Abstract
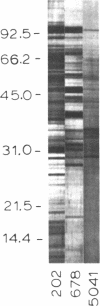
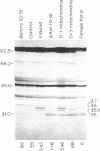
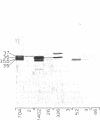
In addition to cytochrome oxidase, plant mitochondria have a second terminal oxidase called the alternative oxidase. The alternative oxidase is of great interest in that energy is not conserved when electrons flow through it. The potential energy of the system is thus lost as heat, and, in plants with high levels of the alternative oxidase, this results in thermogenesis. We have purified the alternative oxidase from mitochondria of the thermogenic spadix of Sauromatum guttatum and have identified its polypeptide constituents by using polyclonal antibodies. A 166-fold purification was achieved through a combination of cation-exchange (carboxymethyl-Sepharose) and hydrophobic-interaction (phenyl-Sepharose) chromatography. Polyclonal antibodies raised to the CM-Sepharose fractions readily immunoprecipitated alternative oxidase activity and immunoprecipitated four of the proteins that copurify with the activity. These proteins have apparent molecular masses of 37, 36, 35.5, and 35 kDa. Polyclonal antibodies raised individually to the 37-, 36-, and 35.5- plus 35-kDa proteins cross-reacted with all of these proteins, indicating the presence of common antigenic sites. The 37-kDa protein appears to be constitutive in Sauromatum, whereas expression of the 36- and 35-kDa proteins was correlated with presence of alternative pathway activity. The 35.5-kDa protein appears with loss of alternative pathway activity during senescence, indicating that this protein may be a degradation product of the 36-kDa protein. Binding of anti-36-kDa protein antibodies to total mitochondrial protein blots of five plant species indicated that similar proteins were always present when alternative pathway activity was observed.
Keywords: cyanide-resistant respiration, thermogenicity
Full text
PDF


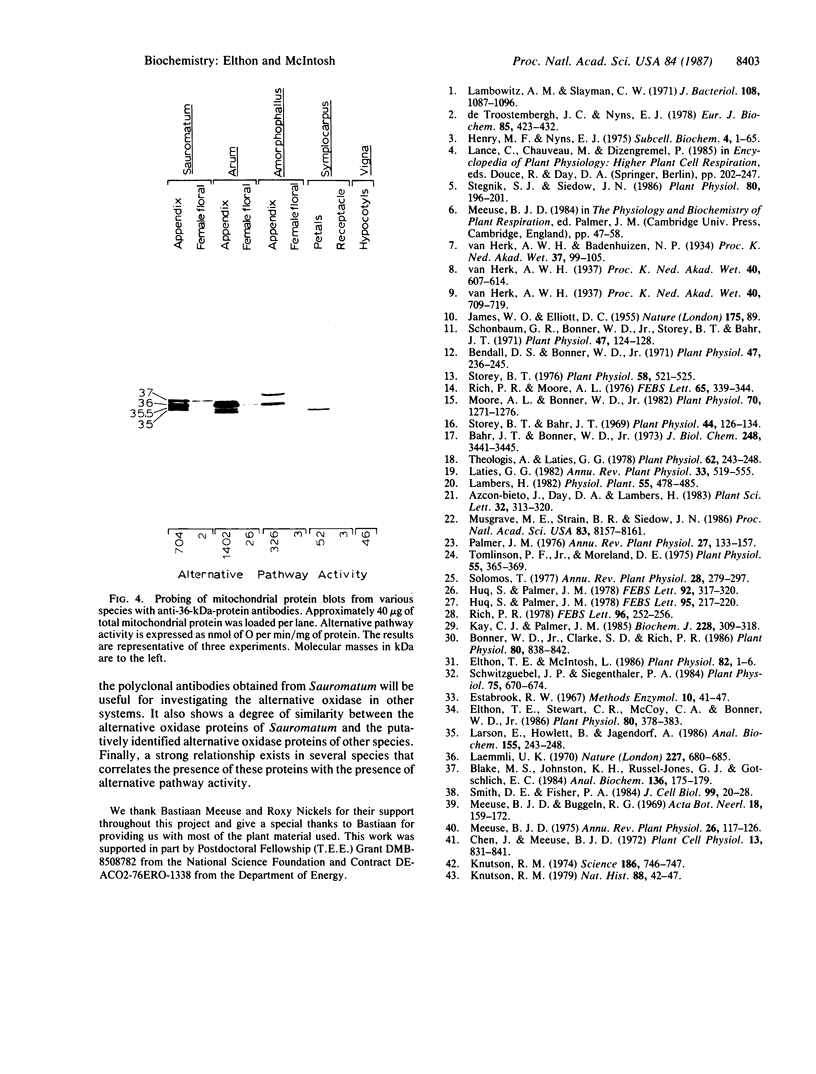
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahr J. T., Bonner W. D., Jr Cyanide-insensitive respiration. I. The steady states of skunk cabbage spadix and bean hypocotyl mitochondria. J Biol Chem. 1973 May 25;248(10):3441–3445. [PubMed] [Google Scholar]
- Bendall D. S., Bonner W. D. Cyanide-insensitive Respiration in Plant Mitochondria. Plant Physiol. 1971 Feb;47(2):236–245. doi: 10.1104/pp.47.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Bonner W. D., Clarke S. D., Rich P. R. Partial Purification and Characterization of the Quinol Oxidase Activity of Arum maculatum Mitochondria. Plant Physiol. 1986 Apr;80(4):838–842. doi: 10.1104/pp.80.4.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon T. E., McIntosh L. Characterization and Solubilization of the Alternative Oxidase of Sauromatum guttatum Mitochondria. Plant Physiol. 1986 Sep;82(1):1–6. doi: 10.1104/pp.82.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon T. E., Stewart C. R., McCoy C. A., Bonner W. D. Alternative Respiratory Path Capacity in Plant Mitochondria: Effect of Growth Temperature, the Electrochemical Gradient, and Assay pH. Plant Physiol. 1986 Feb;80(2):378–383. doi: 10.1104/pp.80.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M. F., Nyns E. D. Cyanide-insensitive respiration. An alternative mitochondrial pathway. Subcell Biochem. 1975 Mar;4(1):1–65. [PubMed] [Google Scholar]
- Huq S., Palmer J. M. Isolation of a cyanide-resistant duroquinol oxidase from Arum maculatum mitochondria. FEBS Lett. 1978 Nov 15;95(2):217–220. doi: 10.1016/0014-5793(78)80997-1. [DOI] [PubMed] [Google Scholar]
- Kay C. J., Palmer J. M. Solubilization of the alternative oxidase of cuckoo-pint (Arum maculatum) mitochondria. Stimulation by high concentrations of ions and effects of specific inhibitors. Biochem J. 1985 Jun 1;228(2):309–318. doi: 10.1042/bj2280309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson R. M. Heat production and temperature regulation in eastern skunk cabbage. Science. 1974 Nov 22;186(4165):746–747. doi: 10.1126/science.186.4165.746. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambowitz A. M., Slayman C. W. Cyanide-resistant respiration in Neurospora crassa. J Bacteriol. 1971 Dec;108(3):1087–1096. doi: 10.1128/jb.108.3.1087-1096.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson E., Howlett B., Jagendorf A. Artificial reductant enhancement of the Lowry method for protein determination. Anal Biochem. 1986 Jun;155(2):243–248. doi: 10.1016/0003-2697(86)90432-x. [DOI] [PubMed] [Google Scholar]
- Moore A. L., Bonner W. D. Measurements of membrane potentials in plant mitochondria with the safranine method. Plant Physiol. 1982 Nov;70(5):1271–1276. doi: 10.1104/pp.70.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrave M. E., Strain B. R., Siedow J. N. Response of two pea hybrids to CO2 enrichment: a test of the energy overflow hypothesis for alternative respiration. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8157–8161. doi: 10.1073/pnas.83.21.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich P. R., Moore A. L. The involvement of the protonmotive ubiquinone cycle in the respiratory chain of higher plants and its relation to the branchpoint of the alternate pathway. FEBS Lett. 1976 Jun 15;65(3):339–344. doi: 10.1016/0014-5793(76)80142-1. [DOI] [PubMed] [Google Scholar]
- Schonbaum G. R., Bonner W. D., Jr, Storey B. T., Bahr J. T. Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol. 1971 Jan;47(1):124–128. doi: 10.1104/pp.47.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwitzguebel J. P., Siegenthaler P. A. Purification of peroxisomes and mitochondria from spinach leaf by percoll gradient centrifugation. Plant Physiol. 1984 Jul;75(3):670–674. doi: 10.1104/pp.75.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegink S. J., Siedow J. N. Binding of Butyl Gallate to Plant Mitochondria : II. Relationship to the Presence or Absence of the Alternative Pathway. Plant Physiol. 1986 Jan;80(1):196–201. doi: 10.1104/pp.80.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. II. Oxidative phosphorylation in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):126–134. doi: 10.1104/pp.44.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. Respiratory Chain of Plant Mitochondria: XVIII. Point of Interaction of the Alternate Oxidase with the Respiratory Chain. Plant Physiol. 1976 Oct;58(4):521–525. doi: 10.1104/pp.58.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Laties G. G. Cyanide-resistant Respiration in Fresh and Aged Sweet Potato Slices. Plant Physiol. 1978 Aug;62(2):243–248. doi: 10.1104/pp.62.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson P. F., Moreland D. E. Cyanide-resistant Respiration of Sweet Potato Mitochondria. Plant Physiol. 1975 Feb;55(2):365–369. doi: 10.1104/pp.55.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Troostembergh J. C., Nyns E. J. Kinetics of the respiration of cyanide-insensitive mitochondria from the yeast Saccharomycopsis lipolytica. Eur J Biochem. 1978 Apr 17;85(2):423–432. doi: 10.1111/j.1432-1033.1978.tb12255.x. [DOI] [PubMed] [Google Scholar]