Abstract
BACKGROUND
Insulin resistance appears to be the best predictor of the development of diabetes in the children of patients with type 2 diabetes, but the mechanism responsible is unknown.
METHODS
We performed hyperinsulinemic–euglycemic clamp studies in combination with infusions of [6,6-2H2]glucose in healthy, young, lean, insulin-resistant offspring of patients with type 2 diabetes and insulin-sensitive control subjects matched for age, height, weight, and physical activity to assess the sensitivity of liver and muscle to insulin. Proton (1H) magnetic resonance spectroscopy studies were performed to measure intramyo-cellular lipid and intrahepatic triglyceride content. Rates of whole-body and subcutaneous fat lipolysis were assessed by measuring the rates of [2H5]glycerol turnover in combination with microdialysis measurements of glycerol release from subcutaneous fat. We performed 31P magnetic resonance spectroscopy studies to assess the rates of mitochondrial oxidative-phosphorylation activity in muscle.
RESULTS
The insulin-stimulated rate of glucose uptake by muscle was approximately 60 percent lower in the insulin-resistant subjects than in the insulin-sensitive control subjects (P<0.001) and was associated with an increase of approximately 80 percent in the intramyocellular lipid content (P=0.005). This increase in intramyocellular lipid content was most likely attributable to mitochondrial dysfunction, as reflected by a reduction of approximately 30 percent in mitochondrial phosphorylation (P=0.01 for the comparison with controls), since there were no significant differences in systemic or localized rates of lipolysis or plasma concentrations of tumor necrosis factor α, interleukin-6, resistin, or adiponectin.
CONCLUSIONS
These data support the hypothesis that insulin resistance in the skeletal muscle of insulin-resistant offspring of patients with type 2 diabetes is associated with dysregulation of intramyocellular fatty acid metabolism, possibly because of an inherited defect in mitochondrial oxidative phosphorylation.
TYPE 2 DIABETES IS RAPIDLY BECOMING a worldwide epidemic.1 Although the primary cause of this disease is unknown, insulin resistance appears to have a major role, as evidenced by cross-sectional studies demonstrating insulin resistance in virtually all patients with type 2 diabetes, as well as prospective studies demonstrating the presence of insulin resistance one to two decades before the onset of the disease.2-4 In addition, insulin resistance in the offspring of patients with type 2 diabetes has been shown to be the best predictor of the development of the disease.5
Despite much work, little is known about the factors responsible for insulin resistance in persons at risk. In this regard, studies measuring triglyceride content of muscle-biopsy specimens6 or intramyocellular lipid content by means of proton (1H) magnetic resonance spectroscopy7-9 indicate a strong relation between intramuscular lipid content and insulin resistance in skeletal muscle. Studies have also identified increases in plasma fatty acid concentrations10 and intramyocellular lipid content8 in the insulin-resistant offspring of patients with type 2 diabetes, suggesting that dysregulation of fatty acid metabolism may mediate the insulin resistance in these persons. Increases in the intramyocellular concentration of fatty acid metabolites in turn have been postulated to activate a serine kinase cascade, which decreases the insulin-stimulated activity of insulin receptor substrate 1–associated phosphatidylinositol 3-kinase11-14 and results in reduced glucose transport12 and glycogen synthesis.15,16
In the present study we examined a potential mechanism for the intramyocellular accumulation of lipids in young, lean, insulin-resistant offspring of patients with type 2 diabetes. These subjects are ideal candidates for studies examining the earliest defects leading to insulin resistance, since in contrast to patients with diabetes, they are young, lean, healthy, and unlikely to have other confounding factors. Since increases in intramyocellular triglyceride content could occur as a result of the increased delivery of fatty acids from lipolysis, decreased rates of mitochondrial oxidative phosphorylation, or both, we examined these processes in the insulin-resistant offspring of patients with type 2 diabetes and in insulin-sensitive control subjects. We assessed rates of whole-body and subcutaneous fat lipolysis by measuring the rates of [2H5]glycerol turnover in combination with microdialysis measurements of the release of glycerol from subcutaneous fat. We determined the rates of in vivo mitochondrial phosphorylation and the ratio of inorganic phosphate to phosphocreatine in skeletal muscle using phosphorus-31 (31P) magnetic resonance spectroscopy. In addition, since studies have also implicated several adipocyte-derived hormones (tumor necrosis factor α,17 interleukin-6,18 resistin,19 and adiponectin20) in causing insulin resistance, we also measured plasma concentration of these factors in this group of insulin-resistant persons.
METHODS
SUBJECTS
All subjects were recruited by means of local advertising over a two-year period (2001 to 2003) and were prescreened to confirm that they were in excellent health, lean, nonsmoking, and taking no medications. A birth weight above 2.3 kg (5 lb) and a sedentary lifestyle, as defined by an activity index questionnaire,21 were also required. Qualifying subjects (more than 150 persons) underwent a three-hour oral glucose-tolerance test (with a 75-g oral glucose load), after which two subgroups of subjects were consecutively selected to identify extreme phenotypes for insulin resistance and increased insulin sensitivity.
Insulin-resistant subjects (3 men and 11 women) were defined as having an insulin sensitivity index22 of less than 4.0 (indicating insulin resistance; lower values indicate greater insulin resistance), at least one parent or grandparent with type 2 diabetes, and at least one other family member with type 2 diabetes. Insulin-sensitive control subjects (five men and seven women) were defined by an insulin sensitivity index of greater than 6.3 (with or without a family history of type 2 diabetes).
All qualifying subjects subsequently underwent a complete medical history taking and a physical examination along with blood tests to verify that the following were normal: blood and platelet counts; concentrations of electrolytes, aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen, creatinine, cholesterol, and triglycerides; prothrombin time; and partial-thromboplastin time. In addition, subjects underwent 1H magnetic resonance spectroscopy studies to determine the triglyceride content of liver and muscle. The subjects then underwent a hyperinsulinemic–euglycemic clamp study to assess the responsiveness of liver, muscle, and fat to insulin or 31P magnetic resonance spectroscopy studies to assess the rates of muscle mitochondrial phosphorylation and the ratio of inorganic phosphate to phosphocreatine. Owing to the complexity of the protocol, not all the subjects were able to complete both the magnetic resonance spectroscopy and clamp studies. Two of the 12 control subjects, who initially qualified on the basis of their insulin sensitivity index, were subsequently excluded after the hyperinsulinemic–euglycemic clamp study identified them as insulin resistant, whereas all subjects who were found to be insulin resistant on the basis of the insulin sensitivity index were also found to be insulin resistant on the basis of the clamp study.
Written consent was obtained from each subject after the purpose, nature, and potential complications of the studies had been explained. The protocol was approved by the human-investigation committee of Yale University.
DIET AND STUDY PREPARATION
The subjects were instructed to eat a regular, weight-maintenance diet containing at least 150 g of carbohydrate per day for three days before admission for either the clamp or magnetic resonance spectroscopy study. All subjects were instructed not to perform any exercise other than normal walking for the three days before the study. To minimize changes in glucose metabolism resulting from ovarian hormonal effects, the female subjects were studied during the follicular phase (days 0 through 12) of the menstrual cycle.23 Subjects were admitted to the Yale–New Haven Hospital General Clinical Research Center the evening before the clamp or 31P magnetic resonance spectroscopy study, and the subjects continued to fast while having free access to regular drinking water until the completion of the study the following day.
MEASUREMENT OF METABOLITES AND HORMONES
Plasma glucose concentrations were measured with the use of a YSI 2700 STAT Analyzer (Yellow Springs Instruments). Plasma concentrations of insulin, glucagon, adiponectin, and resistin were measured with the use of double-antibody radioimmunoassay kits (Linco). Plasma tumor necrosis factor α and interleukin-6 were measured with the use of Quantine High Sensitivity kits (R&D Systems). Plasma fatty acid concentrations were determined with the use of a microfluorometric method.24 Urine nitrogen content was measured at the Mayo Medical Laboratories (Rochester, Minn.). Microdialysate glycerol concentrations (in 0.5-μl samples) were measured with the use of enzyme-linked colorimetry by a CMA 600 microdialysis analyzer (Microdialysis).25 Ethanol concentrations were determined enzymatically with the use of a YSI 2700 STAT Analyzer.25 Gas chromatography–mass spectrometry analyses of the enrichment of [6,6-2H]glucose and [2H5]glycerol in plasma were performed with the use of a Hewlett–Packard Mass Selective Detector (model 5971A) as previously described.25
MAGNETIC RESONANCE SPECTROSCOPY OF INTRAMYOCELLULAR AND INTRAHEPATIC TRIGLYCERIDE CONTENT
On a separate day, after a 12-hour fast, all subjects were transported by wheelchair to the Yale Magnetic Resonance Center, and localized 1H magnetic resonance spectroscopy spectra of the soleus muscle and liver were acquired on a 2.1-T Biospec Spectrometer (Bruker Instruments) as previously described.25
HYPERINSULINEMIC–EUGLYCEMIC CLAMP STUDIES
Basal rates of glucose and glycerol turnover were assessed during a three-hour basal period and insulin-stimulated rates were assessed with a three-hour hyperinsulinemic–euglycemic clamp with the use of 20 mU of insulin per square meter of body-surface area per minute, [6,6-2H]glucose, and [2H5]glycerol as previously described.26 Rates of whole-body energy expenditure and glucose and fat oxidation were assessed by indirect calorimetry (Deltratrack Metabolic Monitor, Sensormedics) during the last 30 minutes of the 3-hour base-line period and during the last 30 minutes of the clamp period.27 Localized rates of in vivo lipolysis were assessed before and during the clamp procedure with the use of microdialysis probes (CMA/60, CMA, Microdialysis) inserted into the fat deposits in two locations on the abdomen, 4 to 6 cm below the umbilicus, as previously described.25
MAGNETIC RESONANCE SPECTROSCOPY OF MITOCHONDRIAL PHOSPHORYLATION
Rates of mitochondrial phosphorylation were assessed by 31P magnetic resonance spectroscopy saturation transfer performed at 36.31 MHz with the use of a flat, concentric probe made of an inner coil 9 cm in diameter (for 31P) and a 13-cm outer coil tuned to proton frequency for scout imaging and shimming as previously described.28 Unidirectional rates of ATP synthesis were measured with the use of the saturation-transfer method applied to the exchange between inorganic phosphate and ATP. The steady-state magnetization of inorganic phosphate was measured in the presence of a selective irradiation of the γ resonance of ATP and compared with the magnetization of inorganic phosphate at equilibrium in a control spectrum (without irradiation of the γ resonance of ATP).28 The total acquisition time for 31P magnetic resonance spectra was about 120 minutes. The ratio of inorganic phosphate to phosphocreatine in the soleus muscle was measured by 31P magnetic resonance spectroscopy as previously described.29,30
STATISTICAL ANALYSIS
Statistical analyses were performed with StatView software (Abacus Concepts). To detect statistically significant differences between control subjects and insulin-resistant subjects, we used unpaired Student's t-tests for independent samples, with a two-sided P value of less than 0.05 considered to indicate statistical significance. Non-normally distributed data (i.e., data regarding the area under the curve) were logarithmically transformed. All data are expressed as means ±SE in the text.
RESULTS
CHARACTERISTICS OF THE SUBJECTS
Insulin-sensitive control subjects and insulin-resistant subjects were frequency matched for age, weight, height, body-mass index, and activity index and had similar fasting plasma concentrations of glycosylated hemoglobin, adiponectin, tumor necrosis factor α, interleukin-6, and resistin (Table 1). In contrast, the insulin sensitivity index was markedly lower in the insulin-resistant subjects than in the insulin-sensitive control subjects (mean [±SE], 2.8±0.2 vs. 10.2±1.4; P<0.001).
Table 1.
Characteristics of the Two Groups of Subjects.*
| Characteristic | Insulin-Sensitive Controls | Insulin-Resistant Subjects |
|---|---|---|
| Age (yr) | 28±7 | 26±7 |
| Weight (kg) | 60±13 | 64±9 |
| Height (m) | 1.69±0.11 | 1.65±0.09 |
| Body-mass index | 21±2 | 23±2 |
| Activity index† | 2.6±0.5 | 2.4±0.4 |
| Glycosylated hemoglobin (%)‡ | 5.1±0.3 | 5.2±0.4 |
| Adipocyte-derived factors | ||
| Adiponectin (μg/ml) | 12±4 | 11±4 |
| Tumor necrosis factor α (pg/ml) | 1.5±0.3 | 1.8±0.9 |
| Interleukin-6 (pg/ml) | 0.52±0.31 | 0.68±0.42 |
| Resistin (ng/ml) | 0.77±0.24 | 0.79±0.24 |
Plus–minus values are means ±SD. The body-mass index is the weight in kilograms divided by the square of the height in meters.
Values can range from 1.7 to 3.3, with higher values indicating greater activity.
Values were measured after an overnight fast.
ORAL GLUCOSE-TOLERANCE TEST
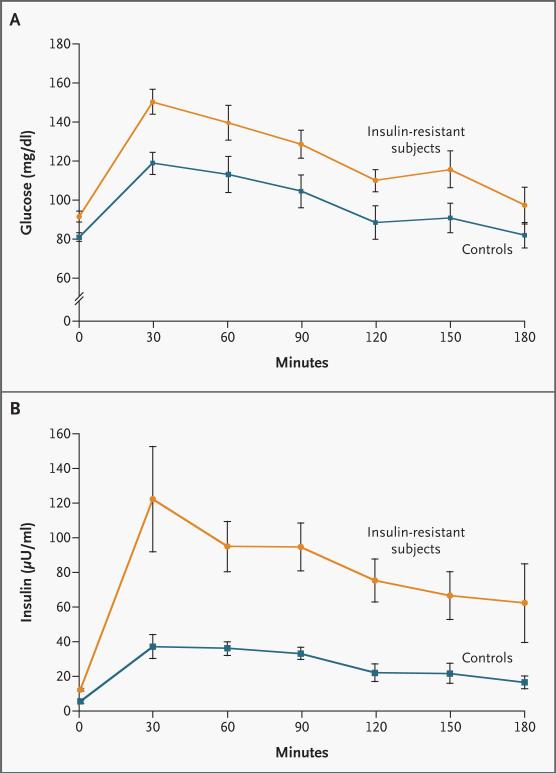
All subjects had normal glucose-tolerance tests, but the plasma concentrations of glucose (Fig. 1A) and insulin (Fig. 1B) before and during the test were significantly higher in the insulin-resistant subjects. Fasting plasma fatty acid concentrations were similar in the insulin-sensitive control subjects (0.37±0.05 mM) and the insulin-resistant subjects (0.47±0.05 mM, P=0.17) and decreased by approximately 80 percent in both groups during the glucose-tolerance test. There were no significant differences in the fasting plasma glucagon concentrations between the insulin-sensitive control subjects (56±4 pg per milliliter) and the insulin-resistant subjects (59±3 pg per milliliter, P=0.64).
Figure 1. Mean (±SE) Plasma Concentrations of Glucose (Panel A) and Insulin (Panel B) before and during an Oral Glucose-Tolerance Test in 9 Insulin-Sensitive Controls and 14 Insulin-Resistant Subjects.
P=0.016 for the comparison of the areas under the curve for glucose concentration of control subjects and insulin-resistant subjects, and P=0.002 for the comparison of the areas under the curve for insulin concentration of control subjects and insulin-resistant subjects. To convert values for glucose to millimoles per liter, multiply by 0.05551. To convert values for insulin to picomoles per liter, multiply by 6.0.
HYPERINSULINEMIC–EUGLYCEMIC CLAMP STUDIES
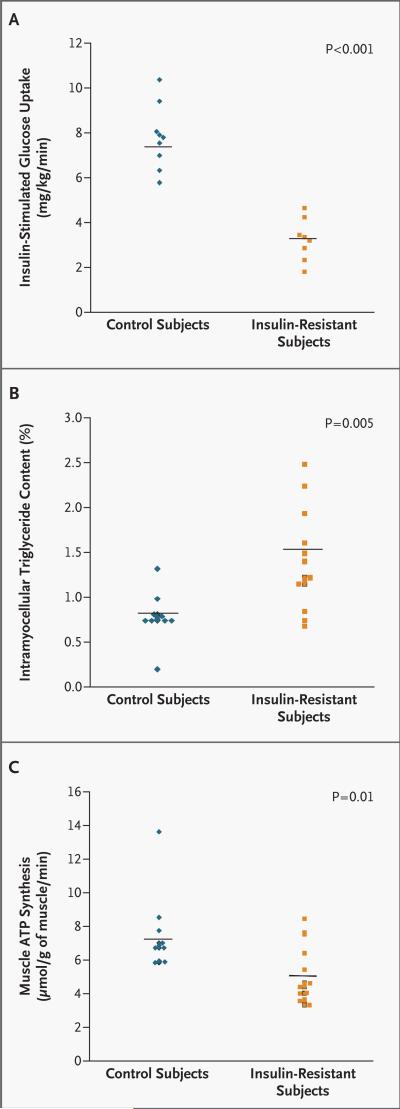
Fasting rates of glucose production were similar in the nine insulin-sensitive control subjects (2.3±0.1 mg per kilogram of body weight per minute) and the eight insulin-resistant subjects (2.0±0.3 mg per kilogram per minute, P=0.41) for whom results were available and were completely suppressed in both groups during the period of hyperinsulinemic–euglycemic clamping. In contrast, the rates of glucose infusion required to maintain euglycemia were approximately 60 percent lower in the insulin-resistant subjects than in the insulin-sensitive control subjects during clamping (3.3±0.3 mg per kilogram per minute vs. 7.7±0.5 mg per kilogram per minute, P<0.001) and the insulin-stimulated rates of peripheral glucose uptake were also approximately 60 percent lower in the insulin-resistant group (P< 0.001) (Fig. 2A). This reduction in peripheral glucose metabolism could be attributed mostly to a reduction of approximately 70 percent (P<0.001) in nonoxidative glucose disposal in the insulin-resistant subjects (data not shown). There were no significant differences in fasting or insulin-stimulated rates of whole-body glucose or fat oxidation between the two groups (data not shown). Fasting rates of whole-body energy expenditure tended to be lower in the insulin-resistant group than in the control group (21.8±0.7 kcal per kilogram per 24 hours vs. 24.6±1.1 kcal per kilogram per 24 hours, P=0.06), and the same was true for insulin-stimulated rates (21.6±0.9 kcal per kilogram per 24 hours and 24.9±0.7 kcal per kilogram per 24 hours, respectively; P=0.01).
Figure 2.
Insulin-Stimulated Rates of Muscle Glucose Metabolism (Panel A), Intramyocellular Lipid Content (Panel B), and Rates of Muscle Mitochondrial Phosphorylation Activity (Panel C) in Insulin-Sensitive Controls and Insulin-Resistant Subjects.
WHOLE-BODY AND LOCALIZED RATES OF GLYCEROL METABOLISM
Fasting rates of glycerol turnover were similar in the control group and the insulin-resistant group (0.21±0.03 μmol per minute and 0.18±0.02 μmol per minute, respectively; P=0.32), as was the insulin-induced suppression of glycerol turnover during the clamp study (0.11±0.01 μmol per minute and 0.09±0.01 μmol per minute, respectively; P= 0.64). Consistent with this finding, the interstitial glycerol concentration, as assessed by microdialysis, decreased by a similar degree in the insulin-sensitive control subjects (36±7 percent) and the insulin-resistant subjects (41±6 percent, P=0.67) during the hyperinsulinemic–euglycemic clamp study.
INTRAMYOCELLULAR AND INTRAHEPATIC TRIGLYCERIDE CONTENT
The intramyocellular lipid content in the soleus muscle was approximately 80 percent higher (P=0.005) in the 12 insulin-resistant offspring in whom it was measured than in the 10 insulin-sensitive control subjects in whom it was measured (Fig. 2B). There was no significant difference with respect to intrahepatic triglyceride content between insulin-resistant subjects (2.35±1.49 percent) and insulin-sensitive control subjects (0.47±0.16 percent, P=0.29).
RATES OF MITOCHONDRIAL PHOSPHORYLATION AND RATIO OF INORGANIC PHOSPHATE TO PHOSPHOCREATINE
Rates of mitochondrial phosphorylation in skeletal muscle were approximately 30 percent lower (P=0.01) in the 13 insulin-resistant subjects in whom it was evaluated than in the 10 control subjects in whom it was evaluated (Fig. 2C). The ratio of inorganic phosphate to phosphocreatine in the soleus muscle was reduced by approximately 20 percent (P=0.002) in the insulin-resistant subjects (0.113±0.004), as compared with the control subjects (0.137±0.005).
DISCUSSION
The lean, insulin-resistant offspring of patients with type 2 diabetes had severe insulin resistance, as compared with insulin-sensitive control subjects matched for age, height, weight, and activity. The difference could be attributed largely to a reduction of approximately 70 percent in insulin-stimulated nonoxidative muscle glucose metabolism. Using 1H magnetic resonance spectroscopy to measure intramyocellular lipid content, we found that insulin resistance in muscle was accompanied by an increase of approximately 80 percent in intramyocellular lipid content in the insulin-resistant subjects, as compared with the insulin-sensitive control subjects. These data are consistent with those of previous studies in humans7-9 and rodents,31,32 which have suggested that dysregulated intramuscular fatty acid metabolism has an important causative role in insulin resistance and may have a similar role in fat-induced insulin resistance in the skeletal muscle of the insulin-resistant offspring of patients with type 2 diabetes.
To assess whether the increase in intramyocellular lipid content in the insulin-resistant subjects was due to increased delivery of fatty acids to the muscle, we measured whole-body and localized rates of lipolysis. Rates of whole-body lipolysis were similar in the control subjects and the insulin-resistant subjects during the basal state and were both suppressed to a similar degree during the hyperinsulinemic–euglycemic clamp study. In a manner consistent with this finding, we found that the extent of insulin-induced suppression of the localized rates of lipolysis in subcutaneous fat, as assessed by microdialysis, was also similar in both groups. Taken together, these data suggest that insulin resistance was confined largely to skeletal muscle and that increased basal rates of peripheral lipolysis and defects in insulin-induced suppression of lipolysis do not have a major role in causing the increased intramyocellular lipid content in the insulin-resistant subjects.
To assess whether decreased mitochondrial activity may contribute to the increased intramyocellular lipid content, we also assessed the rates of muscle mitochondrial phosphorylation using 31P magnetic resonance spectroscopy. We found that the mitochondrial rates of ATP production were reduced by approximately 30 percent in the muscle of the insulin-resistant subjects, as compared with the insulin-sensitive control subjects. Consistent with this finding of altered mitochondrial function, we also found a reduced ratio of inorganic phosphate to phosphocreatine, which may reflect a lower ratio of type I fibers (mostly oxidative) to type II fibers (mostly glycolytic) in the insulin-resistant subjects.29,30 This finding is consistent with those of a biopsy study by Nyholm et al., who found an increased number of type IIb muscle fibers in overweight, insulin-resistant, first-degree relatives of patients with type 2 diabetes.33 Taken together, these data suggest that the insulin-resistant offspring of patients with type 2 diabetes have an inherited reduction in mitochondrial content in muscle, which in turn may be responsible for the reduced rates of mitochondrial oxidative phosphorylation.
Several studies have also implicated a number of novel adipocyte-derived factors in mediating insulin resistance in patients with obesity and in those with type 2 diabetes.17,18,34-37 To address the potential role of resistin, tumor necrosis factor α, interleukin-6, and adiponectin in mediating insulin resistance in our insulin-resistant subjects, we measured the plasma concentrations of these factors and found no significant differences between the two groups. These data suggest that alterations in plasma concentrations of these adipocyte-derived factors do not have a major role in mediating insulin resistance in these persons.
We assessed systemic and localized rates of lipolysis, plasma concentrations of adipocyte-derived factors, and mitochondrial phosphorylation activity in muscle of healthy, young, lean, normoglycemic, insulin-resistant offspring of patients with type 2 diabetes. Taken together, our results support the hypothesis that insulin resistance in these young people is due to dysregulation of intramyocellular fatty acid metabolism, which may be caused by an inherited defect in mitochondrial oxidative phosphorylation. Such a defect might be due to a reduction in mitochondrial content, which in turn might be attributable to a reduced ratio of type I to type II muscle fibers. These results are similar to those in lean, elderly, insulin-resistant subjects, whose insulin resistance, in contrast to that in insulin-resistant off-spring, is most likely attributable to acquired defects in mitochondrial biogenesis, which lead to reductions in skeletal-muscle mitochondrial content.38 Furthermore, since mitochondria have a critical role in mediating glucose-induced insulin secretion,39 the presence of similar inherited defects in beta-cell mitochondrial function or content, in the setting of peripheral insulin resistance, might explain the increased incidence of diabetes in the insulin-resistant offspring of patients with type 2 diabetes.5
In this regard it is of interest that a common Gly482Ser polymorphism of the peroxisome-proliferator–activated receptor γ coactivator 1, a transcriptional regulator of genes responsible for mitochondrial biogenesis and fat oxidation,40 has been linked to an increased relative risk of type 2 diabetes in Danish populations41 as well as to altered lipid oxidation and insulin secretion in Pima Indians.42 These data also identify mitochondrial oxidative phosphorylation as a potential target for the prevention and treatment of type 2 diabetes. In addition, two recent studies involving DNA-microarray analysis suggest that there is a coordinated reduction in the expression of genes encoding peroxi-some-proliferator–activated receptor γ coactivator 1α, which are involved in oxidative phosphorylation, in the skeletal muscle of overweight patients with type 2 diabetes,43 obese Mexican-American patients with type 2 diabetes,44 and overweight nondiabetic subjects with a family history of diabetes.44
Acknowledgments
Supported by grants (K23 DK-02347, R01 AG-23686, R01 DK-063192, R01 DK-49230, P30 DK-45735, and M01 RR-00125) from the Public Health Service and the Yamanouchi USA Foundation.
We are indebted to Drs. Michael Lehrke and Mitch Lazar for assistance in measuring plasma resistin concentrations; to Dr. James Dziura for statistical assistance; to Yanna Kosover, Mikhail Smolgovsky, Anthony Romanelli, Aida Groszmann, Andrea Belous, Jonas Lai, Sandra Alfano, and the staff of the Yale–New Haven Hospital General Clinical Research Center for expert technical assistance with the studies; and to the volunteers for participating in this study.
REFERENCES
- 1.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–7. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 2.Lillioja S, Mott DM, Howard BV, et al. Impaired glucose tolerance as a disorder of insulin action: longitudinal and cross-sectional studies in Pima Indians. N Engl J Med. 1988;318:1217–25. doi: 10.1056/NEJM198805123181901. [DOI] [PubMed] [Google Scholar]
- 3.Lillioja S, Mott DM, Spraul M, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus: prospective studies of Pima Indians. N Engl J Med. 1993;329:1988–92. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- 4.DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM: a balanced overview. Diabetes Care. 1992;15:318–68. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- 5.Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic patients. Ann Intern Med. 1990;113:909–15. doi: 10.7326/0003-4819-113-12-909. [DOI] [PubMed] [Google Scholar]
- 6.Phillips DI, Caddy S, Ilic V, et al. Intramuscular triglyceride and muscle insulin sensitivity: evidence for a relationship in nondiabetic subjects. Metabolism. 1996;45:947–50. doi: 10.1016/s0026-0495(96)90260-7. [DOI] [PubMed] [Google Scholar]
- 7.Krssak M, Falk Petersen K, Dresner A, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–6. doi: 10.1007/s001250051123. Errata, Diabetologia 1999; 42:386, 1269. [DOI] [PubMed] [Google Scholar]
- 8.Perseghin G, Scifo P, De Cobelli F, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48:1600–6. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 9.Szczepaniak LS, Babcock EE, Schick F, et al. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol. 1999;276:E977–E989. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 10.Perseghin G, Ghosh S, Gerow K, Shulman GI. Metabolic defects in lean nondiabetic offspring of NIDDM parents: a cross-sectional study. Diabetes. 1997;46:1001–9. doi: 10.2337/diab.46.6.1001. [DOI] [PubMed] [Google Scholar]
- 11.Griffin ME, Marcucci MJ, Cline GW, et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–4. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 12.Dresner A, Laurent D, Marcucci M, et al. Effect of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253–9. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu C, Chen Y, Cline GW, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–6. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 14.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–11. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 15.Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 1994;93:2438–46. doi: 10.1172/JCI117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roden M, Price TB, Perseghin G, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97:2859–65. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 18.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 19.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–12. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 20.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipo-atrophy and obesity. Nat Med. 2001;7:941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 21.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 23.Diamond MP, Jacob R, Connolly-Diamond M, DeFronzo RA. Glucose metabolism during the menstrual cycle: assessment with the euglycemic, hyperinsulinemic clamp. J Reprod Med. 1993;38:417–21. [PubMed] [Google Scholar]
- 24.Miles J, Glasscock R, Aikens J, Gerich J, Haymond M. A microfluorometric method for the determination of free fatty acids in plasma. J Lipid Res. 1983;24:96–9. [PubMed] [Google Scholar]
- 25.Mayerson AB, Hundal RS, Dufour S, et al. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 2002;51:797–802. doi: 10.2337/diabetes.51.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maggs DG, Buchanan TA, Burant CF, et al. Metabolic effects of troglitazone monotherapy in type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:176–85. doi: 10.7326/0003-4819-128-3-199802010-00002. [DOI] [PubMed] [Google Scholar]
- 27.Petersen KF, Hendler R, Price T, et al. 13C/31P NMR studies on the mechanism of insulin resistance in obesity. Diabetes. 1998;47:381–6. doi: 10.2337/diabetes.47.3.381. [DOI] [PubMed] [Google Scholar]
- 28.Lebon V, Dufour S, Petersen KF, et al. Effect of triiodothyronine on mitochondrial energy coupling in human skeletal muscle. J Clin Invest. 2001;108:733–7. doi: 10.1172/JCI11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer RA, Brown TR, Kushmerick MJ. Phosphorus nuclear magnetic resonance of fast- and slow-twitch muscle. Am J Physiol. 1985;248:C279–C287. doi: 10.1152/ajpcell.1985.248.3.C279. [DOI] [PubMed] [Google Scholar]
- 30.Vandenborne K, Walter G, Ploutz-Snyder L, et al. Energy-rich phosphates in slow and fast human skeletal muscle. Am J Physiol. 1995;268:C869–C876. doi: 10.1152/ajpcell.1995.268.4.C869. [DOI] [PubMed] [Google Scholar]
- 31.Kraegen EW, Clark PW, Jenkins AB, Daley EA, Chisholm DJ, Storlien LH. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes. 1991;40:1397–403. doi: 10.2337/diab.40.11.1397. [DOI] [PubMed] [Google Scholar]
- 32.Kim JK, Fillmore JJ, Chen Y, et al. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci U S A. 2001;98:7522–7. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nyholm B, Qu Z, Kaal A, et al. Evidence of an increased number of type IIb muscle fibers in insulin-resistant first-degree relatives of patients with NIDDM. Diabetes. 1997;46:1822–8. doi: 10.2337/diab.46.11.1822. [DOI] [PubMed] [Google Scholar]
- 34.Steppan CM, Lazar MA. Resistin and obesity-associated insulin resistance. Trends Endocrinol Metab. 2002;13:18–23. doi: 10.1016/s1043-2760(01)00522-7. [DOI] [PubMed] [Google Scholar]
- 35.Tsao TS, Lodish HF, Fruebis J. ACRP30, a new hormone controlling fat and glucose metabolism. Eur J Pharmacol. 2002;440:213–21. doi: 10.1016/s0014-2999(02)01430-9. [DOI] [PubMed] [Google Scholar]
- 36.Pajvani UB, Scherer PE. Adiponectin: systemic contributor to insulin sensitivity. Curr Diab Rep. 2003;3:207–13. doi: 10.1007/s11892-003-0065-2. [DOI] [PubMed] [Google Scholar]
- 37.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 38.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–2. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luft R, Luthman H. Mitokondriernas patofysiologi: från Lufts sjukdom till åldrande och diabetes. Lakartidningen. 1993;90:2770–5. [PubMed] [Google Scholar]
- 40.Wu Z, Puigserver P, Andersson U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–24. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 41.Ek J, Andersen G, Urhammer SA, et al. Mutation analysis of peroxisome proliferator-activated receptor-gamma coactivator-1 (PGC-1) and relationships of identified amino acid polymorphisms to Type II diabetes mellitus. Diabetologia. 2001;44:2220–6. doi: 10.1007/s001250100032. [DOI] [PubMed] [Google Scholar]
- 42.Muller YL, Bogardus C, Pedersen O, Baier L. A Gly482Ser missense mutation in the peroxisome proliferator-activated receptor gamma coactivator-1 is associated with altered lipid oxidation and early insulin secretion in Pima Indians. Diabetes. 2003;52:895–8. doi: 10.2337/diabetes.52.3.895. [DOI] [PubMed] [Google Scholar]
- 43.Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–73. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 44.Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100:8466–71. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]