Wassertheil-Smoller S, Hendrix SL, Limacher M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289:2673–84. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 6.Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA. 2004;292:1573–1580. doi: 10.1001/jama.292.13.1573. [DOI] [PubMed] [Google Scholar]
- 7.Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004:991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- 8.Chlebowski RT, Kuller L, Prentice RL, et al. Breast cancer after estrogen plus progestin use in postmenopausal women. N Engl J Med. 2009;360:573–587. doi: 10.1056/NEJMoa0807684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heiss G, Wallace A, Anderson GL, et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299:1036–45. doi: 10.1001/jama.299.9.1036. [DOI] [PubMed] [Google Scholar]
- 10.Stabile LP, Lyker JS, Gubish CT, et al. Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res. 2005;61:1459–1470. doi: 10.1158/0008-5472.CAN-04-1872. [DOI] [PubMed] [Google Scholar]
- 11.Marquez-Garban DC, Chen HW, Goodglick L, Fishbein MC, Pietras RJ. Targeting aromatase and estrogen signaling in human non-small cell lung cancer. Ann NY Acad Sci. 2009;115:194–205. doi: 10.1111/j.1749-6632.2009.04116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cagle PT, Mody DR, Schwartz MR. Estrogen and progesterone receptors in bronchogenic carcinoma. Cancer Res. 1990;50:6632–6635. [PubMed] [Google Scholar]
- 13.Stabile LP, Davis AL, Gubish CT, et al. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002;62:2141–2150. [PubMed] [Google Scholar]
- 14.Schwartz AG, Wenzlaff AS, Prysak GM, et al. Reproductive factors, hormone use, estrogen receptor expression and risk of non small-cell lung cancer in women. J Clin Oncol. 2007;25:5785–92. doi: 10.1200/JCO.2007.13.3975. [DOI] [PubMed] [Google Scholar]
- 15.Hammoud Z, Tan B, Badve S, Bigsby RM. Estrogen promotes tumor progression in a genetically defined mouse model of lung adenocarcinoma. Endocrine-Related Cancer. 2008;15:475–483. doi: 10.1677/ERC-08-0002. [DOI] [PubMed] [Google Scholar]
- 16.Albain KS, Unger J, et al. Toxicity and survival by sex in patients with advanced non-small cell lung cancer carcinoma (NSCLC) on modern Southwest Oncology Group (SWOG) trials. J Clin Oncol. 2007;25(Part I 18S):7549. [Google Scholar]
- 17.Ross H, Oldham FB, Bandstra B, et al. Serum-free estradiol (E2) levels are prognostic in men with chemothearpy-naive advanced non-small cell lung cacner (NSCLC) and performance status (PS). J Clin Oncol; ASCO Ann Proc; 2007. [Google Scholar]
- 18.The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 19.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–77. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 20.Cox DR. Regression analysis and life tables. JR Stat Soc. 1972;34:187–220. [Google Scholar]
- 21.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–93. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 22.Losordo DW, Isner JM. Estrogen and angiogenesis: a review. Arterioscler Thromb Vasc Biol. 2001;21:6–12. doi: 10.1161/01.atv.21.1.6. [DOI] [PubMed] [Google Scholar]
- 23.Dobrzycka B, Kinalski M, Piechocka D, Terlikowski SJ. The role of estrogens in angiogenesis in the female reproductive system. Endokrynol Pol. 2009;60(3):210–4. [PubMed] [Google Scholar]
- 24.Elkin M, Orgel A, Kleinman HK. An angiogenic switch in breast cancer involves estrogen and soluble vascular endothelial growth factor receptor 1. J Natl Cancer Inst. 2004;96(11):875–8. doi: 10.1093/jnci/djh140. [DOI] [PubMed] [Google Scholar]
- 25.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis: correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 26.Calvo A, Catena R, Noble MS, et al. Identification of VEGF-regulated genes associated with increased lung metastatic potential: functional involvement of tenascin-C in tumor growth and lung metastasis. Oncogene. 2008;27(40):5373–84. doi: 10.1038/onc.2008.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–76. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 28.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 29.Chlebowski RT, Anderson G, Pettinger M, Lane D, Chen C, Walsh BW, McTiernan A. Estrogen plus progestin and breast cancer detection with mammography and breast biopsy. Arch Intern Med. 2008;168(4):382–391. doi: 10.1001/archinternmed.2007.123. [DOI] [PubMed] [Google Scholar]
- 30.International Early Lung Cancer Action Program Investigators. Women’s susceptibility to tobacco carcinogens and survival after diagnosis of lung cancer. JAMA. 2006;296:180–184. doi: 10.1001/jama.296.2.180. [DOI] [PubMed] [Google Scholar]
- 31.Bremes RM, Sundstrom S, Aasebo U, et al. The value of prognostic factors in small cell lung cancer: results from a randomised multicenter study with minimum 5 year follow-up. Lung Cancer. 2003;39:303–13. doi: 10.1016/s0169-5002(02)00508-1. [DOI] [PubMed] [Google Scholar]
- 32.Chlebowski RT, Palomares MR, Lillington L, Grosvenor M. Recent implications of weight loss on lung cancer management. Nutrition. 1996;12:S42–S47. doi: 10.1016/0899-9007(96)90018-0. [DOI] [PubMed] [Google Scholar]
- 33.Wakelee HA, Wang W, Schiller JH, et al. Survival differences by sex for patients with advanced non-small cell lung cancer on Eastern Cooperative Oncology Group trial 1594. J Thorac Oncol. 2006;1:441–6. [PubMed] [Google Scholar]
- 34.Schabath MB, Wu X, Vassilopoulou-Sellin R, Vaporciyan AA, Spitz MR. Hormone replacement therapy and lung cancer risk: a case-control analysis. Clin Cancer Res. 2004;10:113–23. doi: 10.1158/1078-0432.ccr-0911-3. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez C, Spencer Feigelson H, Deka A, et al. Postmenopausal hormone therapy and lung cancer risk in the cancer prevention study II nutrition cohort. Cancer Epidemiol Biomarkers Prev. 2008;17:655–60. doi: 10.1158/1055-9965.EPI-07-2683. [DOI] [PubMed] [Google Scholar]
- 36.Kruezer M, Gerken M, Heinrich J, Kreienbrock L, Wichmann HE. Hormonal factors and risk of lung cancer among women? Int J Epidemiol. 2003;32:263–71. doi: 10.1093/ije/dyg064. [DOI] [PubMed] [Google Scholar]
- 37.Baik CS, Strauss GM, Feskanich D. A prospective study of reproductive factors, hormone use, and risk of lung cancer in postmenopausal women. J Clin Oncol. 2009;27:1501. doi: 10.1158/1055-9965.EPI-10-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taioli E, Wynder EL. Re: Endocrine factors and adenocarcinoma of the lung in women. J Natl Cancer Inst. 1994;86:869–870. doi: 10.1093/jnci/86.11.869. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Inoue M, Sobue T, et al. Reproductive factors, hormone use and the risk of lung cancer among middle-aged never-smoking Japanese women: A large-scale population-based cohort study. Int J Cancer. 2005;117:662–666. doi: 10.1002/ijc.21229. [DOI] [PubMed] [Google Scholar]
- 40.Ganti AK, Sahmoun AE, Panwalker AW, Tendulkar LL, Potti A. Hormone replacement therapy is associated with decreased survival in women with lung cancer. J Clin Oncol. 2006;24:59–63. doi: 10.1200/JCO.2005.02.9827. [DOI] [PubMed] [Google Scholar]
- 41.Huang B, Carloss H, Wyatt SW, Riley E. Hormone replacement therapy and survival from lung cancer in postmenopausal women in a rural population. Cancer. 2009 June 4; doi: 10.1002/cncr.24475. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 42.Hulley S, Furberg C, Barrett-Connor E, et al. Noncardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:58–66. doi: 10.1001/jama.288.1.58. [DOI] [PubMed] [Google Scholar]