Abstract
To examine the mechanism by which fish oil protects against fat-induced insulin resistance, we studied the effects of control, fish oil, and safflower oil diets on peroxisomal content, fatty acyl-CoA, diacylglycerol, and ceramide content in rat liver and muscle. We found that, in contrast to control and safflower oil-fed rats, fish oil feeding induced a 150% increase in the abundance of peroxisomal acyl-CoA oxidase and 3-ketoacyl-CoA thiolase in liver but lacked similar effects in muscle. This was paralleled by an almost twofold increase in hepatic peroxisome content (both P < 0.002 vs. control and safflower). These changes in the fish oil-fed rats were associated with a more than twofold lower hepatic triglyceride/diacylglycerol, as well as intramuscular triglyceride/fatty acyl-CoA, content. In conclusion, these data strongly support the hypothesis that n-3 fatty acids protect against fat-induced insulin resistance by serving as peroxisome proliferator-activated receptor-α ligands and thereby induce hepatic, but not intramuscular, peroxisome proliferation. In turn, an increased hepatic β-oxidative capacity results in lower hepatic triglyceride/diacylglycerol and intramyocellular triglyceride/fatty acyl-CoA content.
Keywords: peroxisome proliferator-activating receptor-α, β-oxidation, diacylglycerol, acyl-CoA oxidase, 3-ketoacyl-CoA thiolase
Insulin resistance is a critical factor in the pathogenesis of type 2 diabetes (16). Recent studies have demonstrated a strong relationship between accumulation of intramyocellular and intrahepatic triglycerides and insulin resistance in these organs (5, 7, 8, 10, 13). In contrast to most long-chain fatty acids, long-chain n-3 fatty acids have been shown to protect against fat-induced insulin resistance (6, 11, 12, 17–19). Recent studies by our group have demonstrated a lower intramyocellular triglyceride content in fish oil-fed rats compared with safflower oil-fed rats (6). One possible explanation for this finding is that n-3 fatty acids promote increased fatty acid oxidation by stimulating peroxisome proliferator-activated receptor-α (PPARα) in liver, which in turn induces peroxisome proliferation in liver. Indeed, it was recently demonstrated in vitro that n-3 polyunsaturated fatty acids, such as docosahexaenoic acid (C22:6n-3) and eicosapentaenoic acid (C20:5n-3), bind to PPARα and serve as primary regulators of transcriptional events (3).
To examine this hypothesis, we pair-fed three groups of rats with isocaloric diets containing standard chow (control, 17% fat-derived calories), menhaden fish oil (fish oil, 59% fat-derived calories), or safflower oil (safflower oil, 59% fat-derived calories) and examined respective increases in peroxisomal and mitochondrial content in liver. Furthermore, because recent studies have implicated fatty acid metabolites as the mediators of fat-induced insulin resistance in muscle and liver by interfering with insulin activation of insulin receptor substrate (IRS)-1- and IRS-2-associated phosphatidylinositol 3-kinase activity (respectively 7, 16), we also measured fatty acyl-CoA, diacylglycerol, and ceramide content in these tissues.
METHODS
Animals and Research Design
Male Sprague-Dawley rats (body wt ~100 g) were purchased from Charles River (Raleigh, NC) and housed under standard vivarium conditions (12:12-h light-dark cycle). Weight-matched rats were divided into control, safflower, and fish oil groups (studies 1 and 2; n = 10 in each group) for 21 days of dietary treatment. Control rats were fed a high-carbohydrate diet (59% carbohydrate-derived calories, 17% soybean oil, 24% protein), whereas rats of the other two groups were supplied with high-fat diets ad libitum (59% fat-derived calories, 23% carbohydrate, 18% protein) consisting of either safflower oil or menhaden fish oil. An overview of the dietary free fatty acid composition is shown in Fig. 1A. All diets (nos. 112245, 112246, and 110700) were supplemented with minerals (no. 210025) and vitamins (no. 310025) and were purchased from Dyets (Bethlehem, PA).
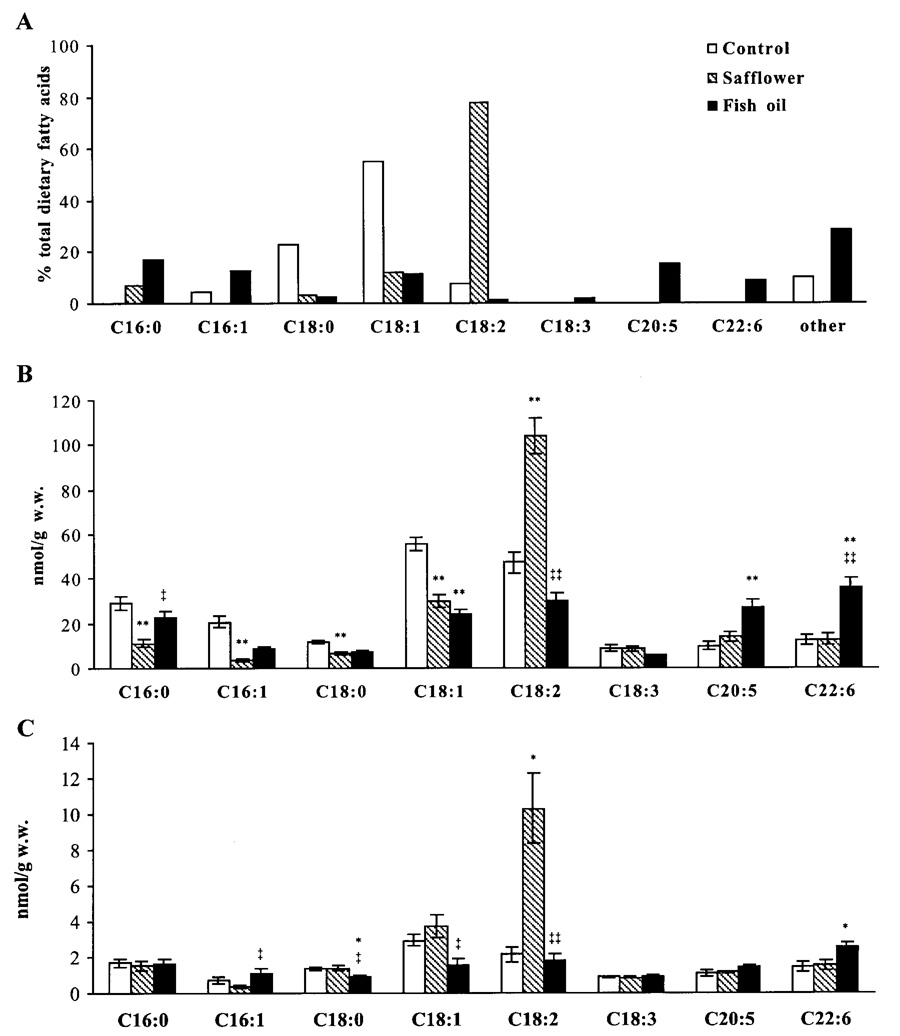
Fig. 1.
Fatty acid profile (A) of soybean oil (control diet), safflower oil, and fish oil and long-chain or very long-chain fatty acyl-CoA species in liver (B) and skeletal muscle (C) from control, safflower oil-fed, and fish oil-fed rats. Approximately 200 mg of tissue were homogenized, and long-chain/very long-chain fatty acyl-CoAs were extracted. With use of a tandem mass spectrometer, fatty acyl-CoAs were ionized in negative electrospray mode. As an internal standard, C17 CoA ester was used. *P < 0.01, **P < 0.0001 vs. control; ‡P < 0.02, ‡‡P < 0.0001 vs. safflower.
Study 1
Rats were housed in groups of five animals per cage. On day 20 of control, safflower oil, or fish oil feeding, rats were fasted overnight (18 h) and were anesthetized the next day with an injection of ketamine-xylazine (100/10 mg/kg ip). Livers and skeletal muscles were freeze-clamped in situ and powdered under liquid nitrogen with a mortar and pestle cooled to −70°C. Powdered tissues were stored at −80°C until further analysis.
Study 2
Rats were housed individually to control feed intake and body weight gain during the dietary regimen. For the 1st wk, rats were fed a regular laboratory chow to stabilize their metabolic conditions; then they were matched for body weight and divided into a control, safflower, or fish oil group for dietary treatment. Body weight and feed intake measurements were taken every 2–3 days, and feed was exchanged in the same intervals. After 20 days of feeding, rats were fasted overnight (18 h) and were anesthetized with ketamine-xylazine (100/10 mg/kg ip) on the next day. Blood samples were obtained via cardiac puncture. Fresh liver sections from peri- and centrilobular regions of the lobus sinister medialis were collected in a fixative for morphological investigations.
Metabolites and Hormone Analysis
Plasma glucose concentrations were determined with a glucose analyzer (Beckman, Fullerton, CA) and the glucose oxidase method. Plasma immunoreactive insulin was measured with a double-antibody radioimmunoassay technique (Linco Research, St. Charles, MO). Plasma fatty acids were determined with an acyl-CoA oxidase-based colorimetric kit (Wako NEFA-C, Wako Pure Chemicals Industries, Osaka, Japan).
Extraction and Measurement of Skeletal Muscle and Liver Triglyceride
The protocol was adapted from methods described previously (4, 17). Tissues (~200 mg) were homogenized for 90 s (Ultra Turrax T25, IKA Works, Wilmington, NC) in ice-cold chloroform-methanol (2:1, vol/vol). Triglycerides were extracted during 5-h shaking at room temperature. For phase separation, H2SO4 was added, samples were centrifuged, and the organic bottom layer was collected. The organic solvent was dried using a SpeedVacPlus (SC210A, Savant Instruments, Farmingdale, NY) and redissolved in chloroform. Triglyceride content of each sample was measured in triplicate by use of an enzymatic method (Sigma Diagnostics, St. Louis, MO).
Solid Phase Extraction of Skeletal Muscle and Liver Long-Chain and Very Long-Chain Fatty Acyl-CoA
Tissues (~200 mg) were homogenized, and long-chain (LC) and very long-chain (VLC) fatty acyl-CoAs were extracted according to methods described previously (1). Stepwise KH2PO4-2-propanol (1:1, vol/vol), saturated (NH4)2SO4, and acetonitrile were added, and the emulsion was vortexed and centrifuged. The supernatant was diluted with KH2PO4 (pH 4.9), and the acyl-CoA fraction was eluted using preconditioned oligonucleotide purification columns (no. 400771; Applied Biosystems, Foster City, CA). Acyl-CoA fractions were dried in a SpeedVacPlus and redissolved in methanol-H2O (1:1, vol/vol) for LC/MS/MS analysis. Total LC/VLC fatty acyl-CoA concentrations are defined by summarizing values obtained from all individual species.
Extraction of Skeletal Muscle and Liver Diacylglycerol and Ceramide
Tissues (~100 mg) were homogenized and lipids extracted according to methods described previously (1). After addition of ice-cold chloroform-methanol (2:1, vol/vol) containing the antioxidant 0.01% butylated hydroxytoluene, samples were extracted by shaking at room temperature. Organic and/or aqueous phase separation was induced by stepwise addition of chloroform and H2O. Samples were centrifuged, dried using a SpeedVacPlus, reconstituted in chloroform-methanol (3:1, vol/vol), and filtered (Acrodisc 13-mm syringe filter with 0.2 mm HAT Taffryn membrane; Pall Gelman laboratory, Ann Arbor, MI) for LC/MS/MS analysis. Total diacylglycerol and ceramide concentrations are defined as the sum of values obtained from all individual species.
LC/MS/MS Analysis
An API 3000 tandem mass spectrometer (Applied Biosystems, Foster City, CA) interfaced with a turbo ion spray or APCI source combined with a Perkin-Elmer Series 200 micropump and autosampler (Perkin-Elmer, Norwalk, CT) were employed. Fatty acyl-CoAs were ionized in negative electrospray mode, and the transition pairs [M-2H]2−/[M-H-80]− were monitored in MRM mode. Both diacylglycerol and ceramide species were ionized in APCI mode, and their transition pairs [M+H-H2O]+/R1+ or R2+ and [M+H-H2O]+/264.4 were monitored in positive MRM mode. As internal standards, C17 CoA ester, 1,3-sn-di-C15 diacylglycerol, and C6 sphingosine were used.
Histocytochemistry and Quantification of Peroxisomes
The alkaline 3,3-diaminobenzidine (DAB) reaction for peroxidase activity of catalase was adapted from LeHir et al. (14). Fresh peri- and centrilobular liver sections (n = 5 each group and region) were fixed by immersion in a fixative containing 1.5% glutaraldehyde and 4% paraformaldehyde in 0.1 M cacodylate buffer (pH 7.2) for 30 min. Liver samples were cut into ~40-µm sections, rinsed in glycine-NaOH buffer containing 7.5% sucrose, and incubated for 60 min with 5 mM DAB in 0.1 M glycine-NaOH buffer (pH 10.5) and 0.15% H2O2. Samples were rinsed thoroughly with 0.1 M glycine-NaOH buffer, postfixed in 2% aqueous osmium tetroxide for 90 min, rinsed with distilled H2O, and dehydrated through a graded series of ethanol. Liver sections embedded in Epon 812 were sectioned and examined in an electron microscope (Philips Tecnai 12, Eindhoven, The Netherlands). From each liver sample a total of 10 micrographs were taken from two separate blocks at a final magnification of ×17,000, and volume density of peroxisomes was estimated by the point-counting method.
Preparation of cDNA Probes
Probes for cytoplasmic β-actin, straight-chain acyl-CoA oxidase, 3-ketoacyl-CoA thiolase, PPARα, liver fatty acid-binding protein (L-FABP), and muscle and liver type carnitine palmitoyltransferase I (CPT-I) were amplified on an MJ Research PCR machine (MJ Research, Waltham, MA) under standard conditions, including 30 cycles at 95°C for 45 s, 58°C for 45 s, and 72°C for 2 min, followed by a 5-min hold at 72°C.
Vectors containing rat acyl-CoA oxidase and 3-ketoacyl-CoA thiolase cDNAs were kind gifts from Dr. Takashi Osumi, Department of Life Science, Himeji Institute of Technology, (Kamigori, Hyogo, Japan). Genes encoding β-actin, PPARα, L-FABP, or CPT-I were amplified from rat liver and muscle cDNA libraries (Clontech Laboratories) as described in Table 1.
Table 1.
Primers employed
| Gene | Sense Primer (5′–3′) | Antisense Primer (5′–3′) | Predicted Size, bp |
GeneBank Accession No. |
|---|---|---|---|---|
| β-actin | GGAATTCATGGTGGGTATGGGTCAG | GCTCTAGATTGATCTTCATGGTGCTA | 1125 | V012176 |
| AAGGACT | GGAGCCAG | |||
| PPARα | GGAATTCACATGAACAAGGTCAAGG | GCTCTAGAATCTCTTGCAACAGTGGG | 8500 | NM_013196 |
| CCCGGGTC | TGCAACG | |||
| L-FABP | CGGGATCCAAGTACCAAGTGCAGAG | GCTCTAGATTGCTGACTCTCTTGTAGA | 491 | V01235 or J00732 |
| CCAAGAGA | CGATGTCA | |||
| CPTI | GCTCTAGAACACCAGGCAGTAGCTT | TCCCCCCGGGTCTTCCCACCAGTCACT | 4700 (liver) | L07736 (liver) |
| TCCAGTTCA | TACATAGTT | 3000 (muscle) | AF029875 (muscle) |
Underlined sequences mark the restriction recognition sites of endonucleases. PPARα, peroxisome proliferator-activated receptor-α; L-FABP, liver fatty acid-binding protein; CPT-I, carnitine palmitoyltransferase I.
Preparation of Total RNA and Northern Blot Analysis
Total liver and skeletal muscle RNA was extracted from frozen tissues with an RNA isolation kit according to the manufacturer’s protocol (Totally RNA, Ambion, Austin, TX). Ten micrograms of total RNA per sample were separated on a denaturing agarose gel and transferred onto nitrocellulose membranes (Hybond-N, Amersham Pharmacia Biotech, Buckinghamshire, UK). Northern blots were hybridized with cDNA probes labeled with [α32P]dCTP (NEN, Boston, MA) by use of a Random Primer DNA labeling system (GIBCO-BRL Life Technologies, Gaithersburg, MD), and quantified using a phosphoimager and ImageQuant software (Molecular Dynamics, Sunnyvale, CA). All results are given in relation to expression of cytoplasmic β-actin mRNA. For reprobing, blots were stripped with boiling 0.1% SDS.
Statistical Analysis
All data are presented as means ± SE. ANOVA was performed on data at a minimum P < 0.05 threshold. A multiple-comparison Bonferroni-Dunn post hoc test was performed to determine significance among the three groups except for muscle acyl-CoA oxidase mRNA content, for which Fisher’s protected least significant difference test was performed, since the group size was six or less than six.
RESULTS
Basal Studies
Rats of all studies were matched according to their initial body weights before the onset of the dietary regimen. When held in groups of five per cage, safflower oil- and fish oil-fed rats gained ~1.2-fold more weight than control rats (P < 0.0001 vs. control, Table 2).
Table 2.
Body weights and basal measurements from 12-h-fasted control, safflower oil-fed, and fish oil-fed rats
| Basal Measurements | Control | Safflower | Fish Oil |
|---|---|---|---|
| Study 1 | |||
| Body weight, g | 290.1±4.9 | 352.4±6.5* | 349.9±5.6* |
| Study 2 | |||
| Body weight, g | 312.8±10.5 | 314.1±10.1 | 310.5±7.5 |
| Fasting plasma glucose, mM | 6.2±0.2 | 6.6±0.2 | 6.2±0.2 |
| Fasting plasma insulin, µU/ml | 20.3±4.9 | 25.1±4.2 | 11.2±4.2† |
Values are means ± SE for 10 rats/group. Animals were either held in groups of 5 rats/cage and fed ad libitum (study 1) or singly housed and pair-fed to match body weights (study 2).
P < 0.0001 vs. control;
P < 0.02 vs. safflower.
Isocaloric feeding (~350 cal·g body wt−1·day−1) of singly housed rats resulted in similar body weights (Table 2). Despite similar fasting plasma glucose concentrations, fish oil-fed rats displayed significantly lower fasting plasma insulin levels compared with control or safflower rats (P < 0.02 vs. control and safflower; Table 2).
Liver and Skeletal Muscle Triglyceride Content
Livers of rats fed safflower oil accumulated about eightfold more triglycerides than those from controls (P < 0.0001 vs. control). In contrast, fish oil feeding protected from this massive increase in hepatic triglycerides compared with the safflower-fed rats (Table 3).
Table 3.
Total triglyceride, long-chain diacylglycerol, ceramide, and long-chain or very long-chain fatty acyl-CoA levels in liver and skeletal muscle from control, safflower-fed, and fish oil-fed rats
| Control | Safflower | Fish Oil | |
|---|---|---|---|
| Liver | |||
| Triglyceride, mg/g | 2.58±0.16 | 20.20±1.64c | 7.87±0.64b,f |
| Diacylglycerol, nmol/g | 28.51±2.15 | 63.80±5.63 | 30.06±2.23f |
| Ceramide, nmol/g | 359.21±12.96 | 327.80±11.36 | 354.28±13.95 |
| LC/VLC fatty acyl-CoA, nmol/g | 166.08±10.54 | 179.36±14.51 | 143.75±8.25 |
| Skeletal muscle | |||
| Triglyceride, mg/g | 0.38±0.04 | 0.69±0.12b | 0.36±0.02d |
| Diacylglycerol, nmol/g | 67.94±5.24 | 58.56±7.26 | 47.49±3.12a |
| Ceramide, nmol/g | 157.28±14.65 | 122.09±8.54 | 106.15±6.03 |
| LC/VLC fatty acyl-CoA, nmol/g | 12.35±1.48 | 20.83±3.10a | 10.81±1.56e |
Values are means ± SE for 10 rats/group, expressed as shown for measures per wet wt. LC, long chain; VLC, very long chain.
P < 0.0135,
P = 0.005,
P = 0.0001 vs. control;
P = 0.01,
P = 0.007,
P = 0.0001 vs. safflower.
Consistent with the findings of our previous study (6), safflower oil-fed rats displayed an ~80% increase in triglyceride content compared with the controls, and fish oil feeding protected against this increase in muscle triglyceride (Table 3).
Liver and Skeletal Muscle Diacylglycerol Content
When animals were treated with 59% safflower oil, total hepatic LC diacylglycerol levels were about twofold higher than in rats fed with fish oil or control chow (P < 0.0001 vs. control and fish oil; Table 3), whereas no difference in total LC diacylglycerol accumulation between the two groups was present in skeletal muscle (Table 3). In contrast, when fish oil was fed, total hepatic LC diacylglycerol levels equaled levels found in control rats (Table 3). In skeletal muscles of fish oil-fed rats, total LC diacylglycerol concentrations were found to be reduced by 21% vs. control (P < 0.02 vs. control; Table 3).
When the individual LC diacylglycerol profiles in liver and skeletal muscle were screened, their fatty acid composition mainly reflected the dietary fatty acid composition. Therefore, when safflower oil, which consists mainly of polyunsaturated linoleic acid (C18:2; Table 2), was fed, the predominant fatty acid incorporated in diacylglycerols was C18:2, with 84% of total fatty acids in liver (P < 0.0001 vs. control and fish oil) and 68% in skeletal muscle (P < 0.0001 vs. control and fish oil). Neither rats of the control nor those of the fish oil group showed an equivalent tendency to accumulate any particulate diacylglycerol species.
Liver and Skeletal Muscle Fatty Acyl-CoA Content
Independent from the dietary regimen, the total hepatic LC and VLC acyl-CoA pools did not differ (Table 3).
Findings in skeletal muscle contrasted, because safflower oil-fed rats accumulated almost twice as much total LC/VLC acyl-CoAs than rats provided with either a control or a fish oil diet (P < 0.02 vs. control; P < 0.007 vs. fish oil). In contrast, when fish oil was the dietary fat source, total skeletal muscle acyl-CoA levels were similar to those of controls (Table 3).
When rats consumed safflower oil, consisting of 78% linoleic acid (C18:2), the corresponding fatty acyl-CoA species accounted for 58% of the total acyl-CoA fraction in liver and for 50% in skeletal muscle (Fig. 1, B and C). Compared with control or safflower oil-fed rats, fish oil feeding increased the acyl-CoA fractions of eicosapentaenoic (C20:5) and docosahexaenoic acid (C22:6) about twofold in liver (P < 0.0001 vs. control and safflower; Fig. 1B). In skeletal muscle of fish oil-fed rats, the docosahexaenoic acid (C22:6) fatty acyl-CoA species was found to be increased by 41% compared with controls (P < 0.02 vs. control; Fig. 1C).
Liver and Skeletal Muscle Ceramide Content
Ceramide, a biosynthetic precursor of sphingomyelin and a product of sphingomyelinase action on sphingomyelin, has been reported to induce differentiation, proliferation, or even cell arrest in certain cell types and to be implicated in the activation of stress kinases, such as c-Jun NH2-terminal kinase (9). Within the three groups, total ceramide levels were approximately twofold higher in livers than in skeletal muscles, but total ceramide levels were not different in liver or skeletal muscle among the three groups (Table 3).
Effect of Fish Oil on mRNA Expression of Peroxisomal Enzymes Involved in β-Oxidation
Peroxisomes contain a variety of enzymes involved in fatty acid degradation, such as acyl-CoA oxidase or 3-ketoacyl-CoA thiolase, controlling key steps of peroxisomal β-oxidation.
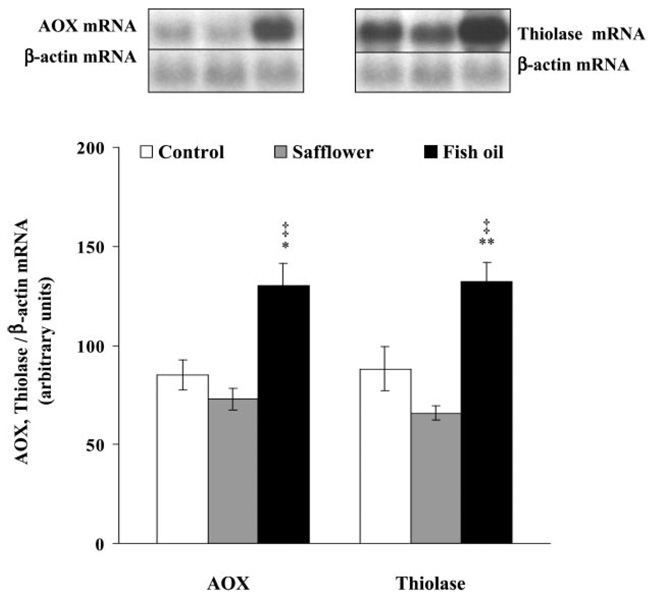
In liver, chronic fish oil feeding induced the transcription of both genes ~150% compared with control or safflower oil-fed rats (acyl-CoA oxidase: P > 0.001 vs. control, P 0.0001 vs. safflower; 3-ketoacyl-CoA thiolase: P > 0.002 vs. control, P > 0.0001 vs. safflower; Fig. 2).
Fig. 2.
Acyl-CoA oxidase (AOX) and 3-ketoacyl-CoA thiolase mRNA expression in livers from control (open bars), safflower oil-fed (gray bars), and fish oil-fed (solid bars) rats. Ten micrograms of total RNA per sample were separated on a denaturing agarose gel and transferred onto nitrocellulose membranes, and transcripts were hybridized with radiolabeled cDNA probes. Quantification was performed by use of phosphoimaging, and results are expressed in relation to cytoplasmic β-actin mRNA. *P < 0.001, **P < 0.002 vs. control; ‡P < 0.0001 vs. safflower.
Determination of peroxisomal acyl-CoA oxidase mRNA abundance in skeletal muscle revealed no difference between control (n = 6; 94 ± 21 arbitrary units) and fish oil-fed rats (n = 6; 107 ± 18 arbitrary units). Compared with both controls and fish oil-fed rats, a more than 50% reduced acyl-CoA oxidase mRNA content was detected in skeletal muscles from safflower oil-fed rats (n = 6; 38 ± 15 arbitrary units; P = 02 vs. fish oil).
Effect of Fish Oil on Hepatic Peroxisomal and Mitochondrial Density
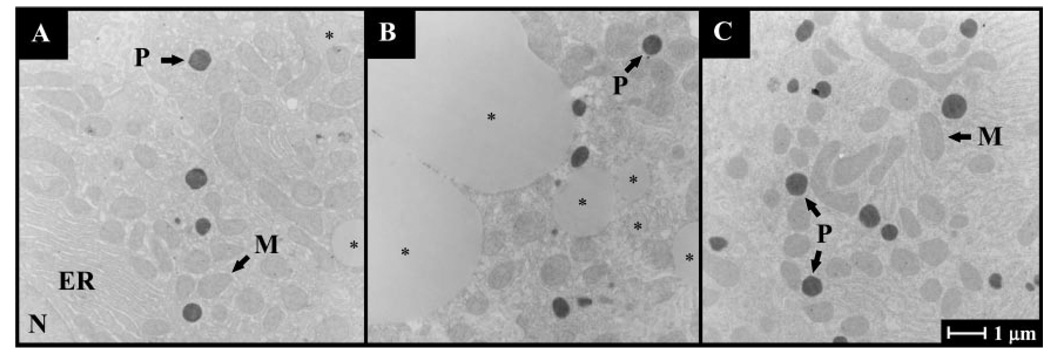
The observed fish oil-induced induction of peroxisomal enzymes was paralleled by an almost twofold increased peroxisomal density in liver compared with either control or safflower oil-fed rats. In liver sections obtained from control rats, peroxisomal volume accounted for 1.8% ± 0.1, in safflower oil-fed rats for 1.6 ± 0.1%, and in fish oil-fed rats 3.4 ± 0.4% of total cell volume (means ± SE for n = 10 each group; P < 0.0001 vs. control and safflower; Fig. 3).
Fig. 3.
Postembedding electron microscopy of centrilobular rat liver sections showing peroxisomes stained for catalase with alkaline 3,3’-diaminobenzidine from control (A), safflower oil-fed (B), and fish oil-fed (C) rats. P, peroxisome; M, mitochondrion; N, nucleus; *lipid droplet; ER, endoplasmic reticulum.
Furthermore, hepatic mitochondrial volume was increased >32% by dietary fish oil treatment. In liver sections obtained from control rats, mitochondrial volume accounted for 16.8% ± 0.8, in safflower oil-fed rats for 16.6% ± 0.9, and in fish oil-fed rats for 22.4% ± 0.2 of total cell volume (n = 10 each group; P < 0.004 vs. control and safflower; Fig. 3).
Effect of Fish Oil on mRNA Expression of Hepatic Proteins Involved in Lipid Metabolism
Both the dietary fat composition and the quantity of dietary fat-derived calories failed to affect hepatic PPARα mRNA abundance. Expression of L-FABP, a protein that has cytosolic distribution and is capable of binding 2 molecules of free fatty acids, was not altered either. In contrast, hepatic CPT I mRNA significantly decreased with safflower oil feeding but was similar in control and fish oil-fed rats (Table 5).
Table 5.
Gene expression (relative to β-actin mRNA expression) of proteins involved in lipid metabolism in livers from control, safflower-fed, and fish oil-fed rats
| mRNA/β-actin mRNA | Control (n = 9) |
Safflower (n = 10) |
Fish Oil (n = 10) |
|---|---|---|---|
| PPARα | 27.7±2.3 | 25.4±2.5 | 29.0±2.7 |
| L-FABP | 10.9±1.2 | 9.7±1.1 | 10.7±0.9 |
| CPT-I | 73.6±7.2 | 47.2±2.7* | 73.1±7.2 |
Gene expression is shown as means ± SE in arbitrary units.
P < 0.005 vs. control and fish oil.
DISCUSSION
In contrast to other types of fat, fish oil has been shown to protect against fat-induced insulin resistance; however, the mechanism responsible for this protective effect remains unknown (6, 18). In this regard, a recent study by our group has shown that rats fed a diet consisting of 59% of calories derived mainly from fish oil had greater insulin responsiveness and lower intramuscular triglyceride content compared with pair-fed rats on an isocaloric safflower oil diet (6). We hypothesized that the protective effect of fish oil on fat-induced insulin resistance in vivo may be due to the rats’ ability to activate PPARα and promote hepatic peroxisome proliferation, which in turn might lead to increased hepatic fatty acid oxidation. Indeed, in this study we found that, in contrast to control and safflower oil-fed rats, fish oil-fed rats had a 150% increase in the abundance of the peroxisome enzymes acyl-CoA oxidase and 3-ketoacyl-CoA thiolase, which was paralleled by an almost twofold increase in hepatic peroxisome content. These changes in the fish oil-fed rats were associated with markedly lower hepatic triglyceride/diacylglycerol content, as well as decreased intramuscular triglyceride/fatty acyl-CoA content. Despite massive hepatic peroxisome proliferation in the fish oil-fed rats, there was no associated increase in PPARα gene expression in liver. Taken together, these data strongly support the notion that n-3 fatty acids act as potent naturally occurring PPARα ligands that can bind and activate PPARα receptors predominantly in the liver to cause peroxisome proliferation and increased hepatic fat oxidation, resulting in lower hepatic triglyceride/diacylglycerol content and secondary intramyocellular triglyceride/fatty acyl-CoA content. In support of this hypothesis, similar intramuscular lipid-lowering and insulin-sensitizing effects have recently been reported for WY-14643, a potent synthetic PPARα agonist (19).
In contrast to liver, the expression of peroxisomal acyl-CoA oxidase mRNA in skeletal muscle was unaffected by fish oil treatment. These data suggest that the ability of fish oil to lower muscle triglyceride and fatty acyl-CoA content in addition to hepatic triglyceride content occurs primarily through activation of hepatic PPARα and is consistent with the reported tissue distribution pattern of rodent PPARα expressed in liver (2).
In view of the increasing evidence demonstrating a strong relationship between accumulation of intramuscular and intrahepatic lipid content and insulin resistance in these tissues (7, 8, 15), these data support the hypothesis that n-3 fatty acids protect against fat-induced insulin resistance by serving as PPARα ligands, which thereby induce hepatic peroxisome and mitochondrial proliferation. This, in turn, increases hepatic β-oxidative capacity, which results in lower hepatic triglyceride/diacylglycerol and intramyocellular triglyceride/fatty acyl-CoA content.
Table 4.
Fatty acid species (% of total) incorporated into LC diacylglycerols extracted from liver and skeletal muscle from control, safflower-fed, and fish oil-fed rats
| Diacylglycerol Species | Control | Safflower | Fish Oil |
|---|---|---|---|
| Liver | |||
| C16:0 | 31.4±1.0 | 17.7±0.4c | 32.4±0.5 |
| C16:1 | 4.7±0.5 | 0.6±0.0c | 4.9±0.2 |
| C18:0 | 5.0±0.2 | 2.8±0.1c | 6.0±0.2d |
| C18:1 | 40.9±0.7 | 17.3±0.5c | 33.8±0.5f |
| C18:2 | 18.1±0.2 | 61.7±0.6c | 23.0±0.4d |
| Skeletal muscle | |||
| C16:0 | 25.7±0.6 | 16.3±0.3c | 29.6±0.7e |
| C16:1 | 7.0±0.4 | 1.8±0.1c | 9.4±0.5e |
| C18:0 | 4.8±0.2 | 4.2±0.2a | 6.1±0.2f |
| C18:1 | 42.2±0.6 | 27.7±0.3c | 37.7±0.4f |
| C18:2 | 20.3±0.3 | 50.1±0.5c | 17.3±0.4f |
Values are means ± SE of 10 rats/group, expressed as % of total.
P < 0.01,
P < 0.001,
P < 0.0001 vs. control and fish oil;
P < 0.01,
P < 0.001,
P < 0.0001 vs. control.
Acknowledgments
We are indebted to Nicole Barucci, Yanna Kosover, Aida Grossmann, and Kim Murphy for expert technical assistance.
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK-49036 and P30 DK-45735. S. Neschen is a research associate, and G. I. Shulman is an investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 2.Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-α, -β, and -γ in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 3.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ. Proc Natl Acad Sci USA. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frayn KN, Maycock PF. Skeletal muscle triacylglycerol in the rat: methods for sampling and measurement, and studies of biological variability. J Lipid Res. 1980;21:139–144. [PubMed] [Google Scholar]
- 5.Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, Vinson C, Eckhaus M, Reitman ML. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest. 2000;105:271–278. doi: 10.1172/JCI7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jucker BM, Cline GW, Barucci N, Shulman GI. Differential effects of safflower oil vs. fish oil feeding on insulin-stimulated glycogen synthesis, glycolysis, and pyruvate dehydrogenase flux in skeletal muscle. Diabetes. 1999;48:134–140. doi: 10.2337/diabetes.48.1.134. [DOI] [PubMed] [Google Scholar]
- 7.Kim JK, Fillmore JJ, Chen Y, Yu C, Moore IK, Pypaert M, Lutz EP, Kako Y, Velez-Carrasco W, Goldberg IJ, Breslow JL, Shulman GI. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci USA. 2001;98:7522–7527. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JK, Gavrilova O, Chen Y, Reitman ML, Shulman GI. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J Biol Chem. 2000;275:8456–8460. doi: 10.1074/jbc.275.12.8456. [DOI] [PubMed] [Google Scholar]
- 9.Kolesnick RN, Goñi FM, Alonso A. Compartmentalization of ceramide signaling: physical foundations and biological effects. J Cell Physiol. 2000;184:285–300. doi: 10.1002/1097-4652(200009)184:3<285::AID-JCP2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Kraegen EW, Clark PW, Jenkins AB, Daley EA, Chisholm DJ, Storlien LH. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes. 1991;40:1397–1403. doi: 10.2337/diab.40.11.1397. [DOI] [PubMed] [Google Scholar]
- 11.Kraegen EW, Cooney GJ, Ye JM, Thompson AL, Furler SM. The role of lipids in the pathogenesis of muscle insulin resistance and beta cell failure in type II diabetes and obesity. Exp Clin Endocrinol Diabetes. 2001;109:S189–S201. doi: 10.1055/s-2001-18581. [DOI] [PubMed] [Google Scholar]
- 12.Kraegen EW, Cooney GJ, Ye J, Thompson AL. Triglycerides, fatty acids and insulin resistance—hyperinsulinemia. Exp Clin Endocrinol Diabetes. 2001;109:516–526. doi: 10.1055/s-2001-15114. [DOI] [PubMed] [Google Scholar]
- 13.Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 14.LeHir M, Herzog V, Fahimi HD. Cytochemical detection of catalase with 3,3’-diaminobenzidine. A quantitative reinvestigation of the optimal conditions. Histochemistry. 1979;64:51–66. doi: 10.1007/BF00493354. [DOI] [PubMed] [Google Scholar]
- 15.Oakes ND, Cooney GJ, Camilleri S, Chisholm DJ, Kraegen EW. Mechanisms of liver and muscle insulin resistance induced by chronic high-fat feeding. Diabetes. 1997;46:1768–1774. doi: 10.2337/diab.46.11.1768. [DOI] [PubMed] [Google Scholar]
- 16.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Storlien LH, Jenkins AB, Chisholm DJ, Pascoe WS, Khouri S, Kraegen EW. Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes. 1991;40:280–289. doi: 10.2337/diab.40.2.280. [DOI] [PubMed] [Google Scholar]
- 18.Storlien LH, Kraegen EW, Chisholm DJ, Ford GJ, Bruce DG, Pascoe SW. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science. 1987;237:885–888. doi: 10.1126/science.3303333. [DOI] [PubMed] [Google Scholar]
- 19.Ye JM, Doyle PJ, Iglesias MA, Watson DG, Cooney GJ, Kraegen EW. Peroxisome proliferator-activated receptor (PPAR)-α activation lowers muscle lipids and improves insulin sensitivity in high fat-fed rats. Diabetes. 2001;50:411–417. doi: 10.2337/diabetes.50.2.411. [DOI] [PubMed] [Google Scholar]