Abstract
1. Detritus that forms the basis for mosquito production in tree hole ecosystems can vary in type and timing of input. We investigated the contributions of plant- and animal-derived detritus to the biomass of Aedes triseriatus (Say) pupae and adults by using stable isotope (15N and 13C) techniques in lab experiments and field collections.
2. Lab-reared mosquito isotope values reflected their detrital resource base, providing a clear distinction between mosquitoes reared on plant or animal detritus.
3. Isotope values from field-collected pupae were intermediate between what would be expected if a single (either plant or animal) detrital source dominated the resource base. However, mosquito isotope values clustered most closely with plant-derived values, and a mixed feeding model analysis indicated tree floral parts contributed approximately 80% of mosquito biomass. The mixed model also indicated that animal detritus contributed approximately 30% of mosquito tissue nitrogen.
4. Pupae collected later in the season generally had isotope values that were consistent with an increased contribution from animal detritus, suggesting this resource became more nutritionally important for mosquitoes as plant inputs declined over the summer.
Keywords: Aedes triseriatus, tree hole, stable isotope, 13C, 15N, detritus
Introduction
Determinants of adult mosquito production from larval habitats include abiotic factors such as temperature and rainfall, and biotic factors such as predation, parasitism, and competition (Blaustein & Chase 2007; Juliano 2009). Although such broad ecological factors affect mosquito production from small, discrete container habitats, resource inputs are often the primary and fundamental limits to larval growth and subsequent adult emergence (Carpenter 1983; Hard et al. 1989; Lounibos et al. 1993; Kitching 2000, 2001; Kaufman et al. 2002; Kneitel 2007). This resource limitation often manifests itself through severe intra- and inter-specific competition that affects numbers of adults, their size, and their vectorial capacity, and ultimately impacts disease transmission dynamics (Hawley 1985; Alto et al. 2005; Bevins 2007). Barrera et al.(2006), for example, concluded that larvae of Aedes aegypti, the primary vector of dengue world-wide, commonly are food limited and compete for resources, leading to reduced body sizes of adult females. This reduced size may in turn affect dispersal and biting rates of adult females (Maciel-de-Freitas et al. 2007).
Aedes triseriatus is a common container breeding mosquito in eastern North America and the primary vector of La Crosse encephalitis virus. Larvae develop in water filled tree holes and tires that are normally heterotrophic microbial habitats, driven largely by particulate inputs and subsequent microbial processing (Walker et al. 1991). Although tree holes are consistent recipients of plant detritus in the form of senescent leaf material (Carpenter 1983, Lounibos et al. 1992; Leonard & Juliano 1995), other inputs include flower parts, twigs, and terrestrial invertebrate carcasses (Lounibos et al. 1992; Yee et al. 2007a, b). Recent studies have emphasized the potential importance of animal (invertebrate) detritus inputs as they relate to container-breeding mosquito nutrition and to outcomes of larval competition (Daugherty & Juliano 2000; Yee & Juliano 2006; Harshaw et al. 2007; Yee et al. 2007a, b; Murrell and Juliano 2008). Insect carcasses appear to be roughly tenfold higher in food value for mosquito larvae compared to senescent leaf material (Yee & Juliano 2006; Yee et al. 2007b), potentially allowing co-existence of competing larval species in tree holes and increased production of Ae. triseriatus from these habitats (Harshaw et al. 2007; Yee et al. 2007b). Tree hole dwelling larvae have even been shown to alter their foraging (browsing) behaviors in response to different types of detritus (Kesavaraju et al. 2007).
Plant material inputs into larval container habitats in the field are typically 10 – 100X those of animal detritus, but invertebrate carcass inputs can periodically exceed those of plant derived material (Daugherty & Juliano 2000; Yee et al. 2007b), and invertebrate material can be the primary nutrient inputs in larval mosquito habitats such as pitcher plants (Gray et al. 2006; Hoekman et al. 2009). Previous experimental investigations of animal versus plant detritus effects on tree hole dwelling mosquitoes have been done in laboratory microcosms and one such study indicated that a 1:10 ratio of insect carcass to senescent leaf material is near optimal for Ae. triseriatus development (Yee et al. 2007b). Path analysis indicated that the insect detritus was largely responsible for the production of mosquito biomass in that study (Yee et al. 2007b). However, the contribution of animal-versus plant-derived detritus to mosquito production from natural tree holes or other container systems has not yet been determined. This represents an important unanswered question in our understanding of how organic inputs into tree hole ecosystems are translated into mosquito biomass and the related vectorial capacity of Ae. triseriatus and similar mosquitoes that breed in a wide variety of detritus-dependent habitats.
Stable isotopes, usually 13C and 15N, are now commonly employed to examine food webs in terrestrial and aquatic systems, and to determine the trophic status of components (Post 2002; Grey 2006; Herbert et al. 2006; Hood-Nowotny & Knols 2007; Pasquad et al. 2007; Layman et al. 2007). δ13C analyses can identify dietary sources of primary consumers because consumer tissue is typically close to the δ13C values of the food source (Goodkoep et al. 2006; Fry 2006). In contrast δ13N values typically increase with trophic level, making them useful for establishing trophic structure. Bi-plots of the isotope values usually help accentuate differences between consumer groups in a mixed food web (Phillips & Koch 2002; Pasquand et al. 2007). Based on food source and consumer isotope values, and elemental concentrations in source and consumer biomass, mass balance-concentration dependent mixing models allow estimation of dietary contributions to consumers (e.g., Phillips & Koch 2002; Fry 2006).
Because animal tissues are typically enriched in both 13C and 15N relative to plant material (Fry 2006), we sought to make use of this distinction to address the question of the detrital dietary resources for Ae. triseriatus in tree holes at our study site in Michigan. Based on the high nutritional content, rapid turnover of, and stimulation of mosquito growth by insect carcasses, we hypothesized that animal detritus would form a substantial portion of the resource base for mosquito biomass in tree holes.
Methods
We used a combination of laboratory studies and field collections to provide material for isotopic analysis. In lab studies, mosquitoes (Ae. triseriatus) were reared with single sources of detrital material in microcosms (e.g, Kaufman & Walker 2006). The microcosms included 300 ml of distilled water, a microbial inoculum from natural tree holes (Kaufman et al. 2002), and the detrital source. The detrital sources were: senescent oak (Quercus alba) leaves, beech (Fagus grandifolia) flower parts, lab-reared fruit flies (Drosophila melanogaster - Diptera) adults, or earthworms (unidentified taxa). Dry mass per microcosm of detritus was: oak leaves, 1 g; beech flowers, 0.6 g; earthworms, 0.4 g; and Drosophila, 0.3 g. Oak leaves, beech flower parts and earthworms were collected from the litter layer at our tree hole study field sites near the MSU campus (E. Lansing, MI). Plant material was added after drying (48 hr, 45° C), and animal material was lyophilized prior to microcosm introduction. Forty neonate mosquito larvae were added to each microcosm and adults were collected as they emerged over a period of several weeks. Adult mosquitoes from previous studies (Kaufman & Walker, 2006; Yee & Juliano 2006) that had been stored with desiccant were also assayed for isotope content. In one of the previous studies (Yee and Juliano 2006), cricket tissue was used as detrital source, and we subsequently obtained lab-reared Grylloides sigillatus from the same source colony (and fed the same diet) used in that study courtesy of Dr. Scott Sakaluk (Illinois State Univ., Normal IL).
Detritus (plant and animal) and Ae. triseriatus pupae were collected from tree holes at our E. Lansing study site periodically in late spring and summer of 2005 and 2006. We collected detritus samples primarily from the surface or near surface of the tree hole water column with the assumption that these reflected recent inputs into the system. In the cases of invertebrate carcasses and flower parts, this assumption was realistic, however; leaf detritus was problematic in that senescent leaves could have entered the system during the previous fall or been blown in anytime hence. We sought to obtain representative detrital inputs at the study site, but did not quantify the relative abundance of detritus categories. All samples were frozen (−80° C), lyophilized, and stored with desiccant before grinding and analysis. Invertebrate samples or mosquitoes collected from individual tree holes were pooled as separate subsamples. Quantities collected were sometimes of insufficient mass to allow for losses in sample preparation prior to isotope analysis, such as in the cases of 2 or fewer mosquito pupae, hence not all samples collected could be analyzed.
All samples were ground to fine powder using stainless steel ball bearings in microcentrifuge tubes on a multiple-sample, high speed shaker (Retsch MM300, Glen Mills, Clifton, NJ). Large bulk samples of plant material (e.g. leaves used in microcosm experiments) were first subsampled by taking small sections from several different leaves or flowers, followed by grinding in a mortar and pestle before being processed with the bead beater. Subsamples of the pulverized material were then weighed into tin cups and stored with desiccant until isotopic analysis.
Because animals partition 13C differently in lipid pathways compared to other tissues (Post et al. 2007), we compared isotope values from mosquito pools that were subjected to lipid extraction with untreated mosquito pools from the same study. The source of the mosquitoes was a microcosm experiment in which we had manipulated levels of added nitrate (Kaufman & Walker 2006). Lipid was extracted using a dichloromethane/methanol biphasic extraction procedure that we’ve used previously for microbial lipid analysis (Kaufman et al. 1999). This method is a modification of the standard Bligh & Dyer (1959) lipid extraction and differs primarily in the replacement of choloroform with dichlomethane as the non-polar lipid extractant (see also Peterson & Klug 1994 and references therein).
Carbon (13C, 12C) and nitrogen (15N, 14N) isotope content of the samples were determined with an elemental analyzer (EA3000, Eurovector, Milan, Italy) coupled to a stable isotope ratio mass spectrometer (Elementar, Mt. Laurel, NI) following procedures detailed in Ostrom et al. (1997) and Ghandi et al. (2004). Total C/N ratios were also determined for each sample. Stable isotope values are expressed in parts per mil (‰) according to the following equation:
where X is 13C or15N, and R is the corresponding ratio 13C/12C or 15N/14N. Rstandard was V-PDB or atmospheric N, for δ13C and δ15N, respectively. For δ13C and δ15N, laboratory standards were analyzed after every 10 unknown samples, with an accuracy and precision of ≤0.2 ‰ for both δ13C and δ15N.
To avoid statistical problems with distribution and transformation of proportionate (ratio) data, we compared isotope values or C/N ratios using the non-parametric Wilcoxon/Kruskal-Wallis rank sum methods. We used JMP® Statistical Discovery Software, V5.1 (www.jmpin.com, SAS Institute, Inc., Cary NC, USA) for theses analyses and for descriptive statistic calculations.
To estimate dietary contributions of detrital sources to mosquito tissue, we used the mixture model of Phillips & Koch (2002), available at www.epa.gov/wed/pages/models.htm. This model takes into account the total carbon and nitrogen concentrations of the diets (detrital inputs) and consumer (mosquito), the isotope values of the dietary sources and consumer, and the trophic fractionation (shifts in isotope ratio between organism and diet) of each isotope by the consumer. Fractionation of food resource isotope values was estimated from the results of lab-reared mosquitoes on single detritus sources. For the mixing model, we used the average isotope values for end members adjusted for fractionation factors determined in the lab studies, and the mixture was the average isotope values of field-collected mosquitoes (Phillips & Koch 2002).
Results
Lab studies
Plant detritus (oak leaves and beech flowers) used as initial sources in microcosm studies had lower δ13C andδ15N values relative to the invertebrate detrital sources (Fig. 1). Isotope values of earthworm tissues were roughly intermediate between the plants and insects examined. In general, adult mosquito tissue was enriched, relative to the detritus sources in 13C when reared on plant detritus, and in 15N when reared on animal sources, but mosquitoes reared on cricket carcasses were enriched in both 13C and 15N (Fig. 1) and there was little evidence of any isotope fractionation in mosquito tissue when Drosophila was the detrital source. Mosquitoes reared on oak leaves with additions of inorganic nitrogen (KNO3) to microcosms were enriched in 15N compared to mosquitoes reared on oak leaves without KNO3 addition (Fig. 1, Table 1).
Figure 1.
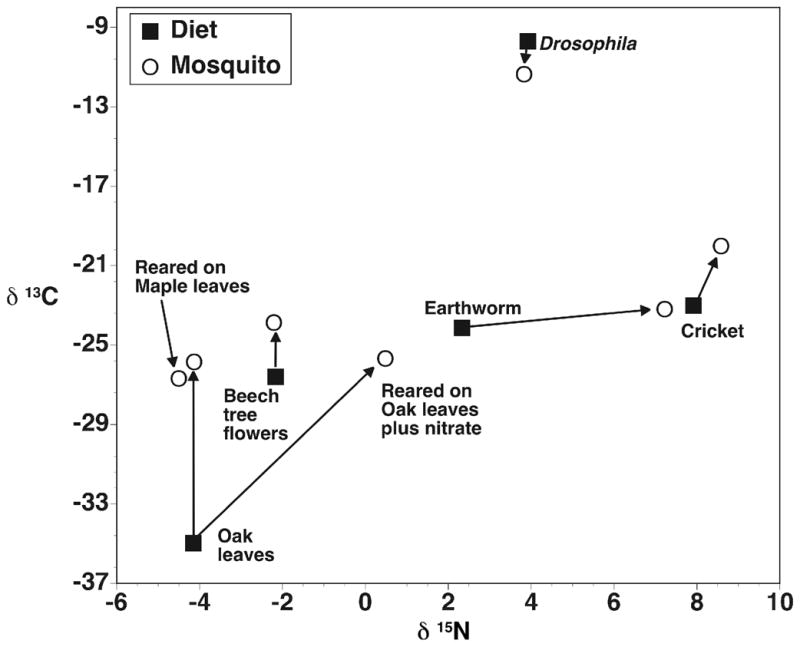
Stable isotope composition of detrital source and mosquitoes from laboratory microcosm experiments. Values are means from 3 – 4 analytical reps of pooled material. Arrows connect diet source with mosquitoes reared on that source. No maple leaf detrital (diet) material was available for analysis.
Table 1.
Nitrate addition during larval growth and lipid extraction effects on isotopic content and carbon/nitrogen (C/N) ratios of Ae. triseriatus adult tissue. Values are mean ± one S.E., n = 3. Non-parametric comparisons are results of Wilcoxon/Kruskal-Wallis rank sums tests.
| Nitrate | Lipid extraction | δ 15N | δ 13C | C/N |
|---|---|---|---|---|
| 0 | 0 | −2.2 + 0.2 | −26.4 + 0.4 | 4.6 + 0.2 |
| 0 | + | −2.1 + 0.1 | −25.9 + 0.2 | 4.0 + 0.1 |
| + | 0 | 0.4 + 0.3 | −25.4 + 0.3 | 4.7 + 0.1 |
| + | + | 0.6 + 0.2 | −26.3 + 0.4 | 4.1 + 0.1 |
| Non-parametric test | p-value | |||
| Nitrate | 0.005* | 0.298 | 0.575 | |
| Lipid Extraction | 0.810 | 0.689 | 0.005* | |
= significant with sequential Bonferroni adjustment
Lipid extraction did not affect δ13C or δ15N values from mosquito tissue, however; extraction significantly lowered the C/N ratio (Table 1).
Field collections
Leaf detritus from tree holes consisted of maple, oak, beech and unidentified leaves, and flower components were from oak and beech trees. Animal detritus from tree holes consisted mainly of small Diptera, other insects (Coleoptera, Lepidoptera), unidentified arthropods, and earthworms. As was seen in the laboratory studies, the two main categories of detrital material (plants and invertebrates) from field collections were distinct, but differences were not as pronounced as seen in the lab studies (Fig. 2). Plant detritus collected directly from tree holes had isotope values very similar to those seen in the lab studies (Fig. 1), and animal detritus isotope values were higher than plants. Field-collected mosquitoes had isotope values (Fig. 2) that were intermediate between those that would be expected if either plant or animal material were the sole resource base. Stable isotope values in field mosquitoes were most similar to those seen in mosquitoes reared in the lab on leaf material with an external source of nitrogen, or with tree flowers, as the detritus base.
Figure 2.
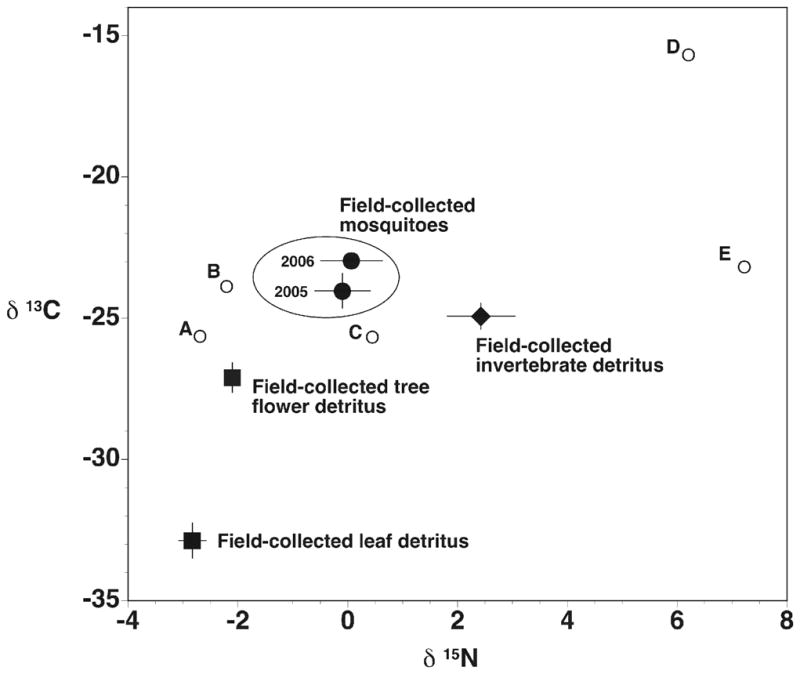
Stable isotope composition of detrital material and mosquitoes collected from field sites. Values are mean ± one SE, n = 16 – 40. Mean values of lab-reared mosquitoes are illustrated for reference with small open circles: A – reared with oak leaves, B – reared with beech flowers, C – reared with oak leaves plus nitrate, D – reared with insect (crickets, Drosophila) carcasses, E – reared with earthworm carcasses.
Carbon and nitrogen isotope values in mosquitoes collected from natural tree holes tended to increase during the course of a season in 2006 (Fig. 3). In 2006, the monthly trend was significant for both C and N isotope values (rank sum test, p = 0.012 for δ13C and p = 0.002 for δ15N). Mosquitoes were collected only in June and July in 2005, and there was no difference between months for either isotope (rank sum test, p = 0.647 for δ13C and p = 0.160 for δ15N).
Figure 3.
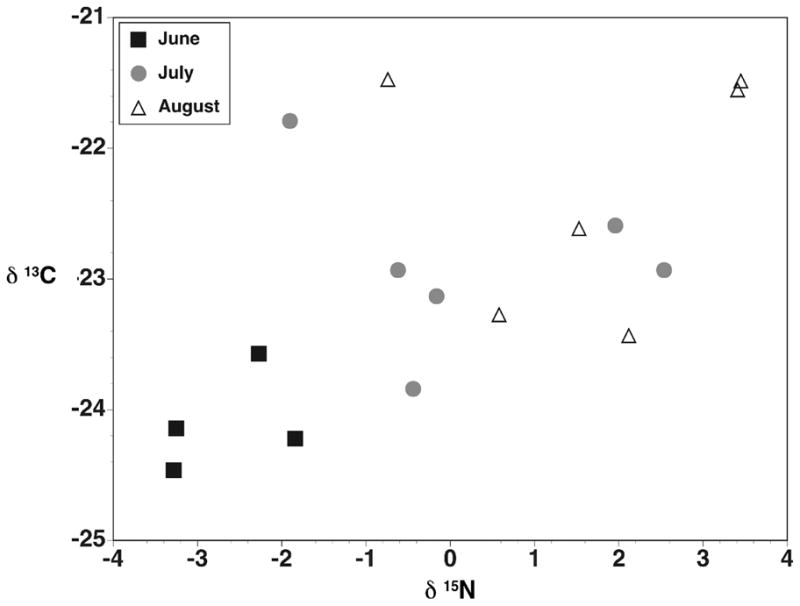
Stable isotope composition of mosquitoes collected from individual tree holes in 2006. Values are single analytical replicates from pooled pupae collected from an individual tree hole.
Because leaf material, tree floral parts, and invertebrates were ubiquitous inputs into tree holes at our study sites, we used isotope values and C and N concentrations for these three resource types in the mixing model. We also used the overall mean of isotope values from all field-collected mosquitoes from both years for the mixture (i.e., consumer) parameters. Estimates of diet contributions to mosquito biomass (Table 2) indicated that 82% of pupal biomass could be attributed to beech flower detritus, but that 31% of the nitrogen in pupal tissue was derived from invertebrate detritus. Surprisingly, the model indicated that leaf material contributed relatively little to mosquito growth. We should point out that the source parameters in Table 2 did not adequately circumscribe (i.e., enclose the mixture values in a triangle formed by the 3 sources - Phillips & Koch 2002) all subsets of the field mosquitoes, and therefore these source estimates do not model the late season (July and August) 2006 mosquitoes. A single isotope (15N), dual source (plant material and invertebrates) version of the model indicated that invertebrate material would have contributed almost nothing to mosquito biomass in June 2006, but approximately 10% in July and 20% in August.
Table 2.
Concentration-dependent mixing model estimates of dietary contributions to field-collected mosquito biomass and components. Isotope values are based on means for each diet type and adjusted for fractionation by mosquitoes determined in lab studies. Concentrations of carbon and nitrogen are mean % C and %N for each diet.
| Mosquito tissue (%) | Detritus values | ||||||
|---|---|---|---|---|---|---|---|
| Biomass | Carbon | Nitrogen | δ13C | δ15N | [C] | [N] | |
| Leaf | 8.6 | 8.8 | 3.4 | −24.3 | −2.8 | 46 | 1 |
| Beech flower | 81.8 | 82.5 | 65.6 | −23.4 | −2.1 | 45 | 2 |
| Invertebrate | 9.6 | 8.7 | 31.0 | −24.1 | 4.7 | 40 | 8 |
Discussion
To our knowledge, this study is the first to use stable isotopes for examining the natural food resource base for mosquito larvae. Ae. triseriatus reared on single sources of detritus had tissue δ13C and δ15N values that reflected the type of detritus, allowing back-calculated estimation of detrital resource bases for mosquitoes emerging from natural tree holes. Field-collected mosquitoes apparently grew mainly on compounds derived from plant detritus with animal detritus supplements. This is consistent with current conceptions about tree hole ecosystems (Kitching 2001) and helps to validate microcosm studies that utilize senescent plant material as the resource base (Carpenter 1983).
The form of plant material resource base, however, might need re-evaluation. Tree floral parts, mainly beech tree flowers, appeared to drive tree hole mosquito production in this study. In 2006 in particular, some tree holes in our study area in May seemed filled to capacity with flower parts. This material did not persist through the summer and was clearly much less refractory than senescent leaf material entering the system. Lounibos et al. (1992) also found tree flower inputs into tree holes in Florida to be substantial, but strongly seasonal and ephemeral. Nitrogen content of beech flowers was double that of typical leaf material (Table 2), indicating a much higher quality microbial substrate. In preliminary growth studies (Kaufman & Pelz-Stelinski, unpub. data), we found that mosquito production from microcosms with beech tree flowers was comparable to that found when Drosophila carcasses are used as the detrital source. The latter source is a very high quality growth substrate for mosquito larvae (Yee & Juliano 2006). Lounibos et al. (1993) also showed that Ae. triseriatus reared on flowers from live oak trees developed significantly faster than larvae reared on similar amounts of live oak leaves, indicating that this form of plant detritus is nutritionally superior for tree hole mosquitoes. Pulse inputs of these higher quality but inconsistent resources may be critical for Ae. triseriatus emergence in the face of intra- and interspecific larval competition (Kaufman and Walker 2006; Yee et al. 2007b).
Field mosquito isotope values were also similar to those of mosquitoes lab-reared on leaf material with an external nitrogen addition. The potassium nitrate used in that experiment was enriched (δ15N = 3.65, Kaufman, unpub. data) compared to the oak leaf material (δ15N = −4.15) and was likely incorporated into microbial biomass harvested by larvae. Nitrogen entering the system via stemflow (Carpenter 1982; Kaufman et al. 1999; Verdonschot et al. 2008) is likely to have very different isotope values than those found in plant detritus, and it has been shown that microbial and larval transformations of N compounds in tree holes are also dynamic (Walker et al. 1991; Kaufman et al. 1999; Kaufman & Walker 2006; Verdonschot et al. 2008). Therefore, the external sources of nitrogen incorporated by the leaf microbial community and subsequently assimilated by larvae could greatly alter the δ15N values in field mosquito tissues compared to original leaf δ15N values.
Another source of nitrogen entering the system would be invertebrate carcasses. Our results indicate that invertebrate detritus may contribute proportionately more to nitrogen-containing compounds in pupal biomass than plant material, consistent with the observations that mosquito isotope values changed during the season and that invertebrate carcass influence on growth of Ae. triseriatus in tree holes is most pronounced when plant material resources are limiting (Harshaw et al. 2007). That carbon may come primarily from one diet source while nitrogen comes from another is not surprising ((Stenroth et al. 2006)), but the ecological consequences of this have not often been addressed. For Ae. triseriatus larvae in tree holes, this may mean that emergence is delayed while waiting for nutritional input provided by a particular detritus category. In the case of nitrogen limitations, this might even be in the form of conspecific larval mortality.
Isotopic evidence here (Fig. 1) would indicate direct incorporation of Drosophila detritus because of the lack of fractionation between diet and mosquito. However, decomposer microorganisms associated with detritus have isotope values that are usually indistinguishable from the substrate (see discussion below). Yee et al. (2007a) suggest that both direct incorporation of animal detritus and harvesting of associated decay microorganisms is important for larval growth. Interestingly, mosquitoes grown on earthworm and cricket carcasses did show isotopic fractionation, possibly because particle size and decay rates differed enough from Drosophila to prevent direct ingestion of tissue. Additionally, gut contents were not removed from any invertebrates tested in this study, and this would influence not only carcass decay rates and associated microbial communities, but also digestion and assimilation processes in larval mosquitoes and resultant isotope values (Fry 2006).
Although our results point to plant derived material as being the dominant resource base for mosquitoes at the study site, animal detritus sources might be expected to increase in importance in other habitats. Invertebrate carcass inputs into tires, a habitat commonly exploited by Ae. triseriatus, occur at rates than can support mosquito production independent of other inputs (Daugherty & Juliano 2000), and animal detritus supports the mosquito, Wyeomyia smithii, in the pitcher plant ecosystem (Gray et al. 2006; Hoekman et al. 2009). It would be expected that many larval habitats would vary greatly in placement and proximity to sources of plant detritus. Additionally, as our results indicate (Fig. 3), the relative importance of animal detritus inputs varies seasonally and year to year, as documented for detrital inputs into tire habitats (Kling et al. 2007; Yee et al. 2010). Tires located in forested areas showed decreasing inputs of plant material during a season, while animal inputs remained constant (Kling et al. 2007), indicating an increased relative importance of animal inputs over time.
Plant detritus samples in this study appear to be atypical from two perspectives. First, the values seen for leaf detritus are relatively low in 13C compared to what has been reported for most C3 plants and are more similar to what might be expected from some algal groups (Post 2002). We found δ13C levels of −28 to −37 for leaf material, which are notably lower than ranges of −29.5 to −26 in leaf litter from similar tree taxa reported by Balesdent et al. (1993). However, Collier et al. (2002) measured δ13C a range of −32 to −30.3 in riparian vegetation at a New Zealand site and the range of values found in C3 plants extends to −34 and lighter (O’Leary 1988). Location and growth conditions can further influence isotope composition of plants (O’Leary 1988; Fry 2006). Second, the 13C fractionation of the plant material by mosquito larvae was much higher (more 13C) than expected, even with the assumption that larvae are consuming microbially transformed material. The isotopic composition of microbial heterotrophs on decaying material is thought to mirror the plant material substrate (Balesdent et al. 1993, Fry 2006) and mosquito larvae harvest this microbial biomass directly (Kaufman et al. 2001). Given the accepted range of fractionation of 13C into the next higher trophic levels (0.5 – 1%), we would need to account for at least 3 trophic levels between tree flower parts and mosquito consumption (difference of + 3.6 δ13C between flowers and mosquito), and 9 trophic levels between leaf and mosquito larvae (difference of + 8.6 δ13C between leaves and mosquito). Although it is clear that the larvae of many mosquito species feed primarily on microorganisms and not directly on leaf material (Kaufman et al. 2001, 2002), and that they also feed upon intermediate microbial grazers such as protozoans and rotifers (Kaufman et al. 2002; Knietel 2007), it’s difficult to conceptualize a food web with that many links in the tree hole system or that mosquitoes harvest only the higher trophic levels. The apparent lack of 15N fractionation between leaves and flowers and the mosquito was also unexpected, but within the range of insects developing in plant-based systems (Spence & Rosenheim 2005).
It seems more likely that the difference in δ13C values between leaf and mosquito reflects an unrecognized fractionation by the microorganisms. It’s been shown, for example, that the relatively low δ13C values from methanotrophic bacteria are detectable in midge larvae and other aquatic organisms that consume benthic detritus (Doi et al., 2006; Deines et al. 2007), but this would not help explain 13C enrichment in the mosquito larvae food web. Nadon & Himmelman (2006) have noted higher than expected enrichment of 13C in primary consumers of marine benthic detritus (+ 4 δ13C). They suggested selective feeding by the macroinvertebrate consumers, but this was not verified. We examined eubacterial and fungal community structures associated with leaf detritus in tree holes and noted differences in relative abundances of microbial groups when larval feeding ceased (Kaufman et al. 2008), but have not yet targeted the Archaea – a group that would be highly active in carbon isotope fractionation (Fry 2006). Because mosquito larvae feed on many microbial groups (bacteria, fungi, protists) associated with detritus, future studies will need to determine how these components alter detritus δ13C values before they reach mosquito tissue. While previous studies indicate that δ13C values of consumers are marginally higher than their diet, such isotope shifts are not necessarily typical of arthropod consumers. For example, trophic fractionation of δ13C by insects ranges from −2.7 to 5.5 % (Ostrom et al., 1997; McCutchan et al. 2003; Scheu & Folger 2004) and amphipods feeding on live or decaying seagrass had δ13C values that differed from the source by 9–10 % (Crawley et al. 2007). While the reasons for this variation are uncertain, it is clear that additional estimates of trophic fractionation for arthropods are needed.
Our results indicate that removal of lipids did not affect δ13C values and adjustment of δ13C values for lipid content of aquatic invertebrates seems to be problematic in general (Kiljunen et al. 2006). Removal of lipids is more of a concern in larger animal samples, where fat tissue can be a considerable portion of biomass (Post et al. 2007). In addition to the impracticality of removing lipids from small samples, extraction of lipids in mosquitoes adds additional steps to sample processing while reducing the mass of the minimal material available for analysis. Because storage lipids in mosquitoes are usually less than 20% of dry weight (Timmermann & Briegel 1996), lipid extraction may be inadvisable forδ13C measurements in these insects (Post et al. 2007). Using C:N normalization models in lieu of lipid extraction for aquatic invertebrates is also tenuous (Kiljunen et al. 2006; Logan et al. 2008). However, additional studies should be conducted to determine if this step is generally unwarranted for mosquitoes.
This study illustrates the utility of stable isotopes in studies of larval mosquito feeding ecology, but also points out some of the limitations. Mixing models for determining diet sources have many caveats (Fry 2006) and the one employed here may not have adequately addressed the particulars of the tree hole system. Specifically, the isotope values of the three observed primary inputs into tree holes, (leaves, flower parts, and invertebrate carcasses) did not adequately circumscribed the targeted consumer (mosquito) values in all cases, with late season 2006 samples falling out of the mixing triangle primarily due to δ13C values. This may have been due, in part, to our estimates of fractionation of 13C from plant material (see above), but may also reflect differences in lab vs. field conditions. δ15N values proved more useful in this study in estimating dietary source contributions from a mixture, dovetailing with our findings that nitrogen dynamics are an important driving force for mosquito production in these habitats (Kaufman & Walker 2006). Additionally, since most of our detrital inputs representatives were collected in the late spring (when larvae had hatched and were starting development), we did not account for possible changes in the isotope values of leaves, flowers, or invertebrates over the course of an entire mosquito season. It’s unlikely that the plant material isotope δ values would change substantially, even if exposed to long periods of decay (e.g., Osono et al. 2008; Lau et al. 2009), but the invertebrate category in our study already showed high variability in spring collection isotope values and later season values are unknown. Finally, this study did not account for all potential inputs into the system as we only collected the larger particulate fractions. Fine particulate inputs into tree holes and similar habitats may influence larval abundance and species composition in container habitats (Kling et al. 2007; Yee et al. 2010), and may have different isotope values from their large particulate counterparts. Inclusion of fine particulates in mixing models might help to explain more of the variation in isotope values we saw in field-collected mosquitoes over the season.
Our observations that ephemeral resources added in pulse inputs could be driving Ae. triseriatus production in these habitats also point out the need to re-consider established perceptions. In the case of mosquitoes and tree holes, the fact that leaf material is most often observed as the most abundant detrital source could be misleading because the important sources fueling mosquito development have disappeared from view due to more rapid decay rates and incorporation into microbial and mosquito biomass.
Acknowledgments
We are very grateful for the technical assistance of Lane Frazier, Renee Bloome, and Robert Burns in field collections, microcosm studies, and sample preparation, and that of Hassand Ghandi for EA and MS analyses. This study was funded by NIH-NIAID awards AI21884 and AI51374-01A1.
References
- Alto BW, Lounibos LP, Higgs S, Juliano SA. Larval competition differentially affects arvovirus infection in Aedes mosquitoes. Ecology. 2005;86:3279–3288. doi: 10.1890/05-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balesdent J, Girardin C, Mariotti A. Site-related 13C of tree leaves and soil organic matter in a temperate forest. Ecology. 1993;74:1713–1721. [Google Scholar]
- Barrera R, Amador M, Clark GG. Ecological factors influencing Aedes aegypti (Diptera: Culicidae) productivity in artificial containers in Salinas, Puerto Rico. Journal of Medical Entomology. 2006;43:484–492. doi: 10.1603/0022-2585(2006)43[484:efiaad]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Blaustein L, Chase JM. Interactions between mosquito larvae and species that share the same trophic level. Annual Review of Entomology. 2007;52:489–507. doi: 10.1146/annurev.ento.52.110405.091431. [DOI] [PubMed] [Google Scholar]
- Bligh ELG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bevins SN. Invasive mosquitoes, larval competition, and indirect effects on the vector competence of native mosquito species (Diptera: Culicidae) Biological Invasions. 2007;10:1109–1117. [Google Scholar]
- Carpenter SR. Resource limitation of larval treehole mosquitoes subsisting on beech detritus. Ecology. 1983;64:219–223. [Google Scholar]
- Collier KJ, Bury S, Gibbs M. A stable isotope study of linkages between stream and terrestrial food webs through spider predation. Freshwater Biology. 2002;47:1651–1659. [Google Scholar]
- Daugherty MP, Alto BM, Juliano SA. Invertebrate carcasses as a resource for competing Aedes albopictus and Aedes aegypti (Diptera: Culicidae) Journal of Medical Entomology. 2000;37:364–372. doi: 10.1093/jmedent/37.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deines P, Bodelier PLE, Eller G. Methane-derived carbon flows through methane-oxidizing bacteria to higher trophic levels in aquatic systems. Environmental Microbiology. 2007;9:1126–1134. doi: 10.1111/j.1462-2920.2006.01235.x. [DOI] [PubMed] [Google Scholar]
- Doi H, Takagi A, Mizota C, Okano J, Nakano S, Kikuchi E. Contribution of chemoautotrophic production to freshwater macroinvertebrates in a headwater stream using multiple stable isotopes. International Review of Hydrobiology. 2006;91:501–508. [Google Scholar]
- Fry B. Stable Isotope Ecology. Springer Science+Business Media, LCC; New York: 2006. p. 308. [Google Scholar]
- Goedkoop W, Akerblom N, Demandt MH. Trophic fractionation of carbon and nitrogen stable isotopes in Chironomus riparius reared on food of aquatic and terrestrial origin. Freshwater Biology. 2006;51:878–886. [Google Scholar]
- Gray SM, Miller TE, Mouquet N, Daufresne T. Nutrient limitation in detritus-based microcosms in Sarracenia purpurea. Hydrobiologia. 2006;573:173–181. [Google Scholar]
- Grey J. The use of stable isotope analyses in freshwater ecology: Current awareness. Polish Journal of Ecology. 2006;54:563–584. [Google Scholar]
- Gandhi H, Wiegner TN, Ostrom PH, Kaplan LA, Ostrom NE. Isotopic (13C) analysis of dissolved organic carbon in stream water using an elemental analyzer coupled to a stable isotope ratio mass spectrometer. Rapid Communications in Mass Spectrometry. 2004;18:903–906. doi: 10.1002/rcm.1426. [DOI] [PubMed] [Google Scholar]
- Hard JJ, Bradshaw WE, Malarkey DJ. Resource- and density-dependent development in tree-hole mosquitoes. Oikos. 1989;54:137–144. [Google Scholar]
- Harshaw L, Chrisawn C, Kittinger B, Carlson J, Metz G, Smith L, Paradise CJ. Decaying invertebrate carcasses increase growth of Aedes triseriatus (Diptera: Culicidae) when leaf litter resources are limiting. Journal of Medical Entomology. 2007;44:589–596. doi: 10.1603/0022-2585(2007)44[589:dicigo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Hawley WA. The effect of larval density on adult longevity of a mosquito, Aedes sierrensis: Epidemiological consequences. Journal of Animal Ecology. 1985;54:955–964. [Google Scholar]
- Hebert CE, Arts TM, Weseloh DVC. Ecological tracers can quantify food web structure and change. Environmental Science and Technology. 2006;40:5618–5623. doi: 10.1021/es0520619. [DOI] [PubMed] [Google Scholar]
- Hoekman D, Winston R, Mitchell N. Top-down and bottom-up effects of a processing detritivore. Journal of the North American Benthological Society. 2009;28:552–559. [Google Scholar]
- Hood-Nowotny R, Knols BG. Stable isotope methods in biological and ecological studies of arthropods. Entomologia Experimentalis et Applicata. 2007;124:3–16. [Google Scholar]
- Juliano SA. Species interactions among larval mosquitoes: Context dependence across habitat gradients. Annual Review of Entomology. 2009;54:37–56. doi: 10.1146/annurev.ento.54.110807.090611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MG, Walker ED, Smith TW, Merritt RW, Klug MJ. The effects of larval mosquitoes Aedes triseriatus and stemflow on microbial community dynamics in container habitats. Applied and Environmental Microbiology. 1999;65:2661–2673. doi: 10.1128/aem.65.6.2661-2673.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MG, Bland SN, Worthen ME, Walker ED, Klug MJ. Bacterial productivity and fungal biomass responses to feeding by larval Aedes triseriatus (Say) (Diptera: Culicidae) Journal of Medical Entomology. 2001;38:711–719. doi: 10.1603/0022-2585-38.5.711. [DOI] [PubMed] [Google Scholar]
- Kaufman MG, Goodfriend W, Kohler-Garrigan A, Walker ED, Klug MJ. Soluble nutrient effects on microbial communities and mosquito production in Ochlerotatus triseriatus habitats. Aquatic Microbial Ecology. 2002;29:73–88. [Google Scholar]
- Kaufman MG, Walker ED. Indirect effects of soluble nitrogen on growth of Ochlerotatus triseriatus larvae in container habitats. Journal of Medical Entomology. 2006;43:677–688. doi: 10.1603/0022-2585(2006)43[677:ieosno]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kaufman MG, Chen S, Walker ED. Leaf-associated bacterial and fungal community shifts in response to larvae of the mosquito, Ochlerotatus triseriatus. Microbial Ecology. 2008;55:673–684. doi: 10.1007/s00248-007-9310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Yee DA, Juliano SA. Interspecific and intraspecific differences in foraging preferences of container-dwelling mosquitoes. Journal of Medical Entomology. 2007;44:215–221. doi: 10.1603/0022-2585(2007)44[215:iaidif]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiljunen M, Grey J, Sinisalo T, Harrod C, Immonen H, Jones RI. A revised model for lipid-normalizing delta C-13 values from aquatic organisms, with implications for isotope mixing models. Journal of Applied Ecology. 2006;43:1213–1222. [Google Scholar]
- Kitching RL. The natural history and ecology of phytotelmata. Cambridge University Press; Cambridge: 2000. Food webs and container habitats; p. 579. [Google Scholar]
- Kitching RL. Food webs in phytotelmata: “bottom-up” and “top-down” explanations for community structure. Annual Review of Entomology. 2001;46:729–760. doi: 10.1146/annurev.ento.46.1.729. [DOI] [PubMed] [Google Scholar]
- Kling LJ, Juliano SA, Yee D. Larval mosquito communities in discarded vehicle tires in a forested and unforested site: detritus type, amount, and water nutrient differences. Journal of Vector Ecology. 2007;32:207–217. doi: 10.3376/1081-1710(2007)32[207:lmcidv]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneitel JM. Intermediate-consumer identity and resources alter a food web with omnivory. Journal of Animal Ecology. 2007;76:651–659. doi: 10.1111/j.1365-2656.2007.01250.x. [DOI] [PubMed] [Google Scholar]
- Lau DCP, Leung KMY, Dudgeon D. What does stable isotope analysis reveal about trophic relationships and the relative importance of allochthonous and autochthonous resources in tropical streams? A synthetic study from Hong Kong. Freshwater Biology. 2009;54:127–141. [Google Scholar]
- Layman CA, Arrington DA, Montana CG, Post DM. Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology. 2007;88:42–48. doi: 10.1890/0012-9658(2007)88[42:csirpf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Leonard PM, Juliano SA. Effect of leaf-litter and density on fitness and population performance of the treehole mosquitoe Aedes triseriatus. Ecological Entomology. 1995;20:125–136. [Google Scholar]
- Logan JM, Jardine TD, Miller TJ, Bunn SE, Cunjak RA, Lutcavage ME. Lipid corrections in carbon and nitrogen stable isotope analyses: comparison of chemical extraction and modeling methods. Journal of Animal Ecology. 2008;77:838–846. doi: 10.1111/j.1365-2656.2008.01394.x. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, Nishimura N, Escher RL. Seasonality and components of oak litterfall in southeastern Florida. Florida Scientist. 1992;55:92–98. [Google Scholar]
- Lounibos LP, Nishimura N, Escher RL. Fitness of a treehole mosquito: influences of food type and predation. Oikos. 1993;66:114–118. [Google Scholar]
- Maciel-de-Freitas R, Codeço CT, Lourenço-de-Oliveira R. Body size-associated survival and dispersal rates of Aedes aegypti in Rio de Janeiro. Medical and Veterinary Entomology. 2007;21:284–292. doi: 10.1111/j.1365-2915.2007.00694.x. [DOI] [PubMed] [Google Scholar]
- McCutchan JH, Lewis WM, Kendall C, McGrath CC. Variation in tropic shift for stable isotope ratios of carbon, nitrogen, and sulphur. Oikos. 2003;102:378–390. [Google Scholar]
- Murrell EG, Juliano SA. The role of detritus type in interspecific competition and population distributions of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) Journal of Medical Entomology. 2008;45:375–383. doi: 10.1603/0022-2585(2008)45[375:dtatoo]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadon MO, Himmelman JH. Stable isotopes in subtidal food webs: Have enriched carbon ratios in benthic consumers been misinterpreted? Limnology and Oceanography. 2006;51:2828–2836. [Google Scholar]
- O’Leary MH. Carbon isotopes in photosynthesis. Bioscience. 1988;38:328–336. [Google Scholar]
- Osono T, Takeda H, Azuma J. Carbon isotope dynamics during leaf litter decomposition with reference to lignin fractions. Ecological Research. 2008;23:51–55. [Google Scholar]
- Ostrom PH, Colunga-Garcia M, Gage SH. Establishing pathways of energy flow for insect predators using stable isotope ratios: field and laboratory evidence. Oecologia. 1997;109:108–113. doi: 10.1007/s004420050064. [DOI] [PubMed] [Google Scholar]
- Pasquaud S, Lobry J, Elie P. Facing the necessity of describing estuarine ecosystems: a review of food web ecology study techniques. Hydrobiologia. 2007;588:159–172. [Google Scholar]
- Peterson SO, Klug MJ. Effects of sieving, storage, and incubation temperature on the phospholipid fatty acid profile of a soil microbial community. Applied and Environmental Microbiology. 1994;60:2421–2430. doi: 10.1128/aem.60.7.2421-2430.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DL, Koch PL. Incorporating concentration dependence in stable isotope mixing models. Oecologia. 2002;130:114–125. doi: 10.1007/s004420100786. [DOI] [PubMed] [Google Scholar]
- Post DM. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology. 2002;83:703–718. [Google Scholar]
- Post DM, Layman CA, Arrington DA, Takimoto G, Quattrochi J, Montaña CG. Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia. 2007;152:179–189. doi: 10.1007/s00442-006-0630-x. [DOI] [PubMed] [Google Scholar]
- Scheu S, Folger M. Single and mixed diets in Collembola: effects on reproduction and stable isotope fractionation. Functional Ecology. 2004;18:94–102. [Google Scholar]
- Spence KO, Rosenheim JA. Isotopic enrichment in herbivorous insects: a comparative field-based study of variation. Oecologia. 2005;146:89–97. doi: 10.1007/s00442-005-0170-9. [DOI] [PubMed] [Google Scholar]
- Stenroth P, Holmqvist N, Nystrom P, Berglund O, Larsson P, Graneli W. Stable isotopes as an indicator of diet in omnivorous crayfish (Pacifastacus leniusculus): the influence of tissue, sample treatment, and season. Canadian Journal of Fisheries and Aquatic Sciences. 2006;63:821–831. [Google Scholar]
- Timmermann SE, Briegel H. Effect of plant, fungal and animal diets on mosquito development. Entomologia Experentia Applicata. 1996;80:173–176. [Google Scholar]
- Verdonschot RCM, Febria CM, Williams DD. Fluxes of dissolved organic carbon, other nutrients and microbial communities in a water-filled treehole ecosystem. Hydrobiologia. 2008;596:17–30. [Google Scholar]
- Walker ED, Lawson DL, Merritt RW, Morgan WT, Klug MJ. Nutrient dynamics, bacterial populations, and mosquito productivity in tree hole ecosystems and microcosms. Ecology. 1991;72:1529–1546. [Google Scholar]
- Yee DA, Juliano SA. Consequences of detritus type in an aquatic microsystem: effects on water quality, micro-organisms, and performance of the dominant consumer. Freshwater Biology. 2006;51:448–459. doi: 10.1111/j.1365-2427.2005.01504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee DA, Keseravaju B, Juliano SA. Direct and indirect effects of animal detritus on growth, survival, and mass of the invasive container mosquito Aedes albopictus (Diptera: Culicidae) Journal of Medical Entomology. 2007a;44:215–221. doi: 10.1603/0022-2585(2007)44[580:daieoa]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee DA, Kaufman MG, Juliano SA. The significance of ratios of detritus types and microorganism productivity to competitive interactions between aquatic insect detritivores. Journal of Animal Ecology. 2007b;76:1105–1115. doi: 10.1111/j.1365-2656.2007.01297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee DA, Kneitel JM, Juliano SA. Environmental correlates of abundances of mosquito species and stages in discarded vehicle tires. Journal of Medical Entomology. 2010;47:53–62. doi: 10.1603/033.047.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]


