Summary
Highly branched Class IV multidendritic sensory neurons of the Drosophila larva function as polymodal nociceptors that are necessary for behavioral responses to noxious heat (>39°C) or noxious mechanical (>30 mN) stimuli. However, the molecular mechanisms that allow these cells to detect both heat and force are unknown. Here, we report that the pickpocket(ppk) gene, which encodes a Degenerin/ Epithelial Sodium Channel (DEG/ENaC) subunit, is required for mechanical nociception but not thermal nociception in these sensory cells. Larvae mutant for pickpocket show greatly reduced nociception behaviors in response to harsh mechanical stimuli. However, pickpocket mutants display normal behavioral responses to gentle touch. Tissue specific knockdown of pickpocket in nociceptors phenocopies the mechanical nociception impairment without causing defects in thermal nociception behavior. Finally, optogenetically-triggered nociception behavior is unaffected by pickpocket RNAi which indicates that ppk is not generally required for the excitability of the nociceptors. Interestingly, DEG/ENaCs are known to play a critical role in detecting gentle touch stimuli in C. elegans and have also been implicated in some aspects of harsh touch sensation in mammals. Our results suggest that neurons which detect harsh touch in Drosophila utilize similar mechanosensory molecules.
Results and Discussion
Two overlapping deficiencies remove pickpocket and elB
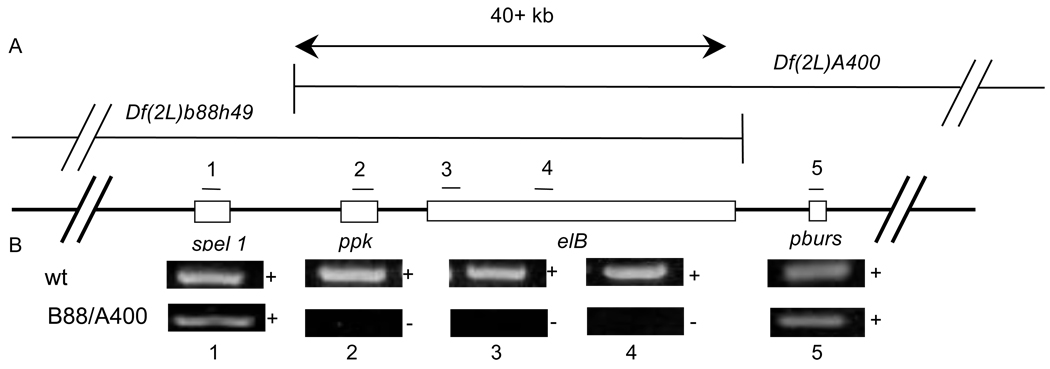
We have previously found that ppk expressing sensory neurons (figure 1A,B) (Class IV multidendritic (md-da)neurons) are required for behavioral responses to harsh (noxious) mechanical stimuli [1]. The pickpocket locus is located on the left arm of chromosome 2 at the cytological position 35B1. ppk is flanked by elbow B (elB), a transcription factor involved in tracheal development [2], and spel1, a gene involved in DNA mismatch repair [3] (Figure 2A). Two overlapping deficiencies, Df(2L)b88h49 and Df(2L)A400, have been shown [4] to overlap and to completely remove ppk. Thus transheterozygotes for these deficiencies are DNA nulls for the ppk gene. It was previously reported that the 44kb overlapping deficiency specifically removed ppk; leaving spel1 and elB intact [4]. However, the ppk containing interval between spel1 and elB is only 22kb suggesting that at least one other gene (spel1 or elB) must be removed if these deficiencies overlap by 44kb (Figure 2A). Therefore we examined this interval first to confirm the absence of ppk, but also to test which other genes are removed in the transheterozygous combination. Polymerase chain reaction amplification of genomic DNA from the Df(2L)b88h49/Df(2L)A400 genotype indicated that two genes, ppk and elB, are removed in the transheterozygote (Figure 2B). Therefore, for the purpose of this discussion, we will refer to transheterozygous larvae of this genotype as ppk elBA400/B88.
Figure 1.
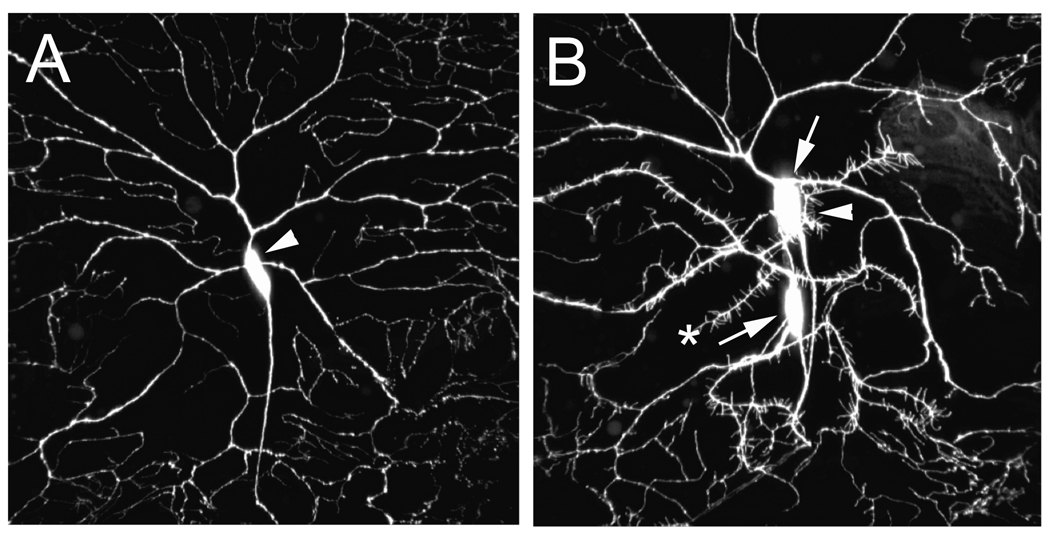
Expression of pickpocket in multidendritic neurons (ppk-GAL4 driving UAS-mCD8::GFP). Confocal micrographs of dorsal cluster peripheral neurons in a third instar larva. (A) When heterozygous for ppk-GAL4 and heterozygous for UAS-mCD8::GFP the driver directed target gene expression solely in Class IV multidendritic neurons (arrowhead indicates the ddaC neuron). (B) When homozygous, the ppk-GAL4 driver targeted both Class IV (arrowhead indicates ddaC) and Class III multidendritic neurons (arrows indicate ddaF and ddaA). The Class III neurons were unambiguously identified by spine like protrusions from the dendrites (asterisk).
Figure 2.
Genomic region containing the pickpocket gene. (A) Schematic representation of Df(2L)B88h49 and Df(2L)A400. The deleted region in the transheterozygous deficiency genotype is indicated by a double arrow (as determined by results Polymerase Chain Reaction (panel B)). The results indicated that Df(2L)B88h49/ Df(2L)A400 animals are deficient for both pickpocket and elbow B. (B) Results of PCR on genomic DNA from wildtype (wt) or Df(2L)B88h49/Df(2L)A400 (B88/A400). On the distal side of pickpocket, spel1 was not deleted. On the proximal side, pburs was present. Within the overlap of the deficiencies, pickpocket and elbow B were both deleted.
Mechanical nociception responses of ppk mutants
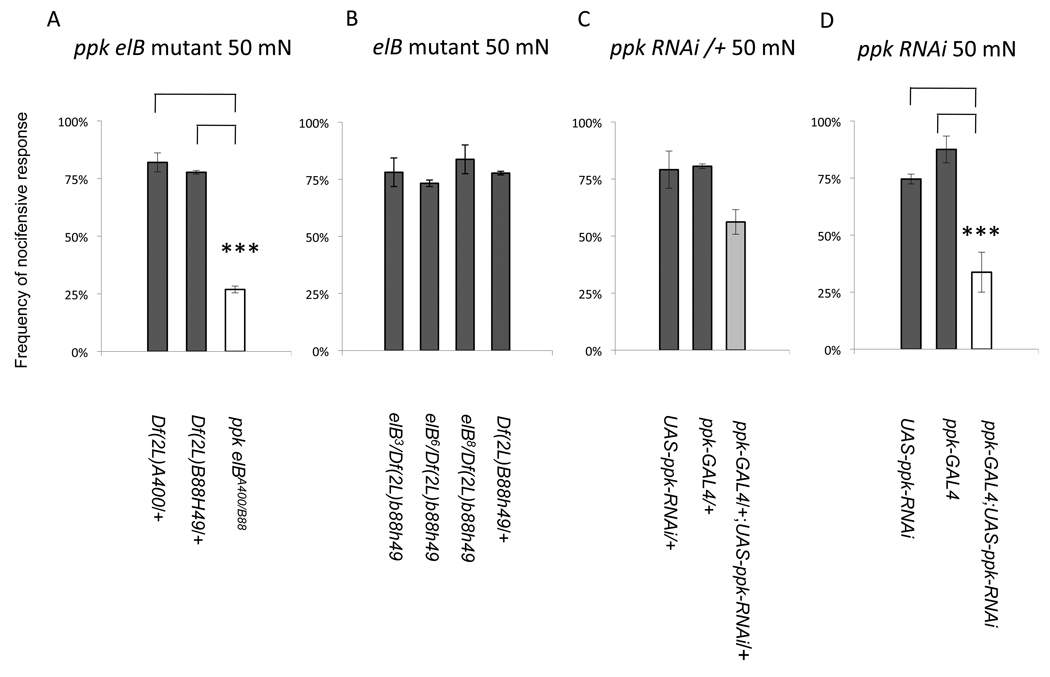
Given the nociceptive function of ppk expressing neurons [1] combined with the known role of DEG/ENaC proteins in C. elegans mechanotransduction, we tested the ppk elBA400/B88 mutants for defects in mechanical nociception. To examine mechanical nociception behavior, ppk elBA400/B88 mutant larvae were exposed to mechanical stimuli delivered by 50 mN Von Frey fibers on the dorsal midline at approximately abdominal segment four (Supplemental Video 1). Following such a stimulus, greater than 75% of wild type larvae responded with nocifensive escape locomotion in which the larvae rotate around their long body axis (Supplemental Video 1). Similarly, both Df(2L)b88h49/+ and Df(2L)A400/+ showed robust nocifensive responses (Figure 3A). In contrast, the ppk elBA400/B88 mutant larvae showed a significant reduction in nocifensive responses to the noxious mechanical stimuli (Figure 3A) and responded with nocifensive behavior in only 27% of the trials. Notably the mutant larvae were not completely unresponsive to the noxious mechanical stimulus. Instead of nocifensive responses the mutant larvae often inappropriately displayed behaviors that resembled the wild type responses to gentle touch. Indeed, behavioral responses to gentle touch were normal in ppk elBA400/B88 mutant animals (Figure S1).
Figure 3.
pickpocket is required for mechanical nociception (A) Response of deficiency lines (Df(2L)A400/+ (5 trials, n=181), Df(2L)b88h49/+ (5 trials, n=186), and Df(2L)A400/Df(2L)B88h49 (ppk null mutant, 3 trials, n=156)). Df(2L)A400/Df(2L)B88h49 animals showed a severe reduction in nocifensive responses (p<0.001 (for both one-way ANOVA and Sheffe’s post-hoc test). (B) Nociception responses in elB mutants and control (elB3/Df(2L)B88h49 (3 trials, n=86) , elB6/Df(2L)B88h49( 3 trials, n=122), elB8/Df(2L)B88h49 (3 trials, n=98) and Df(2L)B88h49/+ (5 trials, n=181)) (p=0.39 one-way ANOVA). (C) Nociception responses with heterozygous ppk-RNAi strains (UAS-ppk-RNAi/+ (3 trials, n=92), ppk-Gal4/+ (3 trials, n=132) and ppk-GAL4/+; UAS-ppk-RNAi/+ (3 trials, n=223)). Although a statistically significant difference was found among the groups (p<0.05 one-way ANOVA) Sheffe’s post-hoc analysis did not detect a significant difference among the means in pair-wise comparisons. (D) Nociception behavior with homozygous ppk-RNAi strains (UAS-ppk-RNAi (7 trials, n=415), ppk-GAL4 (5 trials, n=219), ppk-GAL4; UAS-ppk-RNAi (3 trials, n=96)). (p<0.001 (for both one-way ANOVA and Sheffe’s post-hoc test).
elbowB mutants complement mechanical nociception phenotypes
Although ppk elBA400/B88 mutant larvae were defective for mechanical nociceptive behavior, it was possible that the mutant defect was due to the absence of elB rather than absence of ppk. elB is a zinc-finger containing protein that is involved in several developmental processes. Expression of elB has been detected in a subset of tracheal trunks and elB mutants develop aberrant migration of dorsal and lateral tracheal branches in embryos [2]. In addition, elB is expressed in the leg, wing, eye, and head primordia where it is involved in appendage formation via the repression of body wall genes [5]. Finally, elB plays a role in the regulation of the size of the eye-head primordium [6]. Although elB’s expression has not been found in larval nociceptors, an effect on nociception remained possible. In order to test this, we performed complementation tests with three existing alleles of elB (elB3, elB6, elB8) and Df(2L)b88h49. The elB6 allele is caused by a chromosomal inversion (In(2LR)elB6) with a breakpoint in the first intron of the elB locus, but the molecular lesions of the ethyl methanesulfonate induced elB3 and the γ-ray induced elB8 alleles are unknown. Nevertheless, the three elB alleles complemented the defective mechanical nociception phenotype when placed over Df(2L)b88h49 (Figure 3B) indicating that the nociception defects of ppk elBA400/B88 mutant larvae are unlikely to be a consequence of removing elB.
RNAi knockdown of pickpocket in Class IV multidendritic neurons
In order to further determine whether the mechanical nociception defect observed in ppk elBA400/B88 larvae was due to the removal of ppk, we used the GAL4/UAS system [7] to drive tissue specific expression of ppk-RNAi in neurons that express ppk [8]. With this approach the UAS driven ppk-RNAi is only expressed in cells that express the ppk-GAL4 driver. This allowed us to test if the site of action for pickpocket in mechanical nociception is in the same cells that express GAL4 in the ppk-GAL4 driver line.
With a single copy of ppk-GAL4 expression of UAS transgenes is driven only in Class IV multidendritic neurons (Figure 1A). Indeed, there was a reduction in the larval responses to harsh mechanical stimulation when the RNAi knockdown was performed using a single copy of the ppk-Gal4 driver and a single copy of the UAS-ppk-RNAi (w; ppk-GAL4/+; UAS-ppk-RNAi/+) (Figure 3C). This result implicates the Class IV neurons as playing a pickpocket dependant role in mechanical nociception. However, the reduction of nociception behavior of this genotype was not as severe as in the DNA null allele. The less severe phenotype seen in w; ppk-GAL4/+; UAS-ppk-RNAi/+ could be a consequence of incomplete knockdown of pickpocket in this genotype. To test this possibility, we increased the dosage of RNAi expression by increasing the copy number of the GAL4 driver and UAS transgenes that were used in the experiment.
With two copies of the ppk-GAL4 transgene, UAS reporter transgenes are driven in both the Class III and Class IV multidendritic neurons (Figure 1B). We thus used a homozygous driver strain to test whether expanding expression of ppk-RNAi to include both Class III and Class IV multidendritic neurons would phenocopy the null mutant situation. Indeed, the defect in mechanical nociception observed in the w; ppk-GAL4; UAS-ppk-RNAi strain was nearly as severe as what we observed in the ppk elBA400/B88 null background (Figure 3A,D). The stronger phenotype seen with RNAi knockdown using the homozygous ppk-GAL4 driver may be due to more highly effective knockdown of ppk in the nociceptive Class IV md-da neurons with increased transgene dosage. Alternatively, it is also possible that expression of RNAi in Class III neurons with the homozygous ppk-GAL4 driver strain (Figure 1B) contributes to the severity of the phenotype. Consistent with the latter possibility, we have previously shown that genetic silencing of Class II and III multidendritic neurons causes a mild impairment in mechanical nociception behavior [3]. Thus, it is possible that expression of pickpocket in both Class III as well is in the Class IV multidendritic neurons contributes to its function in mechanical nociception responses.
These data showing the strong phenocopy of ppk elBA400/B88 mechanical nociception defects produced by ppk RNAi strongly support the hypothesis that the phenotype of ppk elBA400/B88 mutants is due to the absence of ppk. Furthermore, these tissue-specific RNAi experiments indicate that the site of action for ppk is in the Class IV (and possibly the Class III) multidendritic neurons which are specifically targeted by the ppk-GAL4 driver line.
Optogenetic activation of Class IV neurons pickpocket RNAi
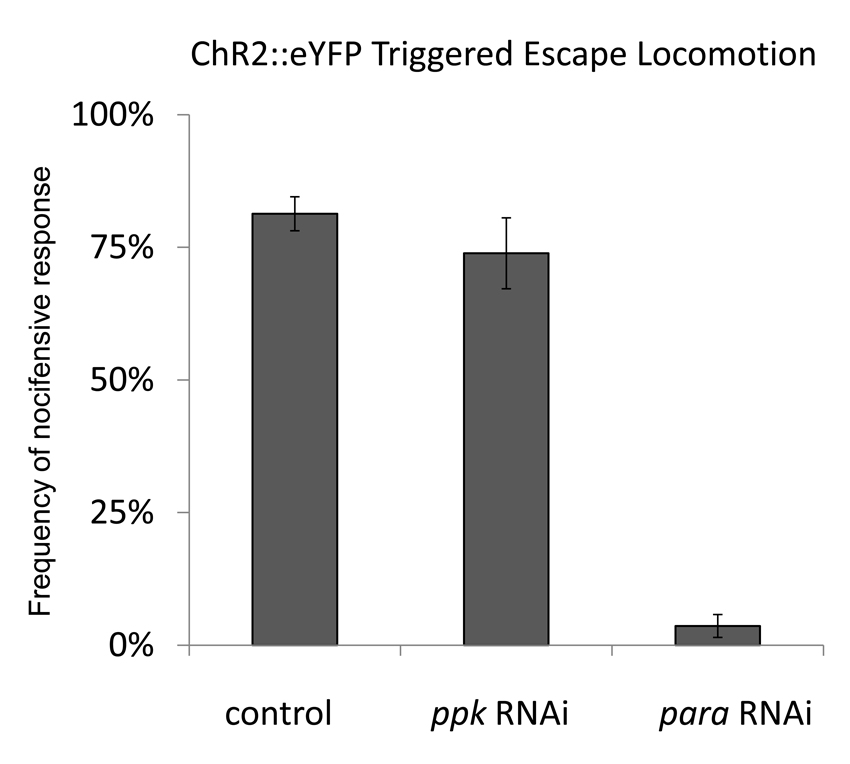
Although mechanical nociception was severely impaired by expression of ppk-RNAi in nociceptive neurons thermal nociception behavior was unaffected by RNAi knockdown of the gene (Figure S2 F,G). In addition, analysis of ppk elBA400/B88 mutant larvae did not clearly implicate ppk in thermal nociception pathways (Figure S2 A–C). The specific requirement for ppk in mechanical nociception behavior suggests a possible role for ppk at the mechanotransduction step in the polymodal nociceptive sensory neurons. To further test the possibility that Pickpocket might be required for the general excitability of these neurons we utilized optogenetic activation of the Class IV cells. We have previously shown that expression of Channelrhodopsin-2YFP (ChR2::eYFP) under control of ppk-GAL4 can be used to activate Class IV md-da neurons with blue light and trigger nocifensive escape locomotion [1]. Blue light activation of the larval nociceptors via ChR2::eYFP should therefore bypass mechanotransduction, but it should still depend on factors that control the general excitability of the neuron. Thus, if ppk has a non-specific or general excitability function in Class IV multidendritic neurons then the blue light triggered escape locomotion should be impaired by ppk knockdown. For example, the para gene encodes a NaV type sodium channel that is essential for action potential propagation in Drosophila [9, 10] and para is required in Class IV multidendritic neurons for ChR2::eYFP triggered nociception behavior (Figure 4). In control transgenic larvae that expressed ChR2::eYFP in nociceptors, escape locomotion was seen in response to 81% of blue light pulses and this was reduced to only 4% when para-RNAi was targeted to the same cells that expressed ChR2::eYFP (Figure 4). Thus, results of knockdown of para showed that optogenetic activation of nociception behavior depends on the generation of action potentials in the nociceptive neurons and upon factors generally involved in neuronal excitability. In contrast, knockdown of ppk did not affect the ChR2::eYFP triggered nocifensive escape locomotion (Figure 4). These data indicate that ppk is not simply required for the general activation of the multidendritic neurons. Instead, combined with the results described above, our results suggest a specific mechanosensory function for pickpocket in larval nociceptors.
Figure 4.
Knockdown of ppk did not affect ChR2 triggered nocifensive behavior. Blue light triggered activation of nociception behavior was seen in 81%(SEM 3%) of animals of the Control optogenetic activation genotype (ppk-GAL4 UAS-ChR2::eYFP Line C / +, UAS-dicer-2/+ (8 trials, n=245)). Knockdown of ppk did not significantly reduce the frequency of escape locomotion in response to blue light (note that UAS-dicer-2 [35]was utilized in this experiment to enhance the effectiveness of RNAi) (ppk-GAL4, UAS-ChR2::eYFP Line C / UAS-ppk-RNAi ; UAS-dicer-2 / +( 3 trials, n=103) ((74% SEM 7%) t-test relative to control, p=0.32). In contrast, knockdown of para dramatically reduced the response to blue light (ppk-GAL4 UAS-ChR2::eYFP Line C / UAS-para-RNAi ; UAS-dicer-2 / +, 4 trials, n=115).
In summary, we have shown that the ppk gene is required for mechanical nociception in Drosophila larvae. The ppk gene is specifically expressed in these cells and genetic null mutants of ppk showed severely impaired mechanical nociception behavior. In addition, RNAi knock-down of ppk gene expression in nociceptive neurons phenocopied this defect. However, RNAi knockdown of ppk did not result in a defect in thermal nociception and also had no effect on optogenetic activation of these cells.
The situation for ppk is distinct from that of the painless gene which is required for both thermal and mechanical nociception responses [11]. In heterologous cells, Painless currents can be activated by heat but not by osmotic pressure [12]. This supports a direct role for Painless in the transduction of thermal nociception stimuli but a direct role as a mechanosensor remains unproven. Interestingly, heat activated Painless currents are strongly affected by intracellular calcium ions. This sensitivity to calcium suggests that the function of Painless in mechanical nociception pathways could feasibly be downstream of Pickpocket. For example, if Pickpocket functions as a direct mechanosensor, calcium influx through downstream voltage gated channels might indirectly sensitize Painless currents. In this model, the function of Painless would serve as an amplifier of mechanically gated Pickpocket currents.
Although our results implicate a specific role for Pickpocket in mechanosensory function, a role for Pickpocket itself as a subunit of a mechanotransduction channel remains unproven. The ultimate proof of this hypothesis would require the detection of mechanically gated currents in a biochemically reconstituted system involving purified Pickpocket protein. However, expression of Pickpocket in heterologous cells has not been found to produce currents [13] and the absence of currents by heterologously Pickpocket proteins likely indicates that additional factors present in vivo are needed for the function of this channel subunit [13]. Indeed, co-expression of MEC-4 and MEC-10 is required for expression of these channels in heterologous expression systems [14]; it is thus likely that another DEG/ENaC subunit in Drosophila is required for Pickpocket to form a functional channel. In future studies, using the mechanical nociception assay described here, it should be possible to identify additional Drosophila DEG/ENaC subunits that are specifically required for mechanical signaling in the multidendritic neurons. These would represent candidates for the additional subunits that may be required for the formation of a functional Pickpocket channel.
Nevertheless, given the specificity of the phenotype, a model where Pickpocket functions as a component of a mechanotransduction complex seems likely especially given the well established functional role of DEG/ENaCs in the C. elegans mechanotransduction complex [14–21]. The sub-cellular localization of the Pickpocket protein is found in discrete varicosities on the dendrites [13] which is reminiscent of the punctate localization of the mechanotransduction complex of the touch receptor neuron processes in C. elegans [20].
Although the mechanical nociception stimulus used in our assays seems qualitatively distinct from the gentle touch stimulus used in C. elegans studies our results suggest that the molecular machinery involved may be similar. This is somewhat surprising since the mec-4 and mec-10 genes of C. elegans are required for gentle touch responses in C. elegans [14, 22, 23], but mec-4 and mec-10 mutants show normal behavioral responses to harsh touch. This may suggest the existence of additional mechanotransduction pathways in the C. elegans touch neurons, or alternatively C. elegans may utilize distinct high threshold mechanosensory neurons. Consistent with the former possibility calcium responses to harsh touch in MEC-4 expressing neurons are still observed in mec-4 mutant ALM neurons [24]. Consistent with the latter possibility worms with laser ablated gentle touch neurons still show behavioral responses to harsh touch [25]. Indeed, although mec-10 itself is not required for harsh touch responses, it has been proposed that harsh touch detection in the worm may be mediated by high-threshold PVD neurons which express MEC-10[25]. Interestingly, mice that are mutant for the ASIC3 DEG/ENaC channel show reduced sensitivity to noxious pinch [26]. Thus, it is possible that the function of Pickpocket in Drosophila neurons could be similar to that of ASIC3 in mouse nociceptive neurons.
It is clear that distinct mechanosensory pathways are likely to exist within organisms. In addition to the role of Pickpocket in harsh touch responses, gentle touch to the body wall of the Drosophila larva is thought to be detected by ciliated chordotonal neurons [27, 28] which utilize the TRP channels Nomp-C[29], Iav, and Nan [30] for mechanotransduction[31, 32]. Similarly in C.elegans, distinct mechanosensory neurons rely on distinct signaling mechanisms [33, 34].
Our identification of Pickpocket as a potential mechanotransducer in the Drosophila nociceptive neurons suggests a widespread and evolutionarily conserved role for DEG/ENaCs in neurosensory mechanotransduction. Furthermore, the pickpocket mutant phenotype genetically separates mechanical nociception from thermal nociception in Drosophila.
Experimental Procedures
Fly Strains and husbandry
The following fly strains were used: w; pickpocket1.9-GAL4 (ppk-GAL4), w; UAS-ppk-RNAi (III) [8]. Df(2L)A400 T(2;3;4)CA4 / CyO P{ActGFP}JMR1, Df(2L)b88h49/ CyO P{ActGFP}JMR1, w1118 ;P{UAS-dicer-2, w[+]} (UAS-dicer-2 on III, VDRC stock number 60009), w1118;P{UAS-para-RNAi} (VDRC stock number 6132), elB8. Transheterozygous larvae (Df(2L)A400T(2;3;4) / Df(2L)b88h49) were identified as GFP negative progeny from the cross Df(2L)A400 T(2;3;4) / CyO P{ActGFP}JMR1 X Df(2L)b88h49/CyO P{ActGFP}JMR1. elB mutant larvae were identified as GFP negative progeny from the cross of elB[3,6 or 8] / CyO P{ActGFP}JMR1 X Df(2L)b88h49/ CyO P{ActGFP}JMR1. Channelrhodopsin experiments were performed with the YFP positive progeny from the cross of ppk-Gal4 UAS-ChR2::eYFP /CyO; UAS-dicer-2 to males of the corresponding UAS-RNAi line or to an isogenic w1118 control (VDRC stock number 60000). Flies were raised on standard cornmeal medium on a 12hour light dark cycle. All stocks were maintained at 25°C and 75% humidity.
Behavioral Assays
The thermal nociception behavioral tests were performed as described previously [1, 11]. The mechanical nociception assays were performed as described previously, with slight modifications[1, 11]. Wandering third-instar larvae were stimulated with a 50mN calibrated Von Frey filament. Omniflex monofilament fishing line Shakespeare (6 lb test, diameter 0.009 inch [0.23 mm]) was cut to a length of 18 mm and attached to a glass pipette such that 8 mm of the fiber protruded from the end of the pipet and 10 mm anchored the fiber. Von Frey fibers were calibrated by using them to depress a balance until the fishing line was seen to bend. The force (in grams) was recorded and converted to millinewtons by multiplying the measured grams by a factor of 9.8. Noxious mechanical stimuli were delivered by rapidly depressing the larva with the fiber on the dorsal side. The stimulus was delivered and released as quickly as possible. The quick release allows the larvae unrestrained freedom to perform escape locomotion behavior. The stimulus was delivered to abdominal segments four, five, or six. A positive response was scored if at least one 360 degree revolution around the A/P axis occurred in response to the mechanical stimulus. Each larva was tested only once.
For each genotype, three to seven trials were performed. For each trial, five vials of crosses were established using six virgin females and three males. These vials were kept at 25°C, 75% humidity and a 12 hour light/dark cycle. Third instar wondering larvae were tested in behavioral assays on the sixth, seventh and eighth day after setting up the crosses. The results of tests on the animals from the five vials were pooled with the goal of obtaining a sample size of approximately 30 larvae for a given trial. The averaged results from the trials for a given genotype were used to obtain a behavioral score and to generate the standard error of the mean. Statistical analyses were performed using the Microsoft Excel one-way Analysis of Variance (ANOVA) tool. In experiments where the between group ANOVA reported a significant p-value post hoc pair-wise comparisons of the means were performed using Sheffe’s test.
The optogenetic activation of ppk-GAL4 neurons was performed as described previously[1]. However, the strain utilized here (ppk-Gal4 UAS-ChR2::eYFP /CyO; UAS-dicer-2) harbored the UAS-dicer-2 trangene which enhances RNAi[35]. When crossed to the UAS-ppkRNAi strain only half of the progeny inherit the ppk-Gal4 UAS-ChR2::eYFP chromosome. These larvae were identified following exposure to blue light by visualizing their YFP fluorescence. The YFP negative (CyO) larvae were used as in internal negative control since they never produce nocifensive escape locomotion in response to blue light.
Molecular biology
Genomic DNA was extracted from adult flies using QIAGEN DNeasy Tissue Kit. The following primers were used to amplify ppk and its neighboring genes. Spel: forward 5' - TCA AGC AAC TGG ACC TAA ACC G - 3', reverse 5' - GTT ATC TCG TGA AAA TGA GTG GCG - 3'. ppk: forward 5' - AGC ACG ACC ATT CAC GGC ATA C - 3' reverse 5' - CCA AAG TTC ACT CAC TGG GCA TC - 3'; elB C-terminus: forward 5' - CGC AAT ACG ACG CTT TTC CG - 3', reverse 5' - TTG ATA GTT CCC CCT GTG AGG TC - 3'; elB : Forward primer 5' - AGC AGT CAT TCA TCC AGC GTT AG - 3', reverse primer 5' - TTC ATT CGG GCG TAG TTC AGA TAC - 3'; Pburs : forward primer 5' - CAA CCA TCG GTG ATA ACG CC – 3’, reverse primer 5' - TCG CCA CAT TTG AAG CAC TTG - 3'.
Supplementary Material
Figure S1 related to Figure3. Behavioral responses to gentle touch in pickpocket mutants Although the class IV md-da neurons that are known to express ppk are nociceptive, it seemed possible that ppk could be expressed at low levels in non-nociceptive neurons. Therefore, we tested whether ppk was required for responses to gentle touch in Drosophila larvae (as DEG/ENaCs are in C. elegans). Using the gentle touch assay previously developed by Kernan et al [36], we tested ppk elBA400/B88 mutant larvae (n=22) for their behavioral response. The ppk elBA400/B88 mutant larvae were indistinguishable from wildtype larvae (n=28). Our results confirm a previous report that which found no requirement for ppk in gentle touch responses [4].
Figure S2 related to Figure 3: pickpocket is not required for thermal nociception. Response times for nocifensive behavior in response to 46°C probe. (A) Df(2L)A400/+ (4 trials, n=173) (B) Df(2L)B88h49/+, (3 trials, n=80) (C) Df(2L)B88h49/Df(2L)A400 (3 trials, n=100). (D) UAS-ppk-RNAi (7 trials, n=207) (E) ppk-Gal4 (4 trials, n=153) (F) ppk-GAL4/+; UAS-ppk-RNAi/+ (2 trials, n=73). (G) ppk-GAL4; UAS-ppk-RNAi (4 trials, n=130).
ppk elBA400/B88 mutant larvae were tested for thermal nociception responses by stimulating them with a probe heated to 46°C and their latency to initiation of larval nocifensive escape behavior was recorded. Wild type larvae initiated escape locomotion within the first 3 seconds of stimulation with the noxious heat probe. Similarly, Df(2L)A400/+ heterozygotes showed a response that was indistinguishable from wild type. The normal thermal nociception response of Df(2L)A400/+ demonstrates that removal of one copy of ppk does not cause a thermal nociception phenotype (Figure S2A). However, Df(2L)b88h49/+ heterozygotes did show an impaired response to noxious heat (Figure S2B). Thus, a mutation(s) in the Df(2)b88h49 strain dominantly impairs thermal nociception. If this were due to deletion of ppk then removal of the remaining copy of ppk in this background would be expected to enhance the Df(2)b88h49/+ phenotype. However, complete removal of ppk, in the ppk elBA400/B88 genotype, if anything, showed improved thermal nociception relative to Df(2L)b88h49/+ (Figure S2C). Since removal of an additional copy of ppk did not enhance the Df(2L)b88h49/+ phenotype and since Df(2L)A400/+ showed wild type thermal nociception behavior, our results do not indicate a role for ppk in thermal nociception. Consistent with this, knockdown of ppk with RNAi did not impair thermal nociception behavior despite the fact that knockdown of ppk effectively blocked mechanical nociception(Figure S2 D–G).
The mechanical nociception assay was performed by delivering a rapid stimulus with a Von Frey fiber to the dorsal midline of a wandering third instar larva (methods). The video shows the wild type escape locomotion response.
Acknowledgments
We thank Wayne Johnson of the University of Iowa, Fen-Biao Gao of the University of California San Francisco, Jonathan Roote of Cambridge University, the Vienna Drosophila Resource Center, and the Bloomington Drosophila Stock Center for providing Drosophila strains. We thank members of the Tracey lab and three anonymous reviewers for helpful comments on the manuscript. Allison Weaver, Kia Walcott, and Melissa Tang provided invaluable technical assistance for this project. This work was supported by an Alfred P. Sloan Fellowship (WDT), a grant from the Whitehall Foundation (WDT), and by funding from the National Institutes of Neurological Disorders and Stroke (5R01NS054899) and the National Institute on Deafness and Other Communication Disorders (1R21DC010222) (WDT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hwang RY, Zhong L, Xu Y, Johnson T, Zhang F, Deisseroth K, Tracey WD. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr Biol. 2007;17:2105–2116. doi: 10.1016/j.cub.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorfman R, Glazer L, Weihe U, Wernet MF, Shilo BZ. Elbow and Noc define a family of zinc finger proteins controlling morphogenesis of specific tracheal branches. Development. 2002;129:3585–3596. doi: 10.1242/dev.129.15.3585. [DOI] [PubMed] [Google Scholar]
- 3.Flores C, Engels W. Microsatellite instability in Drosophila spellchecker1 (MutS homolog) mutants. Proc Natl Acad Sci U S A. 1999;96:2964–2969. doi: 10.1073/pnas.96.6.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ainsley JA, Pettus JM, Bosenko D, Gerstein CE, Zinkevich N, Anderson MG, Adams CM, Welsh MJ, Johnson WA. Enhanced locomotion caused by loss of the Drosophila DEG/ENaC protein Pickpocket1. Curr Biol. 2003;13:1557–1563. doi: 10.1016/s0960-9822(03)00596-7. [DOI] [PubMed] [Google Scholar]
- 5.Weihe U, Dorfman R, Wernet MF, Cohen SM, Milan M. Proximodistal subdivision of Drosophila legs and wings: the elbow-no ocelli gene complex. Development. 2004;131:767–774. doi: 10.1242/dev.00979. [DOI] [PubMed] [Google Scholar]
- 6.Luque CM, Milan M. Growth control in the proliferative region of the Drosophila eye-head primordium: the elbow-noc gene complex. Dev Biol. 2007;301:327–339. doi: 10.1016/j.ydbio.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 7.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 8.Xu K, Bogert BA, Li W, Su K, Lee A, Gao FB. The fragile X-related gene affects the crawling behavior of Drosophila larvae by regulating the mRNA level of the DEG/ENaC protein pickpocket1. Curr Biol. 2004;14:1025–1034. doi: 10.1016/j.cub.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 9.Wu CF, Ganetzky B. Genetic alteration of nerve membrane excitability in temperature-sensitive paralytic mutants of Drosophila melanogaster. Nature. 1980;286:814–816. doi: 10.1038/286814a0. [DOI] [PubMed] [Google Scholar]
- 10.Siddiqi O, Benzer S. Neurophysiological defects in temperature-sensitive paralytic mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1976;73:3253–3257. doi: 10.1073/pnas.73.9.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 12.Sokabe T, Tsujiuchi S, Kadowaki T, Tominaga M. Drosophila painless is a Ca2+-requiring channel activated by noxious heat. J Neurosci. 2008;28:9929–9938. doi: 10.1523/JNEUROSCI.2757-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams CM, Anderson MG, Motto DG, Price MP, Johnson WA, Welsh MJ. Ripped pocket and pickpocket, novel Drosophila DEG/ENaC subunits expressed in early development and in mechanosensory neurons. J Cell Biol. 1998;140:143–152. doi: 10.1083/jcb.140.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodman MB, Ernstrom GG, Chelur DS, O'Hagan R, Yao CA, Chalfie M. MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature. 2002;415:1039–1042. doi: 10.1038/4151039a. [DOI] [PubMed] [Google Scholar]
- 15.Chalfie M, Wolinsky E. The identification and suppression of inherited neurodegeneration in Caenorhabditis elegans. Nature. 1990;345:410–416. doi: 10.1038/345410a0. [DOI] [PubMed] [Google Scholar]
- 16.Driscoll M, Chalfie M. The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature. 1991;349:588–593. doi: 10.1038/349588a0. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Anoveros J, Ma C, Chalfie M. Regulation of Caenorhabditis elegans degenerin proteins by a putative extracellular domain. Curr Biol. 1995;5:441–448. doi: 10.1016/s0960-9822(95)00085-6. [DOI] [PubMed] [Google Scholar]
- 18.Huang M, Gu G, Ferguson EL, Chalfie M. A stomatin-like protein necessary for mechanosensation in C elegans. Nature. 1995;378:292–295. doi: 10.1038/378292a0. [DOI] [PubMed] [Google Scholar]
- 19.Du H, Gu G, William CM, Chalfie M. Extracellular proteins needed for C. elegans mechanosensation. Neuron. 1996;16:183–194. doi: 10.1016/s0896-6273(00)80035-5. [DOI] [PubMed] [Google Scholar]
- 20.Chelur DS, Ernstrom GG, Goodman MB, Yao CA, Chen L, R OH, Chalfie M. The mechanosensory protein MEC-6 is a subunit of the C. elegans touch-cell degenerin channel. Nature. 2002;420:669–673. doi: 10.1038/nature01205. [DOI] [PubMed] [Google Scholar]
- 21.O'Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat Neurosci. 2005;8:43–50. doi: 10.1038/nn1362. [DOI] [PubMed] [Google Scholar]
- 22.Chalfie M, Sulston JE, White JG, Southgate E, Thomson JN, Brenner S. The neural circuit for touch sensitivity in Caenorhabditis elegans. J Neurosci. 1985;5:956–964. doi: 10.1523/JNEUROSCI.05-04-00956.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall DH, Altun ZF. C. elegans atlas. CSHL Press; 2008. [Google Scholar]
- 24.Suzuki H, Kerr R, Bianchi L, Frokjaer-Jensen C, Slone D, Xue J, Gerstbrein B, Driscoll M, Schafer WR. In vivo imaging of C. elegans mechanosensory neurons demonstrates a specific role for the MEC-4 channel in the process of gentle touch sensation. Neuron. 2003;39:1005–1017. doi: 10.1016/j.neuron.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Ernstrom GG, Chalfie M. Genetics of sensory mechanotransduction. Annu Rev Genet. 2002;36:411–453. doi: 10.1146/annurev.genet.36.061802.101708. [DOI] [PubMed] [Google Scholar]
- 26.Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32:1071–1083. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 27.Eberl DF, Hardy RW, Kernan MJ. Genetically similar transduction mechanisms for touch and hearing in Drosophila. J Neurosci. 2000;20:5981–5988. doi: 10.1523/JNEUROSCI.20-16-05981.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caldwell JC, Miller MM, Wing S, Soll DR, Eberl DF. Dynamic analysis of larval locomotion in Drosophila chordotonal organ mutants. Proc Natl Acad Sci U S A. 2003;100:16053–16058. doi: 10.1073/pnas.2535546100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- 30.Gong Z, Son W, Chung YD, Kim J, Shin DW, McClung CA, Lee Y, Lee HW, Chang DJ, Kaang BK, Cho H, Oh U, Hirsh J, Kernan MJ, Kim C. Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. J Neurosci. 2004;24:9059–9066. doi: 10.1523/JNEUROSCI.1645-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albert JT, Nadrowski B, Gopfert MC. Mechanical signatures of transducer gating in the Drosophila ear. Curr Biol. 2007;17:1000–1006. doi: 10.1016/j.cub.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Gopfert MC, Albert JT, Nadrowski B, Kamikouchi A. Specification of auditory sensitivity by Drosophila TRP channels. Nat Neurosci. 2006;9:999–1000. doi: 10.1038/nn1735. [DOI] [PubMed] [Google Scholar]
- 33.Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li W, Feng Z, Sternberg PW, Xu XZ. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature. 2006;440:684–687. doi: 10.1038/nature04538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 36.Kernan M, Cowan D, Zuker C. Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. Neuron. 1994;12:1195–1206. doi: 10.1016/0896-6273(94)90437-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 related to Figure3. Behavioral responses to gentle touch in pickpocket mutants Although the class IV md-da neurons that are known to express ppk are nociceptive, it seemed possible that ppk could be expressed at low levels in non-nociceptive neurons. Therefore, we tested whether ppk was required for responses to gentle touch in Drosophila larvae (as DEG/ENaCs are in C. elegans). Using the gentle touch assay previously developed by Kernan et al [36], we tested ppk elBA400/B88 mutant larvae (n=22) for their behavioral response. The ppk elBA400/B88 mutant larvae were indistinguishable from wildtype larvae (n=28). Our results confirm a previous report that which found no requirement for ppk in gentle touch responses [4].
Figure S2 related to Figure 3: pickpocket is not required for thermal nociception. Response times for nocifensive behavior in response to 46°C probe. (A) Df(2L)A400/+ (4 trials, n=173) (B) Df(2L)B88h49/+, (3 trials, n=80) (C) Df(2L)B88h49/Df(2L)A400 (3 trials, n=100). (D) UAS-ppk-RNAi (7 trials, n=207) (E) ppk-Gal4 (4 trials, n=153) (F) ppk-GAL4/+; UAS-ppk-RNAi/+ (2 trials, n=73). (G) ppk-GAL4; UAS-ppk-RNAi (4 trials, n=130).
ppk elBA400/B88 mutant larvae were tested for thermal nociception responses by stimulating them with a probe heated to 46°C and their latency to initiation of larval nocifensive escape behavior was recorded. Wild type larvae initiated escape locomotion within the first 3 seconds of stimulation with the noxious heat probe. Similarly, Df(2L)A400/+ heterozygotes showed a response that was indistinguishable from wild type. The normal thermal nociception response of Df(2L)A400/+ demonstrates that removal of one copy of ppk does not cause a thermal nociception phenotype (Figure S2A). However, Df(2L)b88h49/+ heterozygotes did show an impaired response to noxious heat (Figure S2B). Thus, a mutation(s) in the Df(2)b88h49 strain dominantly impairs thermal nociception. If this were due to deletion of ppk then removal of the remaining copy of ppk in this background would be expected to enhance the Df(2)b88h49/+ phenotype. However, complete removal of ppk, in the ppk elBA400/B88 genotype, if anything, showed improved thermal nociception relative to Df(2L)b88h49/+ (Figure S2C). Since removal of an additional copy of ppk did not enhance the Df(2L)b88h49/+ phenotype and since Df(2L)A400/+ showed wild type thermal nociception behavior, our results do not indicate a role for ppk in thermal nociception. Consistent with this, knockdown of ppk with RNAi did not impair thermal nociception behavior despite the fact that knockdown of ppk effectively blocked mechanical nociception(Figure S2 D–G).
The mechanical nociception assay was performed by delivering a rapid stimulus with a Von Frey fiber to the dorsal midline of a wandering third instar larva (methods). The video shows the wild type escape locomotion response.